Abstract
Pancreatic fluid collections (PFCs) including pancreatic pseudocyst (PP) and walled-off necrosis (WON) are complications after acute pancreatitis. We aimed to evaluate the efficacy and safety of endoscopic ultrasound (EUS)-guided lumen-apposing metal stent (LAMS) placement to manage PFCs. Between June 2019 and May 2023, patients with symptomatic PFCs who underwent EUS-guided electrocautery-enhanced LAMS drainage were enrolled retrospectively from eight tertiary centers in Taiwan. In total, 33 [14 (42.42%) PP and 19 (57.58%) WON] patients were enrolled. Gallstones (27.27%) and abdominal pain (72.73%) were the most common etiology and indication for drainage. The technical and clinical success rates were 100% and 96.97%, respectively, and the mean procedure time was 30.55 (± 16.17) min. Complications included one (3.03%) case of self-limited bleeding; there were no cases of mortality. Seven (21.21%) patients had recurrence. Patients with disconnected pancreatic duct syndrome (DPDS) had a higher recurrence rate than those without (71.43% vs. 38.46%, p = 0.05). After replacing LAMSs with transmural double-pigtail plastic stents (DPSs) in the DPDS patients, the DPS migration rate was higher in the patients with recurrence (100% vs. 33.33%, p = 0.04). In conclusion, drainage of symptomatic PFCs with EUS-guided electrocautery-enhanced LAMS appears to be efficient and safe. Replacing LAMSs with DPSs in DPDS patients was associated with a lower recurrence rate.
Similar content being viewed by others
Introduction
Pancreatic fluid collections (PFCs) are one of the most common complications of acute pancreatitis (AP). After 3–4 weeks of severe AP, some collections of fluid or necrotic tissue may be encapsulated outside the pancreas, which can lead to the formation of a pancreatic pseudocyst (PP) or walled-off necrosis (WON)1. Approximately 20–30% of patients with AP complicated with persistent PFCs have been reported to have PFC-related symptoms or complications, including fever, infection, abdominal pain, biliary or gastrointestinal tract obstruction, and aneurysm formation, for which interventions are required2. Step-up care for symptomatic PFCs includes cessation and control of the offending factors, accompanied with medical therapy such as proton pump inhibitors, somatostatin, pancreatic enzyme supplements, and antibiotics to control infection2. However, a substantial proportion of patients with persistent symptomatic PFCs fail conservative treatment and are referred for drainage of infective fluid or removal of necrotic tissues. Conventionally, drainage of PFCs is performed by either percutaneous or surgical methods. The former is performed by percutaneous pigtail drainage and the creation of a cutaneous fistula with a self-expandable metal stent followed by necrosectomy, while the latter includes either open or laparoscopic necrosectomy and video-assisted retroperitoneal debridement2,3,4,5. However, percutaneous interventions are associated with a low resolution rate and high re-intervention and recurrence rates, and surgical management is associated with high rates of perioperative mortality and comorbidities, fistula formation, longer hospital stay, and poor quality of life5,6,7.
Endoscopic therapy including transpapillary and transmural internal drainage is an alternative to conventional interventions. These endoscopic techniques have a higher clinical success rate compared with percutaneous drainage, and lower risks of multiorgan failure, fistula formation, and shorter hospital stay than surgery5,6,7. Traditional endoscopic internal drainage involves the creation of a transmural fistula followed by plastic stenting. However, only infective fluid can be drained into the gastrointestinal tract with plastic stents, and the necrotic tissue remains in the walled-off cavity, leading to persistent symptoms and recurrence. In 2012, Binmoeller et al.8 published a porcine study using a novel metal stent, the so-called lumen-apposing metal stent (LAMS), to create a communicating tract between two isolated organs. Subsequently, endoscopic ultrasound (EUS)-guided LAMS placement has been used in several off-label indications with high technical and success rates9. Transoral endoscopic necrosectomy for WON can be achieved with the use of LAMSs, which have been shown to be superior to plastic stents in terms of procedure time, need for surgery, and clinical success and recurrence rates10. However, most previous studies on the application of LAMSs for the management of PFCs have not used electrocautery enhancement, and thus the technique requires a fine needle puncture followed by guidewire insertion and tract dilatation10,11,12,13,14,15,16,17,18,19,20,21,22. In this multicenter study, we aimed to investigate the efficacy and safety of a novel electrocautery-enhanced LAMS technique which avoided the need for a preceding needle puncture and guidewire advancement for the management of symptomatic PFCs. We also evaluated the predictors of clinical resolution and recurrence.
