Abstract
Growth hormone (GH) has a long-standing history of use as an adjunctive therapy in the treatment of poor ovarian response (POR), but the optimal dosage and timing remains unclear. The aim of this study was to evaluate and compare the efficacy of different GH supplementation protocols through a network meta-analysis (NMA) and determine the optimal treatment protocol. This study was reported based on the Preferred Reporting Items for Systematic Reviews for Network Meta-Analysis (PRISMA-NMA) statement. Databases including PubMed, Web of Science, Cochrane Library and Embase were searched until June 2023. A total of 524 records were retrieved in our search, and 23 clinical studies comprising 4889 cycles were involved. Seven different GH protocols were identified. Results showed that compared to the control group, daily administration of 4–8 IU of GH during the follicular phase of the stimulation cycle had the best comprehensive therapeutic effects on improving the number of retrieved oocytes, mature oocytes, endometrial thickness, and reducing gonadotropin requirements in POR patients undergoing assisted reproductive therapy, with a relatively brief treatment duration and a moderate total GH dose. Subgroup analysis demonstrated that this protocol could significantly improve the clinical pregnancy rate of POR patients in the randomized controlled trials (RCT) subgroup and the African subgroup. Therefore, its clinical application is suggested. Besides, the potential advantages of long-term GH supplementation protocol (using GH for at least 2 weeks before oocyte retrieval) has merit for further research. Rigorous and well-designed multi-arm RCTs are needed in the future to confirm the conclusions drawn from this study.
Similar content being viewed by others
Introduction
In recent years, more women are effectively conceiving as a result of the rapid development of assisted reproductive technology (ART). However, poor ovarian response (POR), a pathological condition in which the ovary is unable to develop an adequate number of mature follicles in response to normal gonadotropin (Gn) stimulation, remains one of the major challenges of ART therapy1. Epidemiologic data show that the prevalence of POR is 9–24% in patients undergoing ovarian stimulation for in vitro fertilization (IVF)2. The etiology of POR has not been elucidated yet. It is acknowledged that decline in ovarian reserve caused by aging, genetic factors, ovarian surgery, and certain pelvic infections, is closely related to POR3. Pregnancy rate remains low in patients with POR despite a plethora of interventions, which may be attributed to insufficient oocytes at retrieval, insufficient high-quality embryos for transfer, and poor endometrial receptivity1,4.
The management and treatment of patients with POR is still a controversial issue in ART. Refining controlled ovarian hyperstimulation (COH) protocols and incorporating adjunctive medications are main approaches currently used in the treatment of POR5,6,7,8. Nowadays, many drugs are administrated as adjuvant therapy for POR, such as growth hormone (GH), dehydroepiandrosterone (DHEA), coenzyme Q10 (Co Q10), testosterone, estrogen, letrozole, clomiphene, human chorionic gonadotropin (hCG), and recombinant luteinizing hormone (rLH)7,8. However, no addition of adjuvants is recommended for PORs before and/or during COH to date, according to the recent ESHRE guideline of ovarian stimulation for ART9. Hence, reproductive endocrinologists are still working hard to find evidence of the efficacy of those medications in order to better promote their clinical applications.
GH is a peptide hormone mainly secreted from the pituitary gland10. As a key factor in the processes of cell growth, proliferation, and metabolism11, its value in reproduction and infertility has drawn much attention since it was first used as an adjuvant to Gn in 198812. During the past several decades, the involvement of GH in reproduction and infertility has been extensively studied. Previous researches indicated that GH may modulate reproductive function by binding to GH receptor directly or by promoting the secretion of insulin-like growth factors (IGFs, the major secondary messengers of GH) to play roles in ovarian steroidogenesis, early follicular recruitment, follicular development, oocyte nucleus maturation, preventing follicular atresia, corpus luteum generation and improving endometrial receptivity13,14,15,16. Therefore, it is considered biologically plausible that GH supplementation can bring benefits to people undergoing IVF, especially in cases with diminished ovarian reserve, who are at risk of experiencing POR14.
Although GH has been used in female infertility for over thirty years, there is still a lack of guidelines or consensus on the administration regimen of GH, so clinical protocols are always based on the experience of ART centers or individual clinicians, resulting in significant distinctions in the timing and dosage of administration. Considering the substantially higher cost of GH administration and its potential health risk17, it appears necessary to determine an appropriate dosage and administration schedule to prevent overdose and reduce patients' medical expenses. Besides, the effects of GH on clinical pregnancy rate and live birth rate in patients with POR remains unclear18,19,20, and whether it is related to the discrepancy of GH regimens is uncertain. In addition, previous meta-analyses focusing on this topic demonstrated a high degree of heterogeneity18,21,22, possibly attributable to variations in GH supplementation regimens, inconsistent diagnostic criteria (for example, the Bologna criteria23, the Poseidon criteria24 and diverse self-defined criteria), and other potential confounders, which may affect the strength of the evidence and influence clinical decision-making. All mentioned problems indicate the necessity of exploring differences in efficacy among different GH regimens (including dose and time of administration), in order to provide more evidence for the precise application of GH in patients with POR.
Network meta-analysis (NMA) is a feasible solution to compare the differential effects of multiple interventions and ranking them according to the predicted efficacy by analyzing the results of both direct and indirect comparisons, which can provide important evidence in developing clinical guidelines25. Hence, we performed a NMA to compare the effects of different GH supplementation protocols on the clinical outcomes of patients with POR undergoing ART therapy. Besides, subgroup analyses were further conducted to explore the main source of heterogeneity among the studies.
Materials and methods
Protocol registration
This NMA was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses for Network Meta-analyses (PRISMA-NMA)26. This review protocol was registered in the PROSPERO database before data extraction (CRD42022170119).
