Abstract
Fungicides are frequently used during tree fruit bloom and can threaten insect pollinators. However, little is known about how non-honey bee pollinators such as the solitary bee, Osmia cornifrons, respond to contact and systemic fungicides commonly used in apple production during bloom. This knowledge gap limits regulatory decisions that determine safe concentrations and timing for fungicide spraying. We evaluated the effects of two contact fungicides (captan and mancozeb) and four translaminar/plant systemic fungicides (cyprodinil, myclobutanil, penthiopyrad, and trifloxystrobin) on larval weight gain, survival, sex ratio, and bacterial diversity. This assessment was carried out using chronic oral ingestion bioassays where pollen provisions were treated with three doses based on the currently recommended field use dose (1X), half dose (0.5X), and low dose (0.1X). Mancozeb and penthiopyrad significantly reduced larval weight and survival at all doses. We then sequenced the 16S gene to characterize the larvae bacteriome of mancozeb, the fungicide that caused the highest mortality. We found that larvae fed on mancozeb-treated pollen carried significantly lower bacterial diversity and abundance. Our laboratory results suggest that some of these fungicides can be particularly harmful to the health of O. cornifrons when sprayed during bloom. This information is relevant for future management decisions about the sustainable use of fruit tree crop protection products and informing regulatory processes that aim to protect pollinators.
Similar content being viewed by others
Introduction
The solitary mason bee Osmia cornifrons (Hymenoptera: Megachilidae) was introduced to the United States from Japan in late 1970s and early 1980s1, ever since then, the species has played an essential role as a pollinator in managed ecosystems. Naturalized populations of this bee are part of the approximately 50 wild species of bees that have supplemented the honey bee for pollination of almond and apple orchards in the USA2,3. Mason bees face numerous challenges, including habitat fragmentation, pathogens, and pesticides3,4. Among pesticides, fungicides can reduce energy gain, foraging5 and fitness6,7. Although recent studies indicate mason bee fitness is directly affected by symbiotic and exobiotic microbes8,9 because bacteria and fungi can influence nutrition and immune response, the effects of exposure to fungicides on microbial diversity of mason bees are just beginning to be explored.
Before and during flowering, tree fruit orchards are sprayed with fungicides with varying modes of action (contact and systemic) to treat diseases such as apple scab, bitter rot, brown rot, and powdery mildew10,11. Fungicides were assumed harmless to pollinators; hence, they were recommended to growers during flowering. Contact and ingestion exposure of these fungicides to honey bees are relatively well known since it is part of the pesticide registration process by the U. S. Environmental Protection Agency and many other countries' regulatory agencies12,13,14. However, the effects of fungicides on non-honey bees are less well known as they are not required as part of the registration protocols in the US15. In addition, there is a general lack of standardized testing protocols for solitary bees16,17, and it is challenging to maintain colonies that provide bees for testing18. Various species of managed Osmia in Europe and the US are increasingly being tested to examine pesticide effects on wild bees, and recently, a standardized protocol was developed for O. cornifrons19.
Osmia cornifrons is univoltine and has been commercially used in tree fruit crops to supplement or replace honey bees. These bees emerge between March and April, with protandrous males emerging three to four days ahead of females. After mating, females actively collect pollen and nectar for provisioning a series of brood cells within tubular nest cavities (natural or artificial)1,20. Eggs are laid on the pollen provisions within a cell; the female then builds a mud partition before provisioning the next cell21. The first larval instar is enclosed within the chorion, feeding on embryonic fluids. From the second to fifth instar (prepupa), the larva feeds on pollen provisions22. Once the pollen provision is completely consumed, the larva forms a cocoon, pupates and becomes an adult in the same brood cell, usually by the end of summer20,23. The adult emerges in the following spring22. Adult survival is correlated with net energy gain (weight gain) based on the provisions consumed. Therefore, the nutritional quality of the pollen, as well as other factors such as weather or pesticide exposure, are determinants of survival and fitness24.
Pre-bloom applications of insecticides and fungicides with the ability to move within the plant vascular system to varying degrees from translaminar (e.g., able to move from the top surface of a leaf to the bottom surface as with some fungicides)25 to genuinely systemic neonicotinoid insecticides that can move from root applications up into the canopy have been previously shown to move into the nectar of apple bloom26 where they can kill adult O. cornifrons27. Some pesticides can also move into the pollen, affecting O. cornifrons larval development and causing mortality19. Other studies have shown that some fungicides can dramatically change nesting behavior in a congener, O. lignaria28. Furthermore, laboratory and field studies simulating pesticide (including fungicides) exposure scenarios demonstrated adverse effects on physiology22, morphology29, and survival in honey bees and some solitary bees12. The impact of various fungicide sprays applied directly to open flowers during bloom, which would contaminate the pollen collected by adult O. cornifrons for larval development, has yet to be explored30.
