Abstract
To quantify transplacental transmission of SARS-CoV-2 virus and antibody transfer in pregnant women and their newborns according to the gestational age at maternal infection. A prospective observational multicenter study including pregnant women with a positive RT-PCR or a positive serology for SARS-CoV-2 and compatible symptoms, from April to December 2020, in 11 French maternities. The study was designed to obtain a systematic collection of mother-infant dyad’s samples at birth. SARS-CoV-2 viral load was measured by RT-PCR. IgG and IgM antibodies against the SARS-CoV-2 spike protein were measured by enzyme-linked immunosorbent assay. Antibody concentrations and transplacental transfer ratios were analyzed according to the gestational age at maternal infection. The primary outcome was the rate of SARS CoV-2 materno-fetal transmission at birth. The secondary outcome was the quantification of materno-fetal antibody transfer. Maternal and neonatal outcomes at birth were additionally assessed. Among 165 dyads enrolled, one congenital infection was confirmed {n = 1 (0.63%) IC95% [0.02%; 3.48%]}. The average placental IgG antibody transfer ratio was 1.27 (IC 95% [0.69–2.89]). The transfer ratio increased with increasing time between the onset of maternal infection and delivery (P Value = 0.0001). Maternal and neonatal outcomes were reassuring. We confirmed the very low rate of SARS-CoV-2 transplacental transmission (< 1%). Maternal antibody transfer to the fetus was more efficient when the infection occurred during the first and second trimester of pregnancy.
Similar content being viewed by others
Introduction
At the beginning of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic, it was unclear whether SARS-CoV-2 could be transmitted from the mother to the fetus. Cases of perinatal transmission were described, without knowing whether these occurred through transplacental transmission or through environmental exposure at birth. Vivanti et al.1 demonstrated, in July 2020, the transplacental transmission of SARS-CoV-2 in a neonate born to a mother infected during the third trimester. Very early during the pandemic the expression of the ACE2 receptor, known to play an important role in SARS-CoV-2 replication, was described in the placenta, raising fears of a possible viral replication in the placenta2,3,4,5,6,7. Face with the urgent need to have data regarding this unexpected crisis, the number of publications exploded with many heterogeneities in the clinical presentation of pregnant women and gestational age at infection making it difficult to interpret the risk of vertical transmission.
Today, little is known about the effect of SARS CoV-2 on pregnancy depending on the gestational age at maternal infection and growing evidence suggests that the susceptibility of the human placenta and, perhaps, the fetal tissues to the infection may vary depending on the gestational age8,9,10,11,12,13,14. On the other hand, maternal IgG antibodies are able to cross the placenta and could potentially offer a protection to fetus and the newborn against SARS-CoV-2 infection. Accurate evaluation of materno-fetal transmission during pregnancy and quantification of placental antibody transfer remain controversial because, in most of the studies, a systematic analysis of materno-fetal transmission was lacking15,16,17,18,19. A recent review by Kotlyar et al. suggested that materno-fetal transmission of the virus could reach 3.2% in mothers infected during the third trimester. However, fetal infection could only be convincingly determined by the direct demonstration of the presence of SARS-CoV-2 in amniotic fluid or detection of the virus by PCR in umbilical cord blood/neonatal blood collected within first 12 h of birth (classification of Prakesch20) in a minority of cases. Regarding antibody transfer, results are still discordant21,22,23,24,25. Although, Flannery et al.26 detected specific IgG in 86.7% (72/83) of exposed fetuses, information regarding the potential impact of the delay between maternal infection and delivery regarding materno-fetal antibody transfer is still being explored27,28,29,30,31. Due to the significant protection offered by passive immunization acquired through maternal antibodies to the vulnerable newborn and its potential impact on vaccination campaigns, comparing the efficiency of placental antibody transfer in the first, second, and third trimester of infection with SARS-CoV-2 needs to continue to be explored.
The TRANSCOVID Study aimed to quantify transplacental transmission of SARS-CoV-2 virus and antibody transfer in pregnant women and their newborns according to the gestational age at maternal infection. None of the data from the study has already been published.