Literature search strategy
Databases including PubMed, Web of Science, Cochrane Library and Embase were searched by computer from January 1985 to June 2023. The main search terms were as follows: (“growth hormone” or “pituitary growth hormone” or “somatotropin”) and (“in vitro fertilization” or “intracytoplasmic sperm injection” or “test-tube fertilization” or “embryo transfer” or “IVF” or “ICSI”) and (“poor/low ovarian response/responder” or “POR”). Keywords were searched as both subject terms and free terms. References of eligible articles and related meta-analysis were reviewed manually to avoid missing any relevant literature.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (a) Study design: randomized controlled trials (RCTs) or cohort studies; (b) Language restriction: English; (c) Target population: women with POR undergoing IVF or intracytoplasmic sperm injection (ICSI) treatment; (d) Intervention: the intervention groups must be treated with GH and the control groups be treated without GH or with placebo. (e) Outcomes measures: the primary outcome was the clinical pregnancy rate. The second outcomes were the total dose of Gn for ovarian stimulation, number of retrieved oocytes, number of retrieved mature oocytes (MII oocytes), number of fertilized embryos, serum estradiol (E2) levels on hCG day, endometrial thickness on hCG day, and live birth rate. Clinical pregnancy rate (or live birth rate) was defined as the number of clinical pregnancies (or live births)/the number of embryo transfer cycles * 100%. If the number of embryo transfer cycles was not mentioned, clinical pregnancies (or live birth rate)/number of start cycles*100% was used instead. The continuous data should be reported as mean and standard deviation (SD).
Exclusion criteria were as follows: (a) The details of POR diagnostic criteria or treatment protocol were not reported; (b) Primary outcomes were missing; (c) GH combination with other adjuvant drugs; (d) The data were confusing but the authors could not be contacted; (e) Self-controlled studies, case reports, reviews, comments and replies.
Literature selection and data extraction
Literature selection and data extraction were conducted by two independent researchers (Z.Y.X. and W.Q.T.). Any disagreement was solved by the third researcher (Z.Y.). The title and abstract were scanned first and after excluding irrelevant references, the full text was read to determine whether eligible. If necessary, we would contact the authors by e-mail to obtain important missing information. Study characteristics involving general information, demography information of patients, intervention, major information of methodological quality assessment, and endpoint outcome measures were extracted from each article.
Methodological quality assessment
Methodological quality assessment was performed by two independent researchers (Z.Y.X and W.Q.T). RCTs and cohort studies were assessed by the Cochrane Risk of Bias Tool for randomized controlled trials27 and the Newcastle–Ottawa Scale (NOS)28, respectively. Any disagreement was solved by the third researcher (Z.Y.).
Statistical analysis
A NMA based on the Bayesians was performed, using multiNMA and geMTC packages in R (Version 4.2.3), to simultaneously compare the curative effect of different GH supplementation protocols for the adjuvant treatment of POR. A random effects model was used for data synthesis. Continuous data were summarized as mean difference (MD) with 95% credible interval (95%Crl), and dichotomous data as odds ratio (OR) with 95%Crl. Consistent and inconsistent models were both employed for NMA to analyze global inconsistency. If the difference in deviation information criterion (DIC) values between the two models was less than 5, the consistent model was chosen; otherwise, the inconsistent model was chosen. We also performed pairwise comparisons if direct data were available and used the node-splitting method to estimate local inconsistency. The efficacy of each intervention was predicted according to the surface under the cumulative ranking curve (SUCRA), with a larger area indicating better efficacy. Publication bias was analyzed graphically by funnel plots using the NMA package in Stata (Version 14.0). Subgroup analyses were completed using the Metan command in Stata.
Results
Eligible studies and characteristics
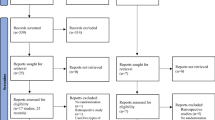
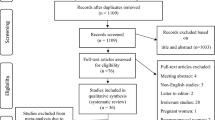
A total of 524 original articles were retrieved. After a series of screening steps, 15 RCTs and 8 cohort studies (comprising a total of 4889 treatment cycles) were finally included29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 (Fig. 1). The characteristics of the eligible studies are presented in Table 1.
According to the GH supplementation protocols reported in the literature, the daily dose of GH ranged from 0.3–15 IU/d (the average value was 6.78 IU/d, and the first and second tertiles were 4 IU/d and 8.5 IU/d, respectively). The administration time of GH ranged from the early follicular phase of the previous menstrual cycle to the mid-follicular phase of the stimulation cycle. Based on the mean and tertiles of the daily dose of GH, we divided the GH daily dose into three groups: low, medium, and high. Furthermore, considering the administration time of GH, we categorized the GH supplementation protocols into seven types (Protocol A-G). The categories are presented in Table 2.
Methodological quality assessment
We utilized stringent quality assessment methodologies to substantiate the integrity of the outcomes. Among the included RCTs, 9 (60.0%) trials had a low risk of bias on random sequence generation and 7 (46.7%) trials were with a low risk of bias on allocation concealment. No study had a high risk for bias on random sequence generation and allocation concealment. Only 6 (40.0%) trials had a low risk of bias on both blinding of participants and personnel and outcome assessment. There were 14 (93.3%) RCTs with low risk and one study with high risk of incomplete outcome data. The risk of bias due to selective reporting in the literature was unclear. One study was at high risk of bias due to insufficient patient recruitment, while other studies were at low or unclear risk. As for cohort studies, the NOS scores ranged from 8 to 9. The methodological quality assessments of studies are summarized in Supplementary Figure S1.
Publication bias assessment
A funnel plot was constructed to assess publication bias for the primary outcome measure. The results showed that the majority of points fall within the funnel, with a symmetrical distribution on both sides, suggesting a lower likelihood of publication bias (Supplementary Figure S2).
Inconsistency testing results
The network maps for the primary and secondary outcome measures can be found in Fig. 2. Global inconsistency was tested for all outcomes. The results showed that there was no inconsistency in any outcome measures, so a consistency model was selected for data synthesis (Supplementary Table S1). Local inconsistency was further evaluated for outcomes with closed loops in the network and the results showed no local inconsistency in outcome measures except for clinical pregnancy rate (Supplementary Table S2).