Methods
Study design
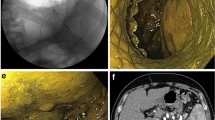
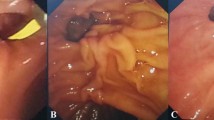
This retrospective multicenter study was conducted at eight tertiary centers in Taiwan, and it was approved by the Research Ethics Review Committees of the study institutes (Far Eastern Memorial Hospital FEMH-111171-E, China Medical University Hospital CMUH111-REC2-114, Kaohsiung Chang Gung Memorial Hospital 202201132B0D001, Changhua Christian Hospital CCH210103, Fu Jen Catholic University Hospital FJUH111205, and Shin Kong Wo Ho-Su Memorial Hospital 20221012R). All study methods were performed in accordance with the Committee on Publication Ethics guidelines. Codes rather than identifying information of the enrolled patients were used in this retrospective study, and the requirement for written informed consent was waived (Research Ethics Review Committee of Far Eastern Memorial Hospital). All data generated or analyzed during this study are included in this published article and its supplementary information files. Patients with AP who developed symptomatic PFCs and were treated by internal drainage with EUS-guided transmural LAMS placement (Figs. 1, 2, 3 and 4) between June 2019 and May 2023 were enrolled consecutively. All procedures were performed by endosonographers with more than 5 years of experience.
Endoscopic ultrasound techniques
In this study, we used Hot AXIOS™ electrocautery-enhanced LAMSs (Boston Scientific, Marlborough, MA, USA) for internal drainage. The Hot AXIOS™ is a saddle-shaped braided flexible metal stent which is flanged at both ends and is fully covered by a silicon membrane. The sizes of the PFCs, defined as the proportion of necrotic area in WON, and the distance between the wall of the encapsulated PFC and gastrointestinal tract, were evaluated by EUS. Color Doppler was used to avoid intervening vessels at the puncture site and trajectory. Under EUS-guidance, an electrocautery-enhanced delivery system was deployed (autocut mode, 100 W, effect 5, VIO200D, ERBE, Germany) from the gastrointestinal lumen into the PFC, and then the bilateral flanges were deployed in order to approximate the PFC and gastrointestinal wall. After endoscopic and fluoroscopic confirmation of the iatrogenic communicating tract created by the LAMS, the delivery system was removed. If clinical indications were present, such as persistent PFC-related symptoms after LAMS placement, transoral direct endoscopic necrosectomy (DEN) using foreign body retrievers was performed through the LAMS to remove necrotic tissues in the pancreatic WON. The LAMS was removed if PFC-related clinical symptoms resolved, and double-pigtail plastic stents (DPSs) were inserted via cysto-gastroenterostomy at the discretion of the endoscopist. Once the diagnosis of disconnected pancreatic duct syndrome (DPDS) was confirmed, transmural DPSs were inserted via cysto-gastroenterotomy or transpapillary pancreatic stenting if possible after removal of LAMS.
Outcome measurements
Clinical, radiological, and endoscopic data were reviewed from medical records. Technical success was defined as the ability to place and deploy a transmural LAMS and observe fluid gushing out under endoscopy after deployment during the index procedure. Clinical success was classified as symptom resolution, defined as disappearance of PFC-related symptoms within the first week of LAMS placement, and radiological resolution, defined as a drained PFC with a size less than 20 mm before LAMS removal. Adverse events (AEs) within 3 days were classified as immediate procedure-related events, and those occurring from 3 days until LAMS removal as delayed complications. DPDS was diagnosed if a disrupted pancreatic duct was shown in EUS, endoscopic retrograde pancreatography, or cross-sectional imaging studies including computed tomography and magnetic resonance imaging. Long-term clinical success was defined as complete resolution of the PFC-related symptoms at the last follow-up date. Recurrence was defined as the development of PFC-related symptoms or when the size of the PFC increased after complete resolution with the initial EUS-guided LAMS management.