Methods
Study design and population
We conducted a prospective observational descriptive longitudinal study on pregnant women with a confirmed SARS-COV-2 infection. Women were approached prospectively for enrollment in 11 obstetrics care centers (Minjoz-Besancon, Dole, Lons-Le-Saunier, Vesoul, Pontarlier, Nord-Franche-Comte, Dijon, Grenoble, Mulhouse, Nancy, Thionville, and Reims hospitals) from April to December 2020. During this period, pregnant women were tested for SARS-CoV-2 by nasopharyngeal reverse transcription polymerase chain reaction (NP-RT-PCR) and/or serology after obtaining their consent in two settings: (1) routinely, upon admission to the hospital for delivery if they had presented any symptoms compatible with a SARS-CoV-2 infection during pregnancy, and (2) at any gestational age during pregnancy if they presented compatible symptoms or a potential SARS-CoV-2 exposure. Pregnant women were eligible for inclusion if they were aged 18 years or older, able to provide informed consent or had a health care proxy able to do so and diagnosed with a confirmed SARS-CoV-2 infection. The TRANSCOVID Study was approved by institutional review boards CPP 8 Ile de France (ID-RCB 2020-A01066-33, N° CPP: 20 04 12), registered on clinical trial (NCT04402918). The study protocol was performed according to the Declaration of Helsinki principles, and the written informed consent containing the details of the study was obtained from all participants.
Diagnosis of maternal SARS CoV-2 infection
Confirmed maternal SARS-CoV-2 infection was defined as a positive RT-PCR for SARS-CoV-2 or a positive serology defined as the presence of either specific IgM and/or specific IgG with a history of maternal symptoms (Fig. 1). Timing of maternal infection diagnosed by a positive serology was determined based on the timing of maternal symptoms. As the study was performed before the availability of vaccination, a positive serology was considered as a definite past exposition to SARS CoV-2. Included women were classified according to the gestational age at maternal infection: before 16 weeks of gestation (WG), from 16 to 28 WG and from 28 to 42 WG. The third trimester was deliberately split into two categories: before and after 37 WG in order to distinguish SARS-CoV-2 infections that occurred very close to delivery.
Definition of outcomes
The primary outcome was the rate of materno-fetal infection. Type of materno-fetal infection ((confirmed, probable, possible, unlikely or not infected) was defined according to Shah et al.20 classification based on clinical features and results of the biological samples at delivery. The secondary objective was the evaluation of materno-fetal IgG antibody transfer assessed by a transfer ratio defined as the neonatal cord blood IgG concentration/maternal serum IgG concentration. Nasopharyngeal (NP) sampling using flocked swabs and serology testing were used to characterize maternal and fetal/neonatal SARS-CoV-2 infection at the time of delivery. Maternal NP was repeated at delivery if the infection had been diagnosed more than one week before the delivery to assess the persistence of SARS-CoV-2 replication. Upon admission to delivery room, maternal blood samples were systematically collected for SARS-CoV-2 RT-PCR and serology testing. In case of abortion, the same biological samples were collected. Swabs were stored at 4 °C when processed within 24h or stored at − 80°C when processed thereafter. A vaginal swab was collected at admission with sterile nylon flocked swabs. To diagnose a congenital infection, a swab was collected from the oropharynx and trachea (if the newborn was intubated) immediately after birth. Umbilical blood samples (5–10 ml) were collected by needle puncture after cord clamping with sterile gloves to limit risk of contamination. The serum and plasma were separated and aliquoted. All samples were stored at − 20 °C until analysis. Placental swabs (both maternal and fetal sides) were obtained with sterile nylon flocked swabs and were stored at 4 °C during 24h or at − 80 °C when processed thereafter (Fig. 2).
Viral RNA was extracted using the NucleoMag Pathogen Kit (Macherey–Nagel) on Nimbus Presto platform (Hamilton) according to the manufacturer’s instructions and amplified by RT-PCR using a protocol developed by Charite (E gene) and Institut Pasteur (RdRp gene) as previously described32 from March to June 2020 or by the TaqPath COVID-19 CE-IVD RT-PCR Kit (Thermofisher) since June 2020. Results were obtained in cycle threshold (Ct).
Serological assays were performed using the ELISA Anti-SARS-CoV-2 QuantiVac (IgG) and the ELISA anti-SARS-CoV-2-NCP IgM kits (Euroimmun) on EVOLIS system (Bio-Rad), according to the manufacturer’s instructions, for IgG and IgM detection, respectively. IgG were directly quantified and expressed in BAU/ml with a positive threshold at 25.6 BAU/ml, according to the manufacturer’s instruction. Information on patients’ demographics and history as well as details of treatments and results from exams were recorded in an electronical database (CleanWeb®).