Network map of different GH supplementation protocol comparisons. The size of the nodes describes the total sample size of GH supplementation protocols. The thickness of the lines shows the number of studies. (a) Clinical pregnancy rate. (b) Total dosage of Gn required for ovarian stimulation. (c) Number of retrieved oocytes. (d) Number of retrieved MII oocytes. (e) Number of fertilized embryos. (f) Serum E2 levels on hCG day. (g) Endometrial thickness on hCG day. (h) Live birth rate.
NMA results
Primary outcome measure: clinical pregnancy rate
All studies29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 reported the clinical pregnancy rate for seven different GH supplementation protocols. Results showed that no protocol could significantly improve the clinical pregnancy rate in POR patients (compared to the control group, > 0.05) (Table 3). The SUCRA values for different GH protocols were as follows: F (0.72), C (0.66), B (0.56), E (0.56), A (0.52), D (0.43), G (0.35), Control (0.21) (Fig. 3).
Secondary outcome measure: total dosage of Gn required for ovarian stimulation
Fourteen studies33,34,35,36,38,40,41,42,44,45,46,48,50,51 reported the total Gn dosage for five different GH supplementation protocols. Protocol D was found to significantly reduce the Gn dosage in POR patients (compared to the control group, MD − 764.31, 95%CrI − 1519.70 to − 12.46) (Table 3). The SUCRA values for different GH protocols were as follows: D (0.78), B (0.60), E (0.52), F (0.51), G (0.45), Control (0.16) (Fig. 3).
Secondary outcome measure: number of retrieved oocytes
Seventeen studies30,34,35,36,38,39,40,41,42,43,44,45,46,47,48,49,51 reported the number of retrieved oocytes for seven different GH supplementation protocols. Protocol A and Protocol D were found to significantly increase the number of retrieved oocytes in POR patients (compared to the control group, MD 3.18, 95%CrI 1.42 to 4.92; MD 1.19, 95%CrI 0.29 to 2.00, respectively) (Table 3). The SUCRA values for different GH protocols were as follows: A (0.98), D (0.64), F (0.56), E (0.48), G (0.47), B (0.46), C (0.26), Control (0.15) (Fig. 3).
Secondary outcome measure: number of retrieved MII oocytes
Twelve studies33,34,35,36,38,39,40,42,46,48,49,50 reported the number of retrieved MII oocyte for five different GH supplementation protocols. Protocol D and Protocol G were found to significantly increase the number of retrieved MII oocytes in POR patients (compared to the control group, MD 1.82, 95%CrI 0.68 to 2.99; MD 1.75, 95%CrI 0.19 to 3.31, respectively) (Table 3). The SUCRA values for different GH protocols were as follows: B (0.72), A (0.72), D (0.64), G (0.48), E (0.40), Control (0.03) (Fig. 3).
Secondary outcome measure: number of fertilized embryos
Ten studies33,34,35,36,38,42,44,46,48,49 reported the fertilized embryo count for four different GH supplementation protocols. Results showed that no protocol could significantly improve the number of fertilized embryos in POR patients (compared to the control group, p > 0.05) (Table 3). The SUCRA values for different GH protocols were as follows: G (0.76), B (0.59), E (0.54), D (0.52), Control (0.09) (Fig. 3).
Secondary outcome measure: serum E2 levels on hCG day
Twelve studies33,34,35,36,37,38,39,40,42,43,48,49 reported the serum E2 levels on hCG day for six different GH supplementation protocols. Results showed that no protocol could significantly improve the serum E2 levels on hCG day in POR patients (compared to the control group, p > 0.05) (Table 3). The SUCRA values for different GH protocols were as follows: D (0.70), E (0.68), A (0.61), G (0.58), B (0.46), Control (0.26), C (0.22) (Fig. 3).
Secondary outcome measure: endometrial thickness on hCG day
Ten studies34,35,36,38,40,42,46,47,48,49 reported the endometrial thickness on hCG day for four different GH supplementation protocols. Protocol D and Protocol E were found to significantly increase the endometrial thickness in POR patients (compared to the control group, MD 0.44, 95%CrI 0.18 to 0.68; MD 0.64, 95%CrI 0.27 to 1.10, respectively) (Table 3). The SUCRA values for different GH protocols were as follows: E (0.94), D (0.75), G (0.45), Control (0.23), B (0.12) (Fig. 3).
Secondary outcome measure: live birth rate
Eleven studies29,31,38,41,42,44,45,46,47,50,51 reported the live birth rate for five different GH supplementation protocols. None of the protocols showed a significant improvement in the live birth rate in POR patients (compared to the control group, p > 0.05) (Table 3). The SUCRA values for different GH protocols were as follows: G (0.80), B (0.67), F (0.64), E (0.43), D (0.25), Control (0.20) (Fig. 3).
Comprehensive ranking results
The comprehensive ranking results for all the outcomes are displayed in Table 3. The asterisk (*) indicated results that had a statistically significant difference compared to the control group (p < 0.05), while the remaining treatment protocols showed no statistically significant difference (p > 0.05) (Table 4).
Subgroup analysis
According to the NMA's results, medium dose, follicular phase protocol (Protocol D) was utilized the most frequently. In addition, it demonstrated greater efficacy than other protocols in terms of minimizing the total dose of Gn, increasing the number of retrieved oocytes, and enhancing endometrial conditions with a moderate administration time and injection dose. Therefore, we conducted a subgroup analysis based on diagnostic criteria, study design, COH protocol, and geographic region, focusing on the primary outcome measure (clinical pregnancy rate), to explore potential sources of heterogeneity among studies (the study by Dor31 was not included in the analysis as both the experimental and control groups had zero events, not contribute to the meta-analysis). The results showed that Protocol D significantly improved the clinical pregnancy rate in the RCT subgroup (OR 1.76, 95% CI 1.12 to 2.76, p = 0.014, I2 = 0.0). In addition, trials in Africa showed relatively positive therapeutic effects (OR 1.65, 95% CI 1.03 to 2.65, p = 0.039, I2 = 0.0). It did not show a significant improvement in the clinical pregnancy rate in the remaining subgroups (p > 0.05) (Table 5).