It is increasingly recognized that larval development is affected by the microbial community present in the pollen and digestive system. The bee microbiome influences parameters such as body mass31, metabolism alterations22, and susceptibility to pathogens32. Prior research has investigated the effects of developmental stages, nutrients, and environment on solitary bee microbiome. These studies revealed similarities in structure and abundance of the microbiome of both larvae and pollen33 and the most abundant bacteria genera, Pseudomonas and Delftia, in solitary bee species34,35. However, the impact of fungicides on the larval microbiome through direct oral exposure remains unexplored despite its relevance for strategies aimed at preserving bee health.
This study tested the effects of field-realistic doses of six commonly used fungicides registered for use on tree fruit throughout the US, which included contact and systemic fungicides via oral exposure to O. cornifrons larvae from contaminated provisions. We found contact and systemic fungicides reduced bee weight gain and increased mortality, with the most severe impact associated with mancozeb and penthiopyrad. Then, we compared the microbial diversity of larvae fed with pollen provisions treated with mancozeb against those fed with control provisions. We discuss potential mechanisms underpinning the lethality as well as implications for integrated pest and pollinator management (IPPM) programs36.
Material and methods
Rearing method and egg collection
Overwintering adults of O. cornifrons in cocoons were obtained from the Fruit Research Center in Biglerville, PA and stored at − 3 to 2 °C (± 0.3 °C). before the experiment (a total of 600 cocoons). In May 2022, groups of 100 cocoons of O. cornifrons were transferred daily to plastic cups (50 cocoons/cup, 5 cm DI × 15 cm length) with a napkin introduced inside the cup to aid emergence and provide a substrate for chewing, which reduces mason bee stress37. Two plastic cups with cocoons were placed inside an insect cage (30 × 30 × 30 cm, BugDorm MegaView Science Co. Ltd., Taiwan) with a 10 ml feeder containing 50% sucrose solution and held for four days to ensure emergence and mating at 23 °C with 60% RH and photoperiod 10 L (low intensity): 14 D. One hundred individual mated females, and males were released every morning for six days at the peak of apple bloom (100 individuals/day) in two artificial nest boxes (trap nests: width 33.66 × height 30.48 × length 46.99 cm; Supplementary Fig. 1) placed at The Arboretum at Pennsylvania State University, PA near cherry (Prunus cerasus 'Eubank' Sweet Cherry Pie™), peach (Prunus persica 'Contender,' Prunus persica 'PF 27A' Flamin Fury®), pear (Pyrus pyrifolia 'Olympic,' Pyrus pyrifolia 'Shinko,' Pyrus pyrifolia 'Shinseiki'), sweet crabapple (Malus coronaria) and numerous apple tree varieties (Malus coronaria, Malus domestica 'Co-op 30' Enterprise™, Malus domestica 'Co-Op 31' Winecrisp™, Malus domestica 'Freedom,' Malus domestica 'Golden Delicious', Malus domestica 'Nova Spy'). Each blue plastic nest box was placed on top of two wooden crates. Each nest box contained 800 empty Kraft tubes (spirally wound open-ended 0.8 cm inside diameter × 15 cm length) (Jonesville Paper Tube Corp, MI) with an inserted opaque glassine tube (0.7 cm outside diameter × 15 cm length) with plastic plugs (T-1X Caplugs) to provide nest sites.
Both nest boxes were faced east, covered with a green plastic garden fence (Everbilt Model# 889250EB12, hole size 5 × 5 cm, 0.95 m × 100 m) to keep rodents and birds out, and provided with mud on the soil surface near the nest boxes (Supplementary Fig. 1a). Osmia cornifrons eggs were harvested daily from the nest boxes by collecting 30 tubes and bringing them to the laboratory. An incision was made on the end of the tube using scissors; then, we unraveled the spiral tubing, revealing the brood cells. Individual eggs and their pollen provisions were removed using a bent spatula (Microslide Tool Set BioQuip Products Inc., CA). The eggs were incubated on moist filter paper and placed in Petri dishes for 2 hours38 and then used in our experiments (Supplementary Fig. 1b–d).
Fungicide exposure
In laboratory we evaluated the oral toxicity of six fungicides applied before and during apple bloom, at three concentrations (0.1X, 0.5X, and 1X, where 1X is the labeled high field dose applied in 100 gal water/acre = field concentration, Table 1). Each concentration was replicated 16 times (n = 16). Toxicity of two contact fungicides (Table S1: mancozeb at 2696.14 ppm and captan at 2875.88 ppm) and four systemic fungicides (Table S1: Penthiopyrad at 250.14 ppm; Trifloxystrobin at 110.06 ppm; Myclobutanil at 75.12 ppm; Cyprodinil at 280.845 ppm) which are widely used in fruits, vegetables, and ornamental crops. We homogenated pollen provisions using a grinder and transferred 0.20 g into a well (24–-Well Falcon Plate), added and mixed 1 µL of fungicide solution, and formed a pyramid-shaped pollen provision with a 1 mm deep hole where the egg was placed using a mini-spatula (Supplementary Fig. 1c,d). Falcon plates were held at room temperature (25 °C) and 70% RH19. We compared them against control larvae fed on homogenized pollen provisions treated with pure water. We recorded mortality and measured larval weight every other day until the prepupal larval instar using an analytical scale (Fisher Scientific, accuracy = 0.0001 g). Finally, the sex ratio was evaluated by dissecting cocoons after 2.5 months.