Statistical analysis
We evaluated the number of patients to include in the cohort to 160 based on a cumulative annual number of deliveries estimated in the participating centers of 29,000 during the study period, a contamination rate in the target population between 5 and 15%, associated with proportion of symptomatic infections of 15%, the realization of a nasopharyngeal swab of 95% in these symptomatic pregnant patients and a sensitivity of the RT-PCR on nasopharyngeal swab of 70%. Applying a 5% loss of unanalyzable data, we expected 150 women to be included in the study. In case of absence of viral transmission (expected proportion equal to zero), the 95% confidence interval of the proportion of children infected with SARS Cov-2 based on a Poisson distribution would be [0; 2.5%], for a 1% viral transmission it would be [0.1; 4.3] and finally for a 5% viral transmission it would be [2.1; 10.1].
Descriptive statistics are presented as means and standard deviations (SD) or as frequencies and percentages (%). Correlations between maternal and infant IgG concentrations and transfer ratio and days between gestational age at maternal infection and delivery were reported using the Pearson correlation coefficient. We used Student tests to explore the transfer ratio function of the gestational age at maternal SARS-CoV-2 infection at.
Ethical approval
Réf CPP: 20 04 12. Réf CNRIPH: 20.04.16.57739. Réf. Protocole: 2020/500—Etude TRANSCOVID. N° ID RCB: 2020-A01066-33. Date: 30th April 2020.
Results
During the study period, 222 pregnant women were included, and matched maternal-cord blood sera were finally available for 165 mother-infant dyads, including 3 twin pregnancies (Fig. 2). 57 patients were excluded from the final analysis. 30 (18%) pregnant women were infected < 16 WG, 48 (29%) between 16 and 27 WG and 6 days, 61 (37%) between 28 and 36 WG and 6 days and 26 (16%) ≥ 37 WG. The characteristics of the 165 mother-infant dyads as well as the neonatal outcomes are presented in Table 1. Among the 165 dyads analyzed, SARS-CoV-2 RNA was amplified from 5 placentas on maternal side, 6 placentas on the fetal side and one vaginal sample (Table 2).
One confirmed congenital infection was observed {(0.63%) IC95% [0.02%; 3.48%]}, none were probable, none were possible. The confirmed infection was reported in a 34-year-old patient who tested positive for SARS-CoV-2 at 37 WG and 2 days. The patient was Caucasian with a normal pregnancy. The patient was asymptomatic and initial diagnosis was made due to close contact with an infected person. She presented at 37 WG and 4 days for reduced fetal movements. During follow-up, fetal monitoring was abnormal with loss of variability and decelerations. The medical team therefore performed a caesarean section before labor for a non-reassuring fetal status. The patient gave birth to a eutrophic female newborn for the gestational age, weighing 2760 g with an Apgar score 7–10–10. The arterial cord blood pH was 7.28. The newborn's condition did not require transfer to neonatal intensive care. All the maternal, newborn and placental swabs were positive for SARS-CoV-2. Following delivery, both maternal and neonatal course were uneventful.
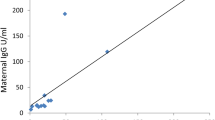
Among 165 maternal sera tested, 106 (64.2%) were positive for specific SARS-CoV-2 IgG. The average placental antibody transfer ratio was 1.27 (IC 95% [38]). Transfer ratios did not differ between infants born to mothers with an asymptomatic or a symptomatic infection (p value = 0.4805) (Table 3). The transfer ratio increased with increasing time between the onset of maternal infection and delivery (P Value = 0.0001) (Table 3, Fig. 3) and placental antibody transfer seemed to be more efficient after a maternal infection during the first trimester. Results of maternal and neonatal IgG and IgM concentration are presented in Table 2.
Regarding maternal outcomes, 12 (7.2%) were hospitalized, 28 (16.9%) had a C-section and 137 (83%) had a vaginal delivery. Neonatal outcomes were favorable with only 14 cases (8.4%) transferred to the neonatology intensive care unit (NICU) and 11 (6.6%) preterm births observed between 29 and 36 WG among which 5 were born after 34 WG. Finally, 165 newborns, only 3 (1.8%) had intra uterine growth restriction (IUGR). We did not observe a correlation between the gestational age at maternal infection and prematurity (p value = 0.35) or IUGR (p value = 0.56), Nevertheless, definite conclusions are difficult to draw due to the low number of IUGR and premature deliveries included, which might not allow to reach statistical significance.