Statistical analysis
The Student’s t-test and χ2 test or Fisher’s exact test were used to compare continuous and categorical variables, respectively. Continuous variables were expressed as mean (± standard deviation), and categorical variables were expressed as count (%). Retrospective analysis of consecutive patients with PFCs who underwent EUS-guided electrocautery-enhanced LAMS placement was conducted without sample size estimation. We divided the patients into two groups according to PFC recurrence, and univariate logistic regression analysis was used to evaluate the predictors, which were expressed as odds ratio (OR) for outcome assessment. Statistical significance was defined as a two-tailed p value < 0.05. The statistical analysis was performed using STATA software (version 11.0; Stata Corp, College Station, TX, USA). All data generated or analyzed during this study are included in this published article and its supplementary information files.
Results
Characteristics of the enrolled patients and PFCs
The demographic data of the enrolled patients are shown in Table 1. Thirty-three patients (13 females; 20 males; mean age 53.93 ± 15.28 years) with symptomatic PFCs were enrolled. Only three (9.09%) patients did not have comorbidities, while six (18.18%) patients had malignancy (one ovarian cancer, one endometrial cancer, one pancreatic ductal adenocarcinoma, one intraductal papillary mucinous neoplasm and two pancreatic neuroendocrine tumors). The most common etiology of AP was gallstones (27.27%), followed by alcohol (24.24%), hypertriglyceridemia (12.12%), malignancy (15.15%), and others (21.21%, two idiopathic, two drug-related pancreatitis, one trauma, one post-endoscopic retrograde pancreatography and one post-endoscopic papillectomy). Abdominal pain was the most common (72.73%) reason for the intervention, followed by infection (21.02%) and gastric outlet obstruction (6.06%). Fourteen (42.42%) patients had a PP, and nineteen (57.58%) had WON. The mean PFC size was 10.77 (± 5.48) cm, and the mean necrotic area in WON was 63.50% (± 23.46%). Most PFCs were unilocular (75.76%), with Balthazar grades of C (15.15%), D (57.58%) and E (27.27%). Seven (21.21%) patients had paracolic gutter extension of inflammatory fluid collection. The mean follow-up period was 421.97 (± 397.75) days.
Efficacy and safety of EUS-guided LAMS placement
Eight (24.24%), 10 (30.30%) and 15 (45.45%) patients underwent EUS-LAMS placement within 1 month, between 1 and 3 months, and more than 3 months after the onset of AP, respectively (Table 2). Most (66.67%) of the patients had not received an intervention before EUS-LAMS placement, while seven (21.21%) patients had undergone external pigtail drainage, two (6.06%) EUS-guided transmural DPS internal drainage, and two (6.06%) transpapillary pancreatic stenting (TPS) before EUS-LAMS placement. The LAMS size was 15 mm in all patients. The total procedure and puncture-to-deployment times were 30.5 (± 16.17) minutes and 5.76 (± 7.75) minutes, respectively. The most common transluminal route was from the gastric body (75.76%), followed by the gastric antrum (6.06%) and gastric fundus (6.06%). The technical success and symptom resolution rates were both 100%, and the radiological resolution rate was 96.97%. One (3.03%) patient had an early AE of procedure-related intracystic bleeding at 8 h after the procedure despite withholding clopidogrel for 7 days preoperatively. Fortunately, the bleeding resolved after conservative treatment with a blood transfusion and intravenous tranexamic acid. Among the patients with WON, a mean 2.65 (± 1.98) DEN sessions were performed. Complications during DEN included three (16.67%) cases of self-limited bleeding, two (11.11%) of stent migration, and one (5.56%) of symptomatic pneumoperitoneum. The mean indwelling LAMS time was 25.27 (± 10.09) days. Seven (21.21%) patients developed PFC recurrence, of whom two (28.57%) were asymptomatic and the others needed further interventions. The mean recurrence to removal of the LAMS time was 200.29 (± 184.11) days.