Metagenome sample preparation and sequencing
DNA was extracted from whole O. cornifrons larvae (n = 3 per treatment condition, mancozeb-treated and untreated pollen provisions), we conducted a microbial diversity analysis on these samples, particularly because the highest mortality rates were observed in larvae fed on mancozeb-treated pollen provisions.. Using the DNAZymoBIOMICS®-96 MagBead DNA Kit (Zymo Research, Irvine, CA), DNA was amplified, purified, and sequenced on Illumina® MiSeq™ with a v3 reagent kit (600 cycles). Bacterial 16S ribosomal RNA gene-targeted sequencing was performed using the Quick-16S™ NGS Library Prep Kit (Zymo Research, Irvine, CA), employing primers that target the V3-V4 region of the 16S rRNA gene. Additionally, 18S sequencing was conducted with 10% PhiX spike-in, and amplification was performed using the primer pair 18S001 and NS4.
Taxonomic profiling
Paired-end reads were imported and processed using the QIIME2 (v2022.11.1) pipeline39. These reads were trimmed and merged, and chimeric sequences were removed using the DADA2 plugin in QIIME2 (qiime dada2 denoise-paired)40. Taxonomic assignment for 16S and 18S was performed with the feature-classifier classify-sklearn plugin and the pre-trained silva-138-99-nb-classifier artifact.
Statistical analysis
All experiments' data were tested for normality (Shapiro-Wilks) and homogeneity of variance (Levene’s test) assumptions. Since data sets did not meet the assumptions for parametric analysis and transformation failed to normalize the residuals, we employed nonparametric two-way ANOVAs (Kruskal–Wallis) with two factors [time (three time points 2, 5 and 8 days) and fungicide] to assess the effect of treatments on larval fresh weight, then posthoc nonparametric pairwise comparisons were conducted using Wilcoxon test. We used a generalized linear model (GLM) with a Poisson distribution to compare the impacts of fungicides on survival at three fungicide concentrations41,42. For differential abundance analysis, amplicon sequence variant counts (ASVs) were collapsed at the genus level. Differential abundance comparisons between groups using 16S (genus level) and 18S relative abundances were performed using a Generalized Additive Model for Location, Scale, and Shape (GAMLSS) with zero-inflated beta (BEZI) family distribution, implemented in the metamicrobiomeR43 (v1.1). Mitochondria and Chloroplast genera were removed before differential analysis. Due to the differing levels of the 18S classification, only the lowest level of each taxon was used for differential analysis. All statistical analyses were conducted using R (v. 3.4.3., CRAN project) (Team 2013).
Results
Larval weight gain
Exposure to mancozeb, penthiopyrad, and trifloxystrobin significantly reduced the weight gain in O. cornifrons (Fig. 1). These effects were consistently observed in the three evaluated doses (Fig. 1a–c). Cyprodinil and myclobutanil did not significantly reduce the larval weight.
Mean fresh weights of Osmia cornifrons larvae measured at three-time points across four diet treatments (homogenized pollen provisions + fungicides: control, 0.1X, 0.5X, and 1X dosage. (a) Low dose (0.1X): First time point (1st day): χ2:30.99, DF = 6; P < 0.0001, second time point (5th day): 22.83, DF = 6; P = 0.0009; third time point (8th day): χ2:28.39, DF = 6; P < 0.0001. (b) Half dose (0.5X): First time point (1st day): χ2:35.67, DF = 6; P < 0.0001, second time point (1st day): χ2:15.98, DF = 6; P = 0.0090; third time point (8th day) χ2:16.47, DF = 6; P = 0.0041. (c) Field or full dose (1X):First time point (1st day) χ2:20.64, DF = 6; P = 0.0326, second time point (5th day): χ2:22.83, DF = 6; P = 0.0009; third time point (8th day): χ2:28.39, DF = 6; P < 0.0001. Two-way nonparametric anovas, followed by pairwise comparisons (α = 0.05) (n = 16). Bars represent mean ± S.E. *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001.
At the lowest dosage (0.1X), larval weight exhibited a reduction of 60% with trifloxystrobin exposure, 49% with mancozeb, 48% with myclobutanil, and 46% with penthiopyrad (Fig. 1a). When exposed to half of the field dose (0.5X), larval weight decreased by 86% with mancozeb, 52% with penthiopyrad, and 50% with trifloxystrobin (Fig. 1b). Full field dose (1X) reduced larval weight in an 82% with mancozeb, 70% with penthiopyrad, and approximately 30% with trifloxystrobin, myclobutanil, and sangard (Fig. 1c).