Discussion
In this study, the SARS-CoV-2 materno-fetal transmission rate was low {(0.63%) IC95% [0.02%; 3.48%]}. On the opposite, we observed an efficient transplacental transfer of specific SARS CoV-2 IgG with an average placental transfer ratio of 1.27 (IC 95% [0.69–2.89]). The transfer ratio increased with increasing time between the onset of maternal infection and delivery (P Value < 0.001). Maternal transplacental antibody transfer did not differ significantly depending on maternal disease severity.
In most studies, the level of vertical transmission was low, estimated to range between 3.2% and 5%25,33,34,35,36. In the largest published meta-analysis including 936 patients from a set of 38 cohorts and case series, the level of vertical transmission reached 3.2% IC 95% [2.2–4.3%]25. However, because of a lack of systematic and standardized samples’ collection at delivery in included studies, definite conclusions are difficult to draw. The present study is a large, multicenter study, designed to obtain a systematic collection of dyad mothers-newborns samples at birth and further strengthen the low risk of placental SARS-CoV-2 transmission, with a rate of less 1% in our study25,33,34,35,36.
Evidence of efficient placental SARS-CoV-2 antibodies transfer had been previously demonstrated25,28, but uncertainties remained regarding the impact of maternal disease severity and the delay required to obtain efficient transfer. Similarly to others, we observed that the placental antibody transfer was proportional to time elapsed since maternal infection27,28,29,30,31,36,37,38. Indeed, Brebant et al.30 showed that a transfer ratio above 1 was positively associated with a longer delay from maternal positive SARS-CoV-2 RT-PCR to delivery. Similarly, recent data from Song et al.28 demonstrated than the transfer ratio of IgG was higher when the first maternal positive RT-PCR occurred 60–180 days before the delivery compared with < 60 days (1.2 vs. 0.6, p < 0.0001). Interestingly, we did not observe a correlation between maternal disease severity and placental antibody transfer ratio. According to Trinité et al.39, hospitalized individuals develop higher titers compared to mild-symptomatic and asymptomatic individuals, which would suggest a more efficient placental antibody transfer among severely ill mothers. In accordance, Song et al.28 demonstrated that the transfer ratio was significantly higher in the mothers who were severely to critically symptomatic (1.6, 95% CI 1.42–2.49, n = 4) compared to mothers who were asymptomatic (1.0, 95% CI 0.62–1.14, n = 23) (1.6 vs. 1.0, p = 0.003) or mildly symptomatic (0.9, 95% CI 0.81–1.09, n = 50) (1.6 vs. 0.9, p = 0.002). The low number of severe cases included in our cohort might explain the absence of association observed in our study.
Our study represents one of the largest cohorts of maternal SARS-CoV-2 infection with a systematic description of fetal/neonatal outcomes. Interestingly, we observed a low rate of prematurity and IUGR compared to previous studies15,21,33,40,41,42,43,44. Similarly, we did not observe an increased risk of C-Sect.15,16,45,46. Nevertheless, our study was not designed to explore the risks of prematurity or IUGR in case of maternal infection and the numbers, especially of severe cases, are too small to draw any conclusion.
Strengths of our study include the prospective inclusion of patients throughout the pregnancy, allowing a representation of the three trimesters of the pregnancy, as well as a large cohort of patients (165 mother-infant dyads) including both symptomatic and asymptomatic form of SARS-CoV-2 infection. Furthermore, the systematic collection of the newborns’ samples immediately after birth limits the risk of contamination and interpretation bias.
A limitation of our study might be the possible underestimation of materno-fetal SARS-CoV-2 transmission due to the use of a restrictive definition of confirmed congenital infection. Our study was carried out in 2020, prior to the publication of the WHO classification of materno-fetal transmission47. By opposition to the WHO classification, which also includes neonatal positive samples for SARS-CoV-2 taken within the first 24 h of life to confirm a congenital infection, we only considered samples taken immediately at birth, as suggested by Shah et al.. Nevertheless, all newborns were hospitalized for a minimum of 48 h after birth and severe adverse outcomes would have been recorded, suggesting that even in the case of missed congenital infection, the outcome would have be favorable. Another limitation is the absence of analysis carried out on patients with adverse outcomes like stillbirths or miscarriages. Miscarriage tissues were not analyzed in our study and fetal cord sera in case of stillbirth were, unfortunately, not available for analysis because fetal blood was clotted at the time of collection. Histopathological analysis of the placenta was not performed. Although we cannot exclude that some cases of stillbirths/ miscarriages were related to a congenital SARS CoV-2 infection, the numbers in our cohort were low (n = 6), suggesting that this would not significantly influence our conclusions. Finally, our reported rate of congenital SARS-CoV-2 infection may not reflect all cases of placental transmission, as a progressive clearance of the virus by placenta may occur during pregnancy which would impair its detection at birth48.