Predictors of recurrence
There were no significant differences in the etiology of pancreatitis, characteristics of the PFCs, and AP onset to LAMS placement time between the patients with and without PFC recurrence (Table 3). However, more of the patients with recurrence had DPDS (71.43% vs. 38.46%, p = 0.047). In addition, the patients with DPDS who developed recurrence had a higher migration rate of the replacement transmural DPS after LAMS removal compared to those with resolution (100% vs. 33.33%, p = 0.038). Univariate analysis showed that older age (OR 1.02), male sex (OR 1.83), WON type (OR 6.00), paracolic gutter extension (OR 4.13), more sessions of DEN (OR 1.38), presence of DPDS (OR 7.50), and those in whom the LAMS was not replaced with a transmural DPS (OR 2.13) were associated with a higher risk of PFC recurrence, although all without statistical significance (Table 4).
Discussion
Pancreatic fluid collections are one of the most common complications after severe AP1. Traditional approaches for symptomatic PFCs such as percutaneous and surgical interventions are often challenging, with high re-intervention and recurrence rates, peri-operative comorbidities, and mortality4,5,7. With advances in interventional EUS techniques, endoscopic treatment has replaced invasive procedures in the treatment of several biliopancreatic disorders13. To our knowledge, the present study is the first to demonstrate the efficacy and safety of managing symptomatic PFCs after severe AP using EUS-guided internal drainage with an electrocautery-enhanced rather than conventional LAMS system in Taiwan. Most previous studies on the application of LAMSs for the management of PFCs have not used an electrocautery-enhanced technique10,12,13,14,15,16,17,18,19,20,21,22. Therefore, tract creation using a fine needle with guidewire advancement and either mechanical or electrocautery-assisted dilatation is necessary. This requires a longer procedure time, and technical failure can occasionally occur when exchanging accessories. In our study, we used freehand placement of the LAMSs with an electrocautery-enhanced tip, and this technique did not require a fine needle puncture, guidewire insertion or dilatation for tract creation. This may have shortened the procedure time and resulted in the high technical (100%) and clinical (96.97%) success rates, with low complication rate (3.03%). The presence of DPDS was associated with recurrence, and we suggest replacing the LAMSs with a transmural DPSs in patients with DPDS to prevent symptomatic PFC recurrence.
Peripancreatic fluid with interstitial edema can occur in patients with severe AP, and transient organ failure and mortality have been reported in the first 2 weeks after onset2. The encapsulated collection of fluid or necrotic tissue has been reported in approximately 6–20% of patients with AP and 20–40% of those with chronic pancreatitis after 4 weeks of the disease, and cross-sectional imaging may disclose well-circumscribed intra- or extra-pancreatic homogeneous fluid density or non-liquid density, with varying degrees of loculation1,2,3,14,15,16. Most patients with chronic PFCs have spontaneous regression and do not require an intervention, however 15 and 30% of patients with PP or WON develop symptoms or complications, such as abdominal pain, infection, and biliary or enteral obstruction2,3. Surgical interventions or percutaneous drainage are the traditional standards of care for symptomatic PFCs, however complication and mortality rates of 64–95% and 6.7–64.1%, respectively, have been reported in patients undergoing a surgical intervention for PFCs4. A meta-analysis of 190 patients reported significantly lower risks of new-onset multiorgan failure (OR 0.31, 95% CI 0.10–0.98), perforations of visceral organs or enterocutaneous fistulae (OR 0.31, 95% CI 0.10–0.93), and pancreatic fistulae (OR 0.09, 95% CI 0.03–0.28) as well as a shorter hospital stay in patients who received endoscopic treatment compared to those who underwent surgery6. Another meta-analysis of six studies comparing endoscopic and percutaneous drainage of PFCs reported a higher re-intervention rate and lower resolution rate among patients receiving percutaneous therapy7. Therefore, a minimally invasive procedure with an endoscopic approach seems to be superior to conventional surgical and percutaneous methods in terms of clinical succuss, complications, recurrence, and quality of life.