Mortality
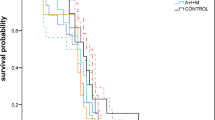
The highest mortality was observed in larvae fed on pollen treated with mancozeb, followed by penthiopyrad and trifloxystrobin. Mortality increased with the dose of mancozeb and penthiopyrad (Fig. 2; Table 2). However, O. cornifrons mortality only slightly increased as the concentration of trifloxystrobin rose; cyprodinil and captan did not significantly increase mortality compared to the control treatment.
Comparison of Osmia cornifrons larvae mortality after ingestion of pollen provision individually treated with six different fungicides. Susceptibility of O. cornifrons larvae by oral exposure was high with mancozeb and penthyopirad (GLM: χ2 = 29.45, DF = 20, P = 0.0059) (line, slope = 0.29, P < 0.001; slope = 0.24, P < 0.00, respectively).
Sex ratio changes
On average, 39.05% of individuals were females, while 60.95% were males across all treatments. In the control treatment, the proportion of females was observed to be 40% in both the low dose (0.1X) and half dose (0.5X) trials, and 30% in the field dose trial (1X). At the 0.1X dose, 33.33% of adults were females in larvae fed on pollen treated with mancozeb and myclobutanil, 22% for penthiopyrad, 44% for myclobutanil,41% for captan, and 31% for the control (Fig. 3a). At 0.5X dose, 33% of adults were females in mancozeb and penthiopyrad, 36% for trifloxystrobin, 41% for myclobutanil, 46% for cyprodinil, 53% for captan, and 38% for the control (Fig. 3b). At 1X dose, 30% were females for mancozeb, 36% for penthiopyrad, 44% for trifloxystrobin, 38% for myclobutanil, 50% for cyprodinil, and 38.5% for the control (Fig. 3c).
Microbiome
Analysis of 16S sequences showed that the bacteriome differed between larvae fed on mancozeb-treated pollen and those fed on untreated pollen (Fig. 4a). The microbial index was higher in untreated pollen-fed larvae than larvae fed on mancozeb-treated pollen (Fig. 4b). While the difference in observed richness between the groups was not statistically significant, it was markedly lower than in larvae fed on untreated pollen (Fig. 4c). The relative abundance showed that the microbiome of larvae fed on control pollen was more diverse than in those fed on mancozeb-treated (Fig. 5a). Descriptive analysis indicated the presence of 28 genera across the control and mancozeb-treated samples (Fig. 5b). c Analysis using 18S sequencing did not exhibit significant differences (Supplementary Fig. 2).
Comparisons of the Shannon richness and observed richness at the phylum level based on SAV profile for 16S sequences. (a) Principal coordinate analysis (PCoA) based on the overall structure of microbial communities in untreated pollen-fed larvae or control (blue) and mancozeb-fed larvae (orange). Each data point represents an individual sample. PCoA was calculated using Bray–Curtis distances with a multivariate t-distribution. Ellipses represent an 80% confidence level. (b) Boxplots, raw data (points) of Shannon richness, and c. observed richness. Box plots display the median line, interquartile range (IQR) boxes, and 1.5 × IQR (n = 3).
Composition of microbial communities in larva fed on mancozeb-treated and untreated pollen provisions. (a) Relative abundance of reads of microbial genera in larvae. (b) Heatmap of microbial communities identified. Delftia (odds ratio (OR) = 0.67, P = 0.0030) and Pseudomonas (OR = 0.3, P = 0.0074), Microbacterium (OR = 0.75, P = 0.0617); Rhodococcus (OR = 1.5, P = 0.0060). Heatmap rows are clustered using correlation distance and average linkage.
Discussion
Our results suggest that oral exposure to contact (mancozeb) and systemic (penthiopyrad and trifloxystrobin) fungicides which are widely applied during bloom significantly reduced weight gain and increased mortality in O. cornifrons larvae. Additionally, mancozeb notably reduced the microbiome's diversity and richness at the pre-pupal stage. Another systemic fungicide, myclobutanil, significantly reduced larval weight gain at all three doses. This effect was notable at the second (5th day) and third (8th day) time points. In contrast, cyprodinil and captan did not significantly reduced weight gain or survival compared to the control group. To our knowledge, this work is the first to determine the effects of field doses of a wide range of fungicides used in crop protection on O. cornifrons using direct exposure through pollen provisions.
All fungicide treatments significantly reduced weight gain compared to control treatments. Mancozeb had the most substantial impact on larval weight gain, declining 51% on average, followed by penthiopyrad. However, other studies did not report the adverse effects of a fungicide field dose on the larval stage44. Although dithiocarbamate fungicides have been shown to have low acute toxicity45, Ethylenebisdithiocarbamate (EBDCS), such as mancozeb, can degrade into ethylene thiourea. Given its reported mutagenic impacts on other animals, this degradation product may be responsible for the observed effects46,47. Previous research indicated that the formation of ethylene thiourea is influenced by factors such as increasing temperature48, moisture levels49, and length of product storage period50. Proper storage conditions for the fungicide could mitigate these adverse effects. In addition, concern about the toxicity of penthiopyrad has been raised by the European Food Safety Authority, which found carcinogenic effects in the digestive system of other animals51.