Discussion
With an increasing number of women delaying childbirth, POR has become a great obstacle in ART, which could lead couples to abandon treatment or seek oocyte donation20. GH has a long-standing history of use as an adjunctive therapy in the treatment of POR. However, there is no standardized protocol for the application of GH to date and the clinical efficacy of GH remains controversial52. Thus, we endeavor to explore whether there is an optimal GH supplementation protocol for enhancing the clinical outcomes of POR patients. This study represents a comprehensive synthesis of data regarding currently GH supplementation protocols for PORs undergoing ART therapy. The results of this NMA can be summarized as follows. First, administering 4–8 IU/d of GH during the follicular phase of the stimulation cycle was the most commonly used protocol in the clinic and it resulted in the best comprehensive improvement in clinical outcomes for POR patients, including increasing the number of retrieved oocytes, the number of mature oocytes, endometrial thickness on hCG day, and reducing total Gn requirements, but had no effect on the number of fertilized embryos, serum E2 levels on hCG day, live birth rate per embryo transfer cycle. Second, subgroup analysis focus on the clinical pregnancy rate showed that the aforementioned treatment protocol could significantly improve the clinical pregnancy rate of POR patients in the RCT subgroup and the African subgroup, but diagnostic criteria and ovarian stimulation protocols appear to not correlate with the efficacy of this protocol. Third, using GH for at least 2 weeks before oocyte retrieval has certain benefits in improving the number of oocytes retrieved and endometrial thickness on the day of hCG administration, however, the advantages are not significantly greater than those of the former protocol, and the optimal dosage remains unclear; therefore, further research is warranted.
Only a handful of prior meta-analyses examined the differences in efficacy among different GH administration protocols on assisted reproductive outcomes in POR patients. Based on the timing of GH administration, Yu et al.21 categorized GH protocols into luteal phase protocols and follicular phase protocols, and the results demonstrated that only GH administration during the follicular phase improved pregnancy rates and live birth rates in patients with POR. Similarly, Cozzolino et al.53 analyzed the influence of GH administration timing on clinical pregnancy rate of PORs and found that positive result was observed only when it was used during the ovarian stimulation period. Nevertheless, neither of the above studies accounted for GH dosage. Shang and colleagues19 also confirmed a dose- and time-dependent association between GH and clinical outcomes in POR patients. They indicated that daily administration of < 5 IU/d of GH or administration starting from the follicular phase of the previous cycle before COH increased endometrial thickness and the likelihood of conception more significantly. In contrast, administration of 5–10 IU/day was associated with better oocyte and embryo quality. However, in this report, they only used traditional meta-analysis methods and treated daily dosage and administration timing of GH as two independent variables, which were not conducive to determining the optimal GH administration protocol.
In response to the aforementioned issues, we placed a significant emphasis on additional research. To our knowledge, this is the first meta-analysis to classify GH supplementation protocols by integrating daily dosage and schedule of administration. In accordance with previous research22,53, our findings demonstrate the efficacy of adding GH during the follicular phase of the stimulation cycle. The present NMA indicates that a dosage of 4–8 IU/day of GH administered during the follicular phase of stimulation cycle is the most widely used protocol in clinical practice and has the best comprehensive therapeutic effects on improving the number of oocytes and endometrial thickness, which were regarded two crucial factors for increasing embryo implantation rates and pregnancy rates in assisted reproduction, and reducing the total dose of Gn for ovarian stimulation, which was considered to be one of the important indicators to evaluate ovarian response. In addition to its clinical efficacy, this method also offers the benefits of a shorter administration time, a more moderate injection dosage, and a more convenient injection process, which can alleviate the inconvenience and pain associated with frequent clinic injections, as well as the financial burden on patients. Thus, this protocol seems to be the optimal protocol and has broad applications and research prospects. However, we did not observe the impact of this protocol on fertilized embryo number and E2 level on the hCG day, which may be related to the inconsistent POR diagnostic criteria of the involved patients and the inclusion of non-RCTs, therefore, the results of those endpoints need further confirmation.
Previous meta-analyses have consistently shown controversial results regarding the effect of adding GH on the clinical pregnancy rate and live birth rate of patients with POR. A traditional meta-analysis by Kolibianakis et al.20 suggested that the addition of GH could improve the probability of clinical pregnancy and live birth in POR patients. Another meta-analysis by Yu et al.21 indicated that the clinical pregnancy rate of POR patients was not associated with the addition of GH. Meta-analysis by Shang et al.19 reported that adding GH could improve clinical pregnancy rates and live births in POR patients. The recent meta-analysis by Cozzolino et al.53 showed that supplementation of GH in POR patients increased the clinical pregnancy rate, but did not affect the ongoing pregnancy rate and live birth rate. Some single studies have also drawn inconsistent conclusions38,49,54. In our NMA, no GH supplementation protocol has been proven effective in improving the clinical pregnancy rate and live birth rate of POR patients according to the overall pooled results. However, it is important to consider that the objective of COH is to recruit a sufficient number of follicles to compensate for the inefficiencies of embryo culture and selection for transfer55, and in assisted reproduction, the number of retrieved oocytes is regarded as a significant prognostic variable with a linear correlation to live birth rates56, so it is possible that, despite the lack of improvement in pregnancy rate and live birth rate within a single transfer cycle, the increased quantity and maturity of oocytes may contribute to a greater likelihood of cumulative pregnancies and live births due to more embryos available for transfer and cryopreservation. We look forward to conducting further research in the future to validate this hypothesis.