Several modalities of endoscopic therapy are available for PFCs, including EUS-guided transmural cystoenterostomy for internal drainage, transoral endoscopic necrosectomy, and TPS11. Binmoeller et al.8 published a landmark report in which they used a novel lumen-apposing stent to create a gastroenterostomy and facilitate intubation with a gastroscope for further endoscopic treatment in a porcine model. Several specialized LAMSs and delivery devices have subsequently been introduced to provide endoscopists with an alternative to surgical interventions. LAMSs were initially approved in 2013 for the drainage of PP and WON with less than 30% solid debris, and thereafter their use has been expanded to many off-label indications, including gastroenterostomy, biliary and gallbladder drainage, and temporary gastric access for endoscopy9. Several clinical studies have demonstrated that EUS-guided internal drainage of symptomatic PFCs with LAMSs is an efficient and safe procedure, with technical and clinical success rates of 91–100% and 79–98%, respectively, and a major AE rate of less than 5%12,13,14,15,16,17,18. Compared with EUS-guided internal drainage using plastic stents, LAMSs have been associated with a higher clinical success rate (88–100% vs. 80–92%), shorter procedure time (10.5–14.9 min vs. 21.4–63.6 min), and lower recurrence rate (6.3–40% vs. 18.8–41.7%) in many clinical studies19,20,21,22,23. A multicenter retrospective study involving 14 institutes and 189 patients found that EUS-guided transmural drainage of WON with LAMSs had a higher clinical success rate (80.4% vs. 57.5%, p = 0.001), shorter procedure time (50.4 min vs. 64.6 min, p = 0.003), lower need for surgery (5.6% vs. 16.1%, p = 0.023), and lower recurrence rate (5.6% vs. 22.9%, p = 0.036) than plastic stents10. When performing DEN for WON, it is sometimes necessary to use multiple plastic stents followed by balloon dilation to create a larger tract for the gastroscope. In contrast, the saddle part of a LAMS has a larger diameter, which provides access for necrosectomy by intubating the transoral gastroscope directly into the PFC. LAMSs have been reported to have a higher DEN success rate compared to plastic stents (80.4% vs. 57.5%, p = 0.001)10. Taken together, these findings suggest that LAMSs can achieve more rapid control of infection and symptoms with less recurrence than plastic stents. In this study, we placed LAMSs with an electrocautery-enhanced tip using a freehand technique without first creating a tract. The technical and clinical success rates were 100% and 96.97%, respectively, which are higher than those using conventional LAMSs without electrocautery-enhanced tips reported in the literature (97.9–100% and 80.4–96%)9,10,12,14,16. In addition, the overall AE rate (3.03%) was lower than that reported for LAMSs without electrocautery-enhanced tips (9.8–24.3%)9,10,12,14,16. Only one patient developed self-limited postprocedural bleeding in our study.
The recurrence of PFCs and clinical success after EUS-guided drainage have been associated with the proportion of necrotic area in WON and the presence of DPDS. Maringhini et al.24 reported that more than 60% of their patients with a solid necrotic area of over 50% required more than three DEN sessions. In addition, the clinical success rate of EUS-guided drainage has been reported to be lower in patients with WON and a necrotic area of more than 40% (OR 0.10, 95% CI 0.02–0.60, p = 0.01)25. Several adjunct methods of DEN for WON have been reported in the literature, including using local instillation or nasocystic tube irrigation with normal saline, hydrogen peroxide, streptokinase or antibiotics26,27. However, none of them are currently recommended as an appropriate technique for DEN due to limited evidence28. After the resolution of PP and complete clearance of necrotic parts in WON with a collapsed cavity, the LAMS should be removed as soon as possible to prevent delayed complications. Regarding the timing of LAMS removal, an expert panel recommended removal at a mean time of 4.59 weeks7. However, the presence of DPDS, which is associated with a higher recurrence rate, should be investigated before LAMS removal. A retrospective review reported that 48 (50%) (6 PP and 42 WON) of 96 PFC patients had DPDS, and that those in whom DPSs were replaced with LAMSs had a lower recurrence rate (5% vs. 37%, p = 0.011)29. In addition, TPS was found to be associated with a higher successful clinical outcome rate in patients with DPDS (76.5% vs. 22.2%, p = 0.014) in a prospective study of 31 patients with DPDS30. Among patients with DPDS in our study, we have found that PFC recurrence was statistically significant higher in those with migration of transmural DPS and numerically higher in those with migration of transpapillary pancreatic stents after LAMS removal (Table 3). Thus, in patients with DPDS, long-term indwelling of transmural DPSs may be necessary to prevent recurrence, and transpapillary bridging of the disrupted main pancreatic duct, if possible, is recommended before removal of the transmural stent.