Oral ingestion of mancozeb, penthiopyrad, and trifloxystrobin increased the mortality of O. cornifrons larvae. In contrast, myclobutanil, cyprodinil, and captan did not affect mortality. These results diverge from Ladurner et al.52, which showed captan significantly reduced survival in O. lignaria and Apis mellifera L. (Hymenoptera, Apidae) adults. Furthermore, fungicides, such as captan and boscalid, were found to induce larval mortality52,53,54 or altered foraging55. These changes could, in turn, affect the nutritional quality of pollen, ultimately impacting the energy gain during the larval stage. The mortality observed in control groups was consistent with those reported in other studies56,57.
The male-biased sex ratio observed in our work can be attributed to factors such as insufficient mating58 and adverse weather conditions during the blooming period59, as previously suggested by Vicens & Bosch60 for O. cornuta. Although females and males in our study were given four days for mating—a period generally considered adequate for successful mating38—we intentionally reduced the light intensity to minimize stress. The modification, however, may have inadvertently hindered the mating process61. Furthermore, the bees were exposed to several days of unfavorable weather, including rain and cold temperatures (< 5 °C), which could have also negatively impacted mating success4,23.
Although our study focuses on the microbiomes of whole larvae, our findings provide insights into potential relationships among bacterial communities that may be crucial for bee nutrition and fungicide exposure. For instance, larvae fed on pollen treated with mancozeb exhibited a significant reduction in both the structure and abundance of their microbial communities compared to those fed on untreated pollen. In larvae that consumed untreated pollen, the dominant bacterial groups were Proteobacteria and Actinobacteria, which are primarily aerobic or facultatively aerobic. Delftia bacteria, often associated with solitary bee62 species, are known to exhibit antibiotic activity, suggesting their potential protective role against pathogens62,63,64. Another bacterial genus that was abundant in larvae fed on untreated pollen but significantly reduced in mancozeb-treated larvae was Pseudomonas. Our results corroborate previous studies that identified Pseudomonas as one of the most abundant genera in O. bicornis35 and other solitary bees34. Although experimental evidence of the Pseudomonas’ role in O. cornifrons health has not been studied, this bacterium has been shown to contribute to the synthesis of a defensive toxin in the beetle, Paederus fuscipes, and promotes arginine metabolism under in vitro conditions35,65. These observations suggest its potential role in viral and bacterial defense during O. cornifrons larval development. Microbacterium was another genus detected in our study, which has been reported in high abundance in black soldier fly larvae under starvation field conditions66. In O. cornifrons larvae, Microbacterium could contribute to the balance and resiliency of the gut microbiome under stress conditions. In addition, Rhodococcus was found in O. cornifrons larvae and is known for its detoxification capabilities67. This genus has also been found in the guts of A. florea but at very low abundance68. Our results indicate a diverse range of genetic variations across numerous microbial taxa that may alter metabolic processes in larvae. However, the functional diversity of bacteria in O. cornifrons needs to be better understood.
To conclude, the results suggest that mancozeb, penthiopyrad, and trifloxystrobin reduced weight gain and increased mortality of O. cornifrons larvae. Although there is increasing interest in the impacts of fungicides on pollinators, the effects of residual metabolites of these compounds need to be better understood. These results may be incorporated into recommendations for integrated pollinator management programs, which can instruct farmers to avoid certain fungicides during the prebloom and bloom period of fruit trees by fungicide selection and application timing change or promot use of less detrimental alternatives36. This information has critical implications for establishing pesticide application recommedations such as adjusting the existing spray program in fungicide selection and spray timing change or promote use of less detrimental alternatives. Additional studies are needed on the side effects of fungicides on sex ratio, foraging behavior, gut microbiome, and the molecular mechanisms underlying O. cornifrons weight loss and mortality.
Data availability
The source data underlying Figs. 1, 2 and 3 has been deposited in the figshare data repository DOI: https://doi.org/10.6084/m9.figshare.24996245 and https://doi.org/10.6084/m9.figshare.24996233. The sequences analysed during the current study (Figs. 4, 5) are available in the NCBI SRA repository, Accession PRJNA1023565.
Change history
18 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-56482-8
References
Bosch, J. & Kemp, W. P. Developing and establishing bee species as crop pollinators: The example of Osmia spp. (Hymenoptera: Megachilidae) and fruit trees. Bull. Entomol. Res. 92, 3–16 (2002).