Medium dose, follicular phase protocol (Protocol D) is extensively employed and relatively effective, so it was the focus of subgroup analyses. We hypothesized, based on existing research, that diverse diagnostic criteria, ovarian stimulation protocols, geographical regions, and ethnicities may contribute to the heterogeneity. In addition, since non-RCTs were included, subgroups were also categorized according to the study design. The results showed low heterogeneity (I2 < 50%) in most subgroups, indicating that the aforementioned factors are likely the main contributors to heterogeneity. We found that when only analyzing RCTs, Protocol D could significantly increase the clinical pregnancy rate of patients, but the overall pooled results showed the opposite effect, partially confirming the efficacy of this protocol but also indicating the uncertainty of the results, which highlights the importance of conducting high-quality RCTs. This protocol received more positive feedback in studies conducted in Africa, which may be attributed to the fact that all African studies were RCTs published within the past decade. Diagnostic criteria appeared to not correlate with the efficacy of Protocol D on clinical pregnancy rate, but standardized diagnostic criteria is still critical because it can effectively reduce population heterogeneity and improve the repeatability of results. Moreover, efficacy of Protocol D on primary outcome measures seems unaffected by the COH regimen. Although the recent studies we included tended to adopt gonadotropin-releasing hormone (GnRH) antagonist protocol, this may not be generalizable. Current COH protocols for PORs mainly include GnRH antagonist protocol, progestion-primed ovarian stimulation (PPOS) protocol, oral superovulation agent protocol, GnRH agonist long protocol, natural/modified-natural cycle protocol, etc.57,58,59,60. Recent studies seem to focus more on the former two protocols. However, the absence of clear evidence indicating which protocol is preferable means that clinical practice relies to some extent on the experience9. A latest meta-analysis of GH supplementation in women with diminished ovarian reserve reported that GH, when used in conjunction with the GnRH antagonist protocol, resulted in a greater increase in the number of oocytes retrieved compared with the GnRH agonist protocol61. But there is still no direct evidence to prove the superiority or inferiority of different COH protocols when GH is used as an adjuvant therapy in POR treatment. The above issues require further exploration. Owing to the small sample sizes, results of subgroup analysis should be interpreted with caution.
It is commonly accepted that the development and maturation of follicles require approximately 75 days, and the role of GH is found to begin at the initial stage of follicle development62,63. Therefore, to enhance oocyte yield, it seems more appropriate to initiate GH co-treatment before commencing ovarian stimulation, which is consistent with the regularity of follicle growth and maturation. Gleicher and colleagues endorsed this hypothesis and suggested that the administration of GH should at least 6 weeks prior to COH64. In this NMA, we have found that compared to the control group, using GH at a dosage > 8 IU/day for at least 2 weeks before oocyte retrieval could improve the number of obtained oocytes in POR patients but did not show significant advantages compared to the medium dose, follicular phase protocol (protocol D). Therefore, the application value of this protocol needs further exploration. We have also found that the administration of GH at a dosage of 4-8 IU/day for at least 2 weeks had advantages in improving endometrial thickness on hCG day (an important indicator for assessing endometrial receptivity65), which may provide an alternative treatment option for POR patients who have thin endometrium or poor endometrial receptivity, with the goal of improving the implantation rate. Besides, some existing studies have focused on the efficacy of administrating GH at a dosage less than 4 IU/day (especially < 1 IU/day), which is considered low-dose, for at least 2 weeks. Lattes et al.66 and Safdarian et al.46 both reported that administration of GH of 0.3–0.5 IU/day from the preceding cycle to hCG day had positive effects on ovarian stimulation and pregnancy outcomes. If this protocol is proven effective, its advantages in reducing GH dosage and lowering medical expenses may position it as a promising treatment regimen in the future. Unfortunately, the current evidence from our NMA do not support the widespread application of the low-dose, long-term protocol in clinical practice, and more research is required to further evaluate its effectiveness and advantages.
Other protocols are utilized on a relatively infrequent basis, and they have not demonstrated any advantages in terms of efficacy or cost-effectiveness, indicating limited clinical viability. However, sample size and study design affect the quality of evidence, so the results need to be interpreted rationally.
It should be noted that none of the RCTs included in this NMA had a high risk of bias for random sequence generation and allocation concealment, and only one had a high risk of incomplete outcome data (the three items mentioned above are deemed to be the key entries67). Additionally, the cohort studies included in this NMA had NOS scores of 8–9. In general, included clinical had a moderately good design and methodological quality. Thus, the findings of our NMA can provide some evidence for clinicians when making decisions about the timing and dosage of GH administration in patients with POR. Nonetheless, as a result of the limited number, small scale and unavoidable clinical heterogeneity of extant clinical studies, the level of existing evidence is not very strong, and more well-designed trials would be necessary to determine the application value of these protocols.
This study has some limitations that should be considered. First, despite undertaking subgroup analyses, there was still substantial heterogeneity within some subgroups, which may be associated with differences in outcomes across reproductive medicine centers. Due to the small number of available studies, we did not further conduct a meta-regression analysis. Second, there were a few indicators with local inconsistency in our NMA, which may reduce the level of evidence. Third, similar to most of the previous studies, the cost-effectiveness ratio of various protocols was not evaluated, thus, it was not possible to provide clinical recommendations that were appropriate from a health economics standpoint. Fourth, an evaluation of safety, including adverse reactions during medication, risk of birth defects, long-term health status of both mothers and progenies with the use of GH co-treatment were not conducted due to insufficient pertinent data. Fifth, the included studies exhibited clinical heterogeneity due to the diverse COH protocols used, which may have influenced the results. However, limited by the number of eligible studies, we failed to perform a NMA by COH protocol to eliminate this confounder and were unable to further explore whether adding GH has different efficacy in different COH protocols. Sixth, the study by Choe et al.40 used a growth hormone sustained release agent and we categorized it as a high-dose, long-term protocol. However, the reasonableness of this classification method remains to be further explored.