There are several limitations to this study. First, it was a retrospective study with a relatively small sample size and heterogeneous data, and we failed to identify independent risk factors for PFC recurrence in multivariate analysis. Because of reimbursement issues with the costly accessories associated with LAMSs in Taiwan, the number of candidate patients was limited. Therefore, we conducted this study at multiple centers, and further studies on the cost-effectiveness compared with other treatment modalities are warranted. Second, the practices used for the management of symptomatic PFCs were unique to each institute. Hence, our findings may not be generalizable to all centers. Third, not all of the enrolled patients underwent investigations for the presence of DPDS before LAMS removal and replacement with transmural DPSs. In addition, more than half (57.58%) of the enrolled patients with PFCs had WON with a mean necrotic area of 63.50%. Thus, the recurrence rate (21.21%) was higher than that reported in the literature.
In conclusion, EUS-guided internal drainage and endoscopic therapy via electrocautery-enhanced LAMSs for symptomatic PFCs appears to be an efficient and safe procedure. Before LAMS removal, investigations for the presence of DPDS and replacement with transmural DPSs are important to avoid recurrence. Further well-designed studies are warranted to compare this method with others and elucidate the appropriate indwelling times of the LAMSs and replacement plastic stents.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Data supporting this study are not publicly available and please contact chungchenshuan_3@yahoo.com.tw to access the original data.
References
Banks, P. A. et al. Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut 62(1), 102–111 (2013).
Forsmark, C. E., Vege, S. S. & Wilcox, C. M. Acute pancreatitis. N. Engl. J. Med. 375(20), 1972–1981 (2016).
Lankisch, P. G., Weber-Dany, B., Maisonneuve, P. & Lowenfels, A. B. The natural course of pancreatic pseudocysts following a first attach of acute pancreatitis (the 39th annual meeting of the american pancreatic association). Pancreas 37, 479 (2008).
Mier, J., León, E. L., Castillo, A., Robledo, F. & Blanco, R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am. J. Surg. 173(2), 71–75 (1997).
Sgaramella, L. et al. Open necrosectomy is feasible as a last resort in selected cases with infected pancreatic necrosis: A case series and systematic literature review. World J. Emerg. Surg. 15(1), 44 (2020).
Haney, C. M. et al. Endoscopic versus surgical treatment for infected necrotizing pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Surg. Endosc. 34(6), 2429–2444 (2020).
Szakó, L. et al. Endoscopic and surgical drainage for pancreatic fluid collections are better than percutaneous drainage: Meta-analysis. Pancreatology 20(1), 132–141 (2020).
Binmoeller, K. F. & Shah, J. N. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: A porcine study. Endoscopy 44(5), 499–503 (2013).
Choi, J. H. et al. Effectiveness and safety of lumen-apposing metal stents in endoscopic interventions for off-label indications. Dig. Dis. Sci. 67(6), 2327–2336 (2022).
Chen, Y. I. et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: A large international multicenter study. Endosc. Int. Open 7(3), E347–E354 (2019).
Jagielski, M. & Jackowski, M. The role of endoscopic transpapillary stenting of the main pancreatic duct during the endoscopic treatment of pancreatic fluid collections. J. Clin. Med. 10(4), 761 (2021).
Aburajab, M., Smith, Z., Khan, A. & Dua, K. Safety and efficacy of lumen-apposing metal stents with and without simultaneous double-pigtail plastic stents for draining pancreatic pseudocyst. Gastrointest. Endosc. 87(5), 1248–1255 (2018).
Adler, D. G. et al. Placement of lumen-apposing metal stents to drain pseudocysts and walled-off pancreatic necrosis can be safely performed on an outpatient basis: A multicenter study. Endosc. Ultrasound 8(1), 36–42 (2019).
Fugazza, A. et al. International multicenter comprehensive analysis of adverse events associated with lumen-apposing metal stent placement for pancreatic fluid collection drainage. Gastrointest. Endosc. 91(3), 574–583 (2020).
Hammad, T. et al. Efficacy and safety of lumen-apposing metal stents in management of pancreatic fluid collections: Are they better than plastic stents? A systematic review and meta-analysis. Dig. Dis. Sci. 63(2), 289–301 (2018).
Kumta, N. A. et al. EUS-guided drainage of pancreatic fluid collections using lumen apposing metal stents: An international, multicenter experience. Dig. Liver Dis. 51(11), 1557–1561 (2019).