Park, M. G. et al. Apple grower pollination practices and perceptions of alternative pollinators in New York and Pennsylvania. Renew. Agric. Food Syst. 35, 1–14 (2020).
Koh, I., Lonsdorf, E. V., Artz, D. R., Pitts-Singer, T. L. & Ricketts, T. H. Ecology and economics of using native managed bees for almond pollination. J. Econ. Entomol. 111, 16–25 (2018).
Lee, E., He, Y. & Park, Y.-L. Effects of climate change on the phenology of Osmia cornifrons: Implications for population management. Clim. Change 150, 305–317 (2018).
Artz, D. R. & Pitts-Singer, T. L. Effects of fungicide and adjuvant sprays on nesting behavior in two managed solitary bees, Osmia lignaria and Megachile rotundata. PloS One 10, e0135688 (2015).
Boff, S. et al. Low toxicity crop fungicide (fenbuconazole) impacts reproductive male quality signals leading to a reduction of mating success in a wild solitary bee. J. Appl. Ecol. 59, 1596–1607 (2022).
Sgolastra, F. et al. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 73, 1236–1243 (2017).
Kueneman, J. G., Gillung, J., Van Dyke, M. T., Fordyce, R. F. & Danforth, B. N. Solitary bee larvae modify bacterial diversity of pollen provisions in the stem-nesting bee, Osmia cornifrons (Megachilidae). Front. Microbiol. 13, 1057626 (2023).
Dharampal, P. S., Danforth, B. N. & Steffan, S. A. Exosymbiotic microbes within fermented pollen provisions are as important for the development of solitary bees as the pollen itself. Ecol. Evol. 12, e8788 (2022).
Kelderer, M., Manici, L. M., Caputo, F. & Thalheimer, M. Planting in the ‘inter-row’to overcome replant disease in apple orchards: A study on the effectiveness of the practice based on microbial indicators. Plant Soil 357, 381–393 (2012).
Martin, P. L., Krawczyk, T., Khodadadi, F., Aćimović, S. G. & Peter, K. A. Bitter rot of apple in the Mid-Atlantic United States: Causal species and evaluation of the impacts of regional weather patterns and cultivar susceptibility. Phytopathology 111, 966–981 (2021).
Cullen, M. G., Thompson, L. J., Carolan, J. C., Stout, J. C. & Stanley, D. A. Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS One 14, e0225743 (2019).
Pilling, E. D. & Jepson, P. C. Synergism between EBI fungicides and a pyrethroid insecticide in the honeybee (Apis mellifera). Pestic. Sci. 39, 293–297 (1993).
Mussen, E. C., Lopez, J. E. & Peng, C. Y. Effects of selected fungicides on growth and development of larval honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environ. Entomol. 33, 1151–1154 (2004).
van Dyke, M., Mullen, E., Wixted, D. & McArt, S. A Pesticide Decision-Making Guide to Protect Pollinators in Tree Fruit Orchards (Cornell University, 2018).
Iwasaki, J. M. & Hogendoorn, K. Non-insecticide pesticide impacts on bees: A review of methods and reported outcomes. Agric. Ecosyst. Environ. 314, 107423 (2021).
Kopit, A. M., Klinger, E., Cox-Foster, D. L., Ramirez, R. A. & Pitts-Singer, T. L. Effects of provision type and pesticide exposure on the larval development of Osmia lignaria (Hymenoptera: Megachilidae). Environ. Entomol. 51, 240–251 (2022).
Kopit, A. M. & Pitts-Singer, T. L. Routes of pesticide exposure in solitary, cavity-nesting bees. Environ. Entomol. 47, 499–510 (2018).
Phan, N. T. et al. A new ingestion bioassay protocol for assessing pesticide toxicity to the adult Japanese orchard bee (Osmia cornifrons). Sci. Rep. 10, 9517 (2020).
Bosch, J., Sgolastra, F. & Kemp, W. P. Timing of eclosion affects diapause development, fat body consumption and longevity in Osmia lignaria, a univoltine, adult-wintering solitary bee. J. Insect Physiol. 56, 1949–1957 (2010).
McKinney, M. I. & Park, Y.-L. Nesting activity and behavior of Osmia cornifrons (Hymenoptera: Megachilidae) elucidated using videography. Psyche 2012, 1–7 (2012).
Bosch, J., Sgolastra, F. & Kemp, W. P. Life cycle ecophysiology of Osmia mason bees used as crop pollinators. In Bee Pollination in Agricultural Ecosystems (ed. James, R.) 83–104 (Oxford University Press, 2008).
Sgolastra, F. et al. The long summer: pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. J. Insect Physiol. 57, 1651–1659 (2011).
Filipiak, Z. M. & Filipiak, M. The scarcity of specific nutrients in wild bee larval food negatively influences certain life history traits. Biology 9, 462 (2020).
Lewis, F. & Hickey, K. Fungicide usage on deciduous fruit trees. Annu. Rev. Phytopathol. 10, 399–425 (1972).