In the future, it would be necessary to conduct larger-scale multi-arm parallel RCTs with rigorous design using the Bologna criteria or the Poseidon criteria to validate the clinical efficacy, cost-effectiveness, and safety profile of various GH supplementation protocols and to provide additional evidence for clinical decision-making. Besides, it would be meaningful to investigate whether the type of COH protocol affects the efficacy of GH supplementation therapy. In addition, further research is warranted to explore the factors which can predict the efficacy of GH, so that to standardize the indications for incorporating GH in clinical practice.
Conclusions
The use of a GH supplementation protocol with a daily dosage of 4-8 IU during the follicular phase of the stimulation cycle has the best comprehensive therapeutic effects on improving clinical outcomes in women with POR undergoing ART therapy with a relatively brief treatment duration and a moderate total GH dose. Therefore, its clinical application is suggested. Besides, the potential advantages of long-term GH supplementation protocol (using GH for at least 2 weeks before oocyte retrieval) has merit for further research. Future clinical trials of higher quality are required to compare the clinical efficacy, cost-effectiveness ratio and safety of various GH supplementation protocols to provide patients with more precise treatment guidance.
Data availability
The datasets generated during and/or analyzed during the current study are available throughout the manuscript.
Abbreviations
- ART:
-
Assisted reproductive technology
- Co Q10:
-
Coenzyme Q10
- COH:
-
Controlled ovarian hyperstimulation
- DHEA:
-
Dehydroepiandrosterone
- DIC:
-
Deviation information criterion
- GH:
-
Growth hormone
- Gn:
-
Gonadotropin
- GnRH:
-
Gonadotropin-releasing hormone
- hCG:
-
Human chorionic gonadotropin
- ICSI:
-
Intracytoplasmic sperm injection
- IGF:
-
Insulin-like growth factor
- IVF:
-
In vitro fertilization
- MD:
-
Mean difference
- NMA:
-
Network meta-analysis
- NOS:
-
Newcastle-Ottawa Scale
- OR:
-
Odds ratio
- POR:
-
Poor ovarian response
- PRISMA-NMA:
-
Preferred Reporting Items for Systematic Reviews for Network Meta-Analysis
- RCT:
-
Randomized controlled trials
- rLH:
-
Recombinant luteinizing hormone
- SUCRA:
-
Surface under the cumulative ranking curve
References
Abu-Musa, A., Haahr, T. & Humaidan, P. Novel physiology and definition of poor ovarian response; Clinical recommendations. Int. J. Mol. Sci. 21, 2110 (2020).
Drakopoulos, P. et al. Update on the management of poor ovarian response in IVF: The shift from Bologna criteria to the Poseidon concept. Ther. Adv. Reprod. Health. 14, 2633494120941480. https://doi.org/10.1177/2633494120941480 (2020).
Jirge, P. R. Poor ovarian reserve. J. Hum. Reprod. Sci. 9, 63–69 (2016).
Monteiro, C. S., Scheffer, B. B., Carvalho, R. F. & Scheffer, J. B. The impact of dehydroepiandrosterone in poor ovarian responders on assisted reproduction technology treatment. JBRA Assist Reprod. 23, 414–417 (2019).
Vaiarelli, A., Cimadomo, D., Ubaldi, N., Rienzi, L. & Ubaldi, F. M. What is new in the management of poor ovarian response in IVF?. Curr. Opin. Obstet. Gynecol. 30, 155–162 (2018).
Blumenfeld, Z. What is the best regimen for ovarian stimulation of poor responders in ART/IVF?. Front. Endocrinol. (Lausanne) 11, 192 (2020).
Zhang, Y. et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: A systematic review and network meta-analysis. Hum. Reprod. Update 26, 247–263 (2020).
Zhu, F. et al. TEAS, DHEA, CoQ10, and GH for poor ovarian response undergoing IVF-ET: A systematic review and network meta-analysis. Reprod. Biol. Endocrinol. 21, 64 (2023).
The Eshre Guideline Group On Ovarian Stimulation et al. Ovarian stimulation, T. et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI(†). Hum. Reprod. Open 2020, 009. https://doi.org/10.1093/hropen/hoaa009 (2020).
Hull, K. L. & Harvey, S. Growth hormone and reproduction: A review of endocrine and autocrine/paracrine interactions. Int. J. Endocrinol. 2014, 234014. https://doi.org/10.1155/2014/234014 (2014).
Altmae, S. et al. Effect of growth hormone on uterine receptivity in women with repeated implantation failure in an oocyte donation program: A randomized controlled trial. J. Endocr. Soc. 2, 96–105 (2018).
Homburg, R., Eshel, A., Abdalla, H. I. & Jacobs, H. S. Growth hormone facilitates ovulation induction by gonadotrophins. Clin. Endocrinol. (Oxf) 29, 113–117 (1988).
Bachelot, A. et al. Growth hormone is required for ovarian follicular growth. Endocrinology 143, 4104–4112 (2002).
Dosouto, C., Calaf, J., Polo, A., Haahr, T. & Humaidan, P. Growth hormone and reproduction: Lessons learned from animal models and clinical trials. Front. Endocrinol. (Lausanne) 10, 404 (2019).
Xue-Mei, W., Hong, J., Wen-Xiang, Z. & Yang, L. The effects of growth hormone on clinical outcomes after frozen-thawed embryo transfer. Int. J. Gynaecol. Obstet. 133, 347–350 (2016).
Pan, P. & Huang, X. The clinical application of growth hormone and its biological and molecular mechanisms in assisted reproduction. Int. J. Mol. Sci. 23, 10768. https://doi.org/10.3390/ijms231810768 (2022).
Fantini, C. et al. Short-term, supra-physiological rhGH administration induces transient DNA damage in peripheral lymphocytes of healthy women. J. Endocrinol. Investig. 40, 645–652 (2017).
Hart, R. J., Rombauts, L. & Norman, R. J. Growth hormone in IVF cycles: any hope?. Curr. Opin. Obstet. Gynecol. 29, 119–125 (2017).