Teoh, A. W. B. et al. Prospective multicenter international study on the outcomes of a newly developed self-approximating lumen-apposing metallic stent for drainage of pancreatic fluid collections and endoscopic necrosectomy. Dig. Endosc. 32(3), 391–398 (2020).
Yang, D. et al. Safety and rate of delayed adverse events with lumen-apposing metal stents (LAMS) for pancreatic fluid collections: A multicenter study. Endosc. Int. Open 6(10), E1267–E1275 (2018).
Falk, V. et al. The evolution of EUS-guided transluminal drainage for the treatment of pancreatic fluid collections: A comparison of clinical and cost outcomes with double-pigtail plastic stents, conventional metal stents and lumen-apposing metal Stents. J. Can. Assoc. Gastroenterol. 3(1), 26–35 (2018).
Ge, P. S. et al. Comparative study evaluating lumen apposing metal stents versus double pigtail plastic stents for treatment of walled-off necrosis. Pancreas 49(2), 236–241 (2020).
Guzmán-Calderón, E. et al. Head-to-head comparison between endoscopic ultrasound guided lumen apposing metal stent and plastic stents for the treatment of pancreatic fluid collections: A systematic review and meta-analysis. J. Hepatobil. Pancreat. Sci. 29(2), 198–211 (2022).
Shin, H. C., Cho, C. M., Jung, M. K. & Yeo, S. J. comparison of clinical outcomes between plastic stent and novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of peripancreatic fluid collections. Clin. Endosc. 52(4), 353–359 (2019).
Yang, J. et al. Lumen-apposing stents versus plastic stents in the management of pancreatic pseudocysts: A large, comparative, international, multicenter study. Endoscopy 51(11), 1035–1043 (2019).
Seicean, A. et al. What is the impact of the proportion of solid necrotic content on the number of necrosectomies during EUS-guided drainage using lumen-apposing metallic stents of pancreatic walled-off necrosis?. J. Gastrointestin. Liver Dis. 29(4), 623–628 (2020).
Zhu, H. et al. The role of solid debris in endoscopic ultrasound-guided drainage of walled-off necrosis: A large cohort study. J. Gastroenterol. Hepatol. 35(12), 2103–2108 (2020).
Lariño-Noia, J. et al. Endoscopic drainage with local infusion of antibiotics to avoid necrosectomy of infected walled-off necrosis. Surg. Endosc. 35(2), 644–651 (2021).
Messallam, A. A. et al. direct endoscopic necrosectomy with and without hydrogen peroxide for walled-off pancreatic necrosis: A multicenter comparative study. Am. J. Gastroenterol. 116(4), 700–709 (2021).
Guo, J. et al. A multi-institutional consensus on how to perform endoscopic ultrasound-guided peri-pancreatic fluid collection drainage and endoscopic necrosectomy. Endosc. Ultrasound. 6(5), 285–291 (2017).
Pawa, R. et al. Long-term transmural drainage of pancreatic fluid collections with double pigtail stents following lumen-apposing metal stent placement improves recurrence-free survival in disconnected pancreatic duct syndrome. Dig. Endosc. 34(6), 1234–1241 (2022).
Chen, Y. et al. Endoscopic transpapillary drainage in disconnected pancreatic duct syndrome after acute pancreatitis and trauma: Long-term outcomes in 31 patients. BMC Gastroenterol. 19(1), 54 (2019).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: C-S C, H-P W. Generation, collection, assembly, analysis and/or interpretation of data: C-S C, Y-T K, Y-C C, Y-C L, C-Y Y, K-C C, S-C L, C-K S, Y-C L, H-P W. Drafting or revision of the manuscript: C-S C, H-P W. Approval of the final version of the manuscript: C-S C, Y-T K, Y-C Chiu, Y-C L, C-Y Y, K-C C, S-C L, C-K S, Y-C L, H-P W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, CS., Kuo, YT., Chiu, YC. et al. Multicenter study of the efficacy and safety of electrocautery-enhanced lumen-apposing metal stents for the internal drainage of pancreatic fluid collections. Sci Rep 14, 5481 (2024). https://doi.org/10.1038/s41598-024-53785-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53785-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.