Sanchez-Bayo, F. & Goka, K. Impacts of pesticides on honey bees. Beekeep. Bee Conserv.-Adv. Res. 4, 77–97 (2016).
S. E. Shugrue, Pesticide use, habitat manipulation, and management changes as factors in pollinator sustainability in Pennsylvania apple orchards. (2016).
Ladurner, E., Bosch, J., Kemp, W. & Maini, S. Foraging and nesting behavior of Osmia lignaria (Hymenoptera: Megachilidae) in the presence of fungicides: cage studies. J. Econ. Entomol. 101, 647–653 (2008).
Hansted, L., Grout, B. W., Toldam-Andersen, T. B. & Eilenberg, J. An assessment of Osmia rufa (syn. bicornis) as a pollinator of the sour cherry (Prunus cerasus) cv. Stevnsbaer in eastern Denmark. J. Apic. Res. 53, 177–182 (2014).
Lehmann, D. M. & Camp, A. A. A systematic scoping review of the methodological approaches and effects of pesticide exposure on solitary bees. PLoS One 16, e0251197 (2021).
Kwong, W. K. & Moran, N. A. Gut microbial communities of social bees. Nat. Rev. Microbial. 14, 374–384 (2016).
Raymann, K. & Moran, N. A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 26, 97–104 (2018).
McFrederick, Q. S. et al. Flowers and wild megachilid bees share microbes. Microb. Ecol. 73, 188–200 (2017).
Fernandez De Landa, G. et al. The gut microbiome of solitary bees is mainly affected by pathogen assemblage and partially by land use. Environ. Microb. 18, 1–17 (2023).
Leonhardt, S. D., Peters, B. & Keller, A. Do amino and fatty acid profiles of pollen provisions correlate with bacterial microbiomes in the mason bee Osmia bicornis?. Philos. Trans. R. Soc. B 377, 20210171 (2022).
Biddinger, D. J. & Rajotte, E. G. Integrated pest and pollinator management—Adding a new dimension to an accepted paradigm. Curr. Opin. Insect Sci. 10, 204–209 (2015).
Porras, M. F. et al. Extreme heat alters the performance of hosts and pathogen. Front. Ecol. Evol. 11, 1186452 (2023).
Porras, M. F., Meza, J. S., Rajotte, E. G., Bourtzis, K. & Cáceres, C. Improving the phenotypic properties of the Ceratitis capitata (Diptera: Tephritidae) temperature-sensitive lethal genetic sexing strain in support of sterile insect technique applications. J. Econ. Entomol. 113, 2688–2694 (2020).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Callahan, B. et al. High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. https://doi.org/10.1038/nmeth (2016).
Hellerstein, D. & Mendelsohn, R. A theoretical foundation for count data models. Am. J. Agric. Econ. 75, 604–611 (1993).
Schmoll, T., Schurr, F. M., Winkel, W., Epplen, J. T. & Lubjuhn, T. Lifespan, lifetime reproductive performance and paternity loss of within-pair and extra-pair offspring in the coal tit Periparus ater. Proc. R. Soc. B Biol. Sci. 276, 337–345 (2009).
Ho, N. T., Li, F., Wang, S. & Kuhn, L. metamicrobiomeR: an R package for analysis of microbiome relative abundance data using zero-inflated beta GAMLSS and meta-analysis across studies using random effects models. BMC Bioinform. 20, 1–15 (2019).
Dharampal, P. S., Carlson, C. M., Diaz-Garcia, L. & Steffan, S. A. In vitro rearing of solitary bees: A tool for assessing larval risk factors. JoVE (J. Vis. Exp.) 137, e57876 (2018).
Vermeulen, L., Reinecke, A. & Reinecke, S. Evaluation of the fungicide manganese-zinc ethylene bis (dithiocarbamate)(Mancozeb) for sublethal and acute toxicity to Eisenia fetida (Oligochaeta). Ecotoxicol. Environ. Saf. 48, 183–189 (2001).
Crnogorac, G. & Schwack, W. Residue analysis of dithiocarbamate fungicides. TrAC Trends Anal. Chem. 28, 40–50 (2009).
García-Gutierrez, A. R., Poblano-Bata, R., Flores-Merino, M. V. & Castillo-Cadena, J. In vitro evaluation of the mutagenic and cytostatic effect of Tamaron, Lannate and Manzate alone and in mixture. J. Environ. Sci. Health B 51, 731–735 (2016).
Camoni, I., Di Muccio, A., Pontecorvo, D. & Citti, P. Survey of ethylenethiourea (ETU) in ethylenebis (dithiocarbamate)(EBDC) fungicides. Ecotoxicol. Environ. Saf. 16, 176–179 (1988).
López-Fernández, O., Pose-Juan, E., Rial-Otero, R. & Simal-Gándara, J. Effects of hydrochemistry variables on the half-life of mancozeb and on the hazard index associated to the sum of mancozeb and ethylenethiourea. Environ. Res. 154, 253–260 (2017).