Shang, Y., Wu, M., He, R., Ye, Y. & Sun, X. Administration of growth hormone improves endometrial function in women undergoing in vitro fertilization: A systematic review and meta-analysis. Hum. Reprod. Update 28, 838–857 (2022).
Kolibianakis, E. M., Venetis, C. A., Diedrich, K., Tarlatzis, B. C. & Griesinger, G. Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in-vitro fertilization: A systematic review and meta-analysis. Hum. Reprod. Update 15, 613–622 (2009).
Yu, X. et al. Efficacy of growth hormone supplementation with gonadotrophins in vitro fertilization for poor ovarian responders: An updated meta-analysis. Int. J. Clin. Exp. Med. 8, 4954–4967 (2015).
Li, X. L. et al. The influence of different growth hormone addition protocols to poor ovarian responders on clinical outcomes in controlled ovary stimulation cycles: A systematic review and meta-analysis. Medicine (Baltimore) 96, e6443. https://doi.org/10.1097/MD.0000000000006443 (2017).
Ferraretti, A. P. et al. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 26, 1616–1624 (2011).
Alviggi, C. et al. A new more detailed stratification of low responders to ovarian stimulation: From a poor ovarian response to a low prognosis concept. Fertil. Steril. 105, 1452–1453 (2016).
Mills, E. J., Thorlund, K. & Ioannidis, J. P. Demystifying trial networks and network meta-analysis. BMJ 346, f2914. https://doi.org/10.1136/bmj.f2914 (2013).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162, 777–784 (2015).
Higgins, J. P. et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, 5928. https://doi.org/10.1136/bmj.d5928 (2011).
Wells, G. A., Shea, B. J., O’Connell, D., Peterson, J. & Tugwell, P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Evidence-based Public Health http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (2000).
Owen, E. J., Shoham, Z., Mason, B. A., Ostergaard, H. & Jacobs, H. S. Cotreatment with growth hormone, after pituitary suppression, for ovarian stimulation in in vitro fertilization: A randomized, double-blind, placebo-control trial. Fertil. Steril. 56, 1104–1110 (1991).
Bergh, C. et al. Adjuvant growth hormone treatment during in vitro fertilization: A randomized, placebo-controlled study. Fertil. Steril. 62, 113–120 (1994).
Dor, J. et al. Adjuvant growth hormone therapy in poor responders to in-vitro fertilization: A prospective randomized placebo-controlled double-blind study. Hum. Reprod. 10, 40–43 (1995).
Suikkari, A., MacLachlan, V., Koistinen, R., Seppala, M. & Healy, D. Double-blind placebo controlled study: Human biosynthetic growth hormone for assisted reproductive technology. Fertil. Steril. 65, 800–805 (1996).
Kucuk, T., Kozinoglu, H. & Kaba, A. Growth hormone co-treatment within a GnRH agonist long protocol in patients with poor ovarian response: A prospective, randomized, clinical trial. J. Assist. Reprod. Genet. 25, 123–127 (2008).
Eftekhar, M., Aflatoonian, A., Mohammadian, F. & Eftekhar, T. Adjuvant growth hormone therapy in antagonist protocol in poor responders undergoing assisted reproductive technology. Arch. Gynecol. Obstet. 287, 1017–1021 (2013).
Zhi-Ping, H. U. et al. Effects of growth hormone supplementation in patients undergoing IVF/ICSI-ET with poor ovarian response to gonadotropin. J. Reprod. Contracept. 25, 32–40 (2014).
Bayoumi, Y. A., Dakhly, D. M., Bassiouny, Y. A. & Hashish, N. M. Addition of growth hormone to the microflare stimulation protocol among women with poor ovarian response. Int. J. Gynaecol. Obstet. 131, 305–308 (2015).
Dunne, C., Seethram, K. & Roberts, J. Growth hormone supplementation in the luteal phase before microdose GnRH agonist flare protocol for in vitro fertilization. J. Obstet. Gynaecol. Can. 37, 810–815 (2015).
Bassiouny, Y. A., Dakhly, D. M. R., Bayoumi, Y. A. & Hashish, N. M. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil. Steril. 105, 697–702 (2016).
Ho, Y. K. et al. Effects of growth hormone plus gonadotropins on controlled ovarian stimulation in infertile women of advanced age, poor responders, and previous in vitro fertilization failure patients. Taiwan J. Obstet. Gynecol. 56, 806–810 (2017).
Choe, S. A. et al. Increased proportion of mature oocytes with sustained-release growth hormone treatment in poor responders: A prospective randomized controlled study. Arch. Gynecol. Obstet. 297, 791–796 (2018).
Chu, K., Pang, W., Sun, N., Zhang, Q. & Li, W. Outcomes of poor responders following growth hormone co-treatment with IVF/ICSI mild stimulation protocol: A retrospective cohort study. Arch. Gynecol. Obstet. 297, 1317–1321 (2018).
Dakhly, D. M. R. et al. The addition of growth hormone adjuvant therapy to the long down regulation protocol in poor responders undergoing in vitro fertilization: Randomized control trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 228, 161–165 (2018).
Lee, Y. X., Shen, M. S. & Tzeng, C. R. Low dose growth hormone adjuvant treatment with ultra-long ovarian stimulation protocol in poor responders showed non-inferior pregnancy outcome compared with normal responders. Front. Endocrinol. (Lausanne) 10, 892. https://doi.org/10.3389/fendo.2019.00892 (2019).
Cai, M. H. et al. The effect of growth hormone on the clinical outcomes of poor ovarian reserve patients undergoing in vitro fertilization/intracytoplasmic sperm injection treatment: A retrospective study based on POSEIDON criteria. Front. Endocrinol. (Lausanne) 10, 775. https://doi.org/10.3389/fendo.2019.00775 (2019).