We chose to include both women with positive RT-PCR for SARS-CoV-2 and women with a negative PCR test but a positive serology for SARS-Cov-2 and compatible symptoms. Although we cannot exclude a memory bias and a potential infection before the inclusion, the presence of compatible symptoms during the current pregnancy lowers the risk.
The population included was an unvaccinated population allowing the evaluation of the transfer ratio following maternal infection. According to Beharier et al.49, the transfer ratio of IgG from a vaccinated population of pregnant women is similar to the one from an infected population. Similarly to what we observed in a natural infection, Atyeo et al.50 demonstrated a higher transfer efficiency following first trimester vaccination. Others have even demonstrated efficient antibody transfer, even when the vaccination took place prior to conception51,52,53. Considering these results, effective antibody transfer following maternal vaccination might be higher during the first part of the pregnancy and vaccination campaigns should target the first and second trimester of the pregnancy54. Some studies have raised the possibility of increased passive neonatal immunity when mothers have both been vaccinated and infected during pregnancy; adjunctive vaccination following maternal infection needs to be further explored55.
Conclusions
In this large multicenter prospective study, we demonstrated a low rate of materno-fetal transmission. The placental IgG antibody transfer from the mother to the fetus seems to be higher when SARS-CoV-2 infection occurs during the first and second trimesters. These findings support SARS-CoV-2 vaccination of pregnant women during the first and the second trimester to ensure efficient neonatal protection through passive immunity.
Data availability
The data used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Vivanti, A. J. et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 11(1), 3572 (2020).
Levy, A. et al. ACE2 expression and activity are enhanced during pregnancy. Am. J. Physiol-Regul. Integr. Comp. Physiol. 295(6), R1953–R1961 (2008).
Mourad, M. et al. Placental response to maternal SARS-CoV-2 infection. Sci. Rep. 11(1), 14390 (2021).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2), 271-280.e8 (2020).
Mahyuddin, A. P. et al. Mechanisms and evidence of vertical transmission of infections in pregnancy including sars-CoV -2s. Prenat. Diagn. 40(13), 1655–1670 (2020).
Chilamakuri, R. & Agarwal, S. COVID-19: Characteristics and therapeutics. Cells. 10(2), 206 (2021).
SeyedHosseini, E. et al. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology. 551, 1–9 (2020).
Beesley, M. et al. COVID-19 and vertical transmission: assessing the expression of ACE2/TMPRSS2 in the human fetus and placenta to assess the risk of SARS-CoV-2 infection. BJOG Int. J. Obstetr. Gynaecol. 129(2), 256–266 (2021).
Zhou, J. et al. Is SARS-CoV-2 infection a risk factor for early pregnancy loss ACE2 and TMPRSS2 coexpression and persistent replicative infection in primitive trophoblast. J. Infect. Dis. 224(Supplement_6), S660–S669 (2021).
Rubio, R. et al. Maternal and neonatal immune response to SARS-CoV-2, IgG transplacental transfer and cytokine profile. Front. Immunol. 13, 999136 (2022).
Crovetto, F. et al. Impact of severe acute respiratory syndrome coronavirus 2 infection on pregnancy outcomes: a population-based study. Clin. Infect. Dis. 73(10), 1768–1775 (2021).
Villar, J. et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 175(8), 817 (2021).
Fabre, M. et al. Frequent placental SARS-CoV-2 in patients with COVID-19-associated hypertensive disorders of pregnancy. Fetal. Diagn. Ther. 48(11–12), 801–811 (2021).
Fallach, N. et al. Pregnancy outcomes after SARS-CoV-2 infection by trimester: A large, population-based cohort study. PLOS ONE. 17(7), e0270893 (2022).