Emara, A. R., Ibrahim, H. M. & Masoud, S. A. The role of storage on Mancozeb fungicide formulations and their antifungal activity against Fusarium oxysporium and Rhizoctonia solani. Arab. J. Chem. 14, 103322 (2021).
Authority, E. F. S. Conclusion on the peer review of the pesticide risk assessment of the active substance penthiopyrad. EFSA J. 11, 3111 (2013).
Ladurner, E., Bosch, J., Kemp, W. P. & Maini, S. Assessing delayed and acute toxicity of five formulated fungicides to Osmia lignaria Say and Apis mellifera. Apidologie 36, 449–460 (2005).
E. Mussen. (2003).
Wade, A., Lin, C.-H., Kurkul, C., Regan, E. R. & Johnson, R. M. Combined toxicity of insecticides and fungicides applied to California almond orchards to honey bee larvae and adults. Insects 10, 20 (2019).
Sprayberry, J. The effect of olfactory exposure to non-insecticidal agrochemicals on bumblebee foraging behavior. PLoS ONE https://doi.org/10.1371/journal.pone.0076273 (2013).
J. Bosch, W. P. Kemp, How to manage the blue orchard bee as an orchard pollinator. (2001).
Nicholls, E., Fowler, R., Niven, J. E., Gilbert, J. D. & Goulson, D. Larval exposure to field-realistic concentrations of clothianidin has no effect on development rate, over-winter survival or adult metabolic rate in a solitary bee, Osmia bicornis. PeerJ 5, e3417 (2017).
Seidelmann, K. & Rolke, D. Advertisement of unreceptivity–Perfume modifications of mason bee females (Osmia bicornis and O. cornuta) and a non-existing antiaphrodisiac. Plos One 14, e0215925 (2019).
Conrad, T., Stöcker, C. & Ayasse, M. The effect of temperature on male mating signals and female choice in the red mason bee, Osmia bicornis (L.). Ecol. Evol. 7, 8966–8975 (2017).
Vicens, N. & Bosch, J. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 29, 413–420 (2000).
K. Y. Lee, H. J. Yoon, M. A. Kim, Y. M. Kim, I. G. Park, in Proceedings of the Korean Society of Applied Entomology Conference. vol. 98, (2010).
Mohr, K. I. & Tebbe, C. C. Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ. Microbiol. 8, 258–272 (2006).
Kačániová, M., Terentjeva, M., Žiarovská, J. & Kowalczewski, P. Ł. In vitro antagonistic effect of gut bacteriota isolated from indigenous honey bees and essential oils against Paenibacillus larvae. Int. J. Mol. Sci. 21, 6736 (2020).
Huang, W. et al. Delftia tsuruhatensis TC1 symbiont suppresses malaria transmission by anopheline mosquitoes. Science 381, 533–540 (2023).
Silby, M. W., Winstanley, C., Godfrey, S. A., Levy, S. B. & Jackson, R. W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbial. Rev. 35, 652–680 (2011).
Yang, F., Tomberlin, J. K. & Jordan, H. R. Starvation alters gut microbiome in black soldier fly (Diptera: Stratiomyidae) larvae. Front. Microbiol. 12, 601253 (2021).
Ganeshprasad, D. et al. Gut bacterial flora of open nested honeybee, Apis florea. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2022.837381 (2022).
Mohr, K. I. & Tebbe, C. C. Field study results on the probability and risk of a horizontal gene transfer from transgenic herbicide-resistant oilseed rape pollen to gut bacteria of bees. Appl. Microbial. Biotechnol. 75, 573–582 (2007).
Acknowledgements
We thank the Pennsylvania Department of Agriculture (#C940000552) and State Horticultural Association of Pennsylvania. Drs. N. Phan and N. Joshi thank USDA-NIFA Project (# ARK02710). We also thank Dr. Mónica Medina and Dr. Claude De Pamphilis, and Dr. Paula Ralph for generously providing access to equipment, to Dr. H. Patch, H. Desorcie, and S. Edelson for their support during fieldwork. We also thank anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Contributions
D.B., E.G.R., K.P. conceived the idea and designed fungicide exposure experiment; D.B., K.P., N.K.J., N.J., selected fungicides; M.F.P. designed the microbiome experiment and provided ecological context to the experiment; M.F.P., M.B., T.L.L., A.C., and M.C. conducted the experiments; M.F.P., J.A.R.G., and T.L.L. analyzed data. M.F.P. and E.G.R. wrote the first draft. All authors contributed and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the name of author Neelendra K. Joshi, which was incorrectly given as Neelandra K. Joshi. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Porras, M.F., Raygoza Garay, J.A., Brought, M. et al. Fungicide ingestion reduces net energy gain and microbiome diversity of the solitary mason bee. Sci Rep 14, 3229 (2024). https://doi.org/10.1038/s41598-024-53935-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53935-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.