Norman, R. J. et al. Human growth hormone for poor responders: A randomized placebo-controlled trial provides no evidence for improved live birth rate. Reprod. Biomed. Online 38, 908–915 (2019).
Safdarian, L. et al. Growth hormone (GH) improvement of ovarian responses and pregnancy outcome in poor ovarian responders: A randomized study. Asian Pac. J. Cancer Prev. 20, 2033–2037 (2019).
Zhu, J. et al. Growth hormone supplementation may not improve live birth rate in poor responders. Front. Endocrinol. (Lausanne) 11, 1. https://doi.org/10.3389/fendo.2020.00001 (2020).
Gong, Y. et al. Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: A randomized controlled trial. Reprod. Biol. Endocrinol. 18, 91 (2020).
Mohammad, E. H. et al. Efficacy of growth hormone supplementation with ultrashort GnRH antagonist in IVF/ICSI for poor responders; Randomized controlled trial. Taiwan J. Obstet. Gynecol. 60, 51–55 (2021).
Zafardoust, S., Ansaripor, S., Karimi, A., Hosseinirad, H. & Ataei, M. Effects of adjuvant growth hormone therapy on poor ovarian responders in assisted reproductive technology. Maedica (Bucur) 17, 336–343 (2022).
Bender, R. A., Ozcan, C., Aslancan, R., Akar, B. & Caliskan, E. The effect of growth hormone addition protocols to poor ovarian responders in in vitro fertilization cycles. Eur. Rev. Med. Pharmacol. Sci. 26, 5503–5508 (2022).
Norman, R. J. & Hart, R. J. Human growth hormone use in poor ovarian response—Caution and opportunities. Ther. Adv. Reprod. Health. 15, 2633494121999420. https://doi.org/10.1177/2633494121999420 (2021).
Cozzolino, M., Cecchino, G. N., Troiano, G. & Romanelli, C. Growth hormone cotreatment for poor responders undergoing in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 114, 97–109 (2020).
Liu, X., Xu, J., Bi, L., Liu, P. & Jiao, X. Growth hormone cotreatment for low-prognosis patients according to the POSEIDON criteria. Front. Endocrinol. (Lausanne) 12, 790160. https://doi.org/10.3389/fendo.2021.790160 (2021).
Macklon, N. S., Stouffer, R. L., Giudice, L. C. & Fauser, B. C. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr. Rev. 27, 170–207 (2006).
Artini, P. G. et al. Difficult-to-treat women for controlled ovarian hyperstimulation: Tips and tricks. Expert Rev. Endocrinol. Metab. 6, 617–627 (2011).
Duan, X. Y., Li, Z., Li, M. M. & Ma, X. Efficacies of different ovarian hyperstimulation protocols in elderly patients with poor ovarian response. Eur. Rev. Med. Pharmacol. Sci. 27(23), 11606–11613 (2023).
Practice Committee of the American Society for Reproductive Medicine. Comparison of pregnancy rates for poor responders using IVF with mild ovarian stimulation versus conventional IVF: A guideline. Fertil. Steril. 109, 993–999 (2018).
Di Guardo, F. et al. Poor ovarian response and the possible role of natural and modified natural cycles. Ther. Adv. Reprod. Health 16, 26334941211062024. https://doi.org/10.1177/26334941211062026 (2022).
Di, M., Wang, X., Wu, J. & Yang, H. Ovarian stimulation protocols for poor ovarian responders: A network meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 307, 1713–1726 (2023).
Lin, G., Zhong, X., Li, S. & Xu, L. Clinical evidence of growth hormone for infertile women with diminished ovarian reserve undergoing IVF: A systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 14, 1215755. https://doi.org/10.3389/fendo.2023.1215755 (2023).
Silva, J. R., Figueiredo, J. R. & van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 71, 1193–1208 (2009).
Abir, R. et al. Growth hormone and its receptor in human ovaries from fetuses and adults. Fertil. Steril. 90, 1333–1339 (2008).
Gleicher, N., Darmon, S. K., Molinari, E., Patrizio, P. & Barad, D. H. Importance of IGF-I levels in IVF: Potential relevance for growth hormone (GH) supplementation. J. Assist. Reprod. Genet. 39, 409–416 (2022).
Tong, R. et al. Analysis of the guidance value of 3D ultrasound in evaluating endometrial receptivity for frozen-thawed embryo transfer in patients with repeated implantation failure. Ann. Transl. Med. 8, 944 (2020).
Lattes, K., Brassesco, M., Gomez, M. & Checa, M. A. Low-dose growth hormone supplementation increases clinical pregnancy rate in poor responders undergoing in vitro fertilisation. Gynecol. Endocrinol. 31, 565–568 (2015).
Karam, G. et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: Systematic review and network meta-analysis. BMJ 380, e072003. https://doi.org/10.1136/bmj-2022-072003 (2023).
Acknowledgements
We sincerely thank Professor Xinbin Zhou from The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine) for providing instructive support for this paper.
Funding
This research received funding from the Project of Medicine Science and Technology Program of Zhejiang Province: 2024KY135.
Author information
Authors and Affiliations
Contributions
Z.Y.X. contributed to proposal of research topics, design of the study, literature search, data collection and organization, literature quality evaluation, statistical analysis, preparing the figures and tables, and drafting the manuscript. W.Q.T. contributed to literature selection, literature quality evaluation, data extraction, and polishing of language. Z.Y. contributed to literature selection, literature quality evaluation, data extraction, and arbitration of disputes during the research process. H.Y.Z. contributed to proposal of research topics, quality control of this research and revising the manuscript critically. X.B.C. contributed to revision and final review of the paper. All authors confirm that the manuscript has been read and approved to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Z., Tong, W., Yang, Z. et al. Comparative efficacy of different growth hormone supplementation protocols in improving clinical outcomes in women with poor ovarian response undergoing assisted reproductive therapy: a network meta-analysis. Sci Rep 14, 3377 (2024). https://doi.org/10.1038/s41598-024-53780-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53780-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.