Ciapponi, A. et al. COVID-19 and pregnancy: An umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLOS ONE. 16(6), e0253974 (2021).
Juan, J. et al. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound. Obstet. Gynecol. 56(1), 15–27 (2020).
Mannar, D. et al. SARS-CoV-2 omicron variant: Antibody evasion and cryo-EM structure of spike protein-ACE2 complex. Science. 375(6582), 760–764 (2022).
Rangchaikul, P. & Venketaraman, V. SARS-CoV-2 and the immune response in pregnancy with delta variant considerations. Infect. Dis. Rep. 13(4), 993–1008 (2021).
Wang, S. et al. A Case report of neonatal 2019 coronavirus disease in China. Clin. Infect. Dis. 71(15), 853–857 (2020).
Shah, P. S., Diambomba, Y., Acharya, G., Morris, S. K. & Bitnun, A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. Gynecol. Scand. 99(5), 565–568 (2020).
Schwartz, D. A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal Coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 144(7), 799–805 (2020).
Edlow, A. G. et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open. 3(12), e2030455 (2020).
Walker, K. et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. R Coll. Obstet. Gynaecol. 127(11), 1324–1336 (2020).
Bwire, G. M., Njiro, B. J., Mwakawanga, D. L., Sabas, D. & Sunguya, B. F. Possible vertical transmission and antibodies against SARS-CoV-2 among infants born to mothers with COVID-19: A living systematic review. J. Med. Virol. 93(3), 1361–1369 (2021).
Kotlyar, A. M. et al. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 224(1), 35-53.e3 (2021).
Flannery, D. D. et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 175(6), 594 (2021).
Houhou-Fidouh, N. et al. Preliminary results on transmission of SARS-CoV-2 antibodies to the fetus and serum neutralizing activity. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 158(2), 476–478 (2022).
Song, D. et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open. 11(7), e053036 (2021).
Matsui, Y. et al. Neutralizing antibody activity against SARS-CoV-2 variants in gestational age-matched mother-infant dyads after infection or vaccination. JCI Insight. 7(12), e157354 (2022).
Brebant, D. et al. Transplacental transfer of anti-SARS-CoV-2 neutralizing antibodies in comparison to other pathogens total antibodies. J. Clin. Virol. Off. Publ. Pan. Am. Soc. Clin. Virol. 165, 105495 (2023).
Rottenstreich, M. et al. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. Int. J. Obstetr. Gynaecol. 8, 248 (2021).
Biguenet, A. et al. SARS-CoV-2 respiratory viral loads and association with clinical and biological features. J. Med. Virol. 93(3), 1761–1765 (2021).
Lassi, Z. S. et al. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: Clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J. Glob. Health. 11, 05018 (2021).
Jafari, M. et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 31(5), 1–16 (2021).
Ward, J. D. et al. The clinical impact of maternal COVID-19 on mothers, their infants, and placentas with an analysis of vertical transfer of maternal SARS-CoV-2-specific IgG antibodies. Placenta. 123, 12–23 (2022).
Karimi, H., Mansouri, V. & Rezaei, N. Vertical transmission and maternal passive immunity post-SARS-CoV-2. Fut. Virol. 18(13), 895–912 (2023).
Nielsen, S. Y. et al. Transplacental transfer of SARS-CoV-2 antibodies: a cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 42(3), 277–285 (2023).
González-Mesa, E. et al. Transmitted fetal immune response in cases of SARS-CoV-2 infections during pregnancy. Diagnostics. 12(2), 245 (2022).
Trinité, B. et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci. Rep. 11(1), 2608 (2021).
Timircan, M. et al. Exploring pregnancy outcomes associated with SARS-CoV-2 infection. Medicina 57(8), 796 (2021).
Ville, Y. The placenta in COVID -19 infection in pregnancy. BJOG Int. J. Obstet. Gynaecol. 129(8), 1375–1375 (2022).
Papageorghiou, A. T. et al. Preeclampsia and COVID-19: Results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 225(3), 289.e1-289.e17 (2021).
Zaigham, M. & Andersson, O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 99(7), 823–829 (2020).
Girardelli, S., Mullins, E. & Lees, C. C. COVID-19 and pregnancy: Lessons from 2020. Early Hum. Dev. 162, 105460 (2021).
Yu, N. et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 20(5), 559–564 (2020).
Chen, C. Y., Yu, C., Chang, C. C. & Lin, C. W. Comparison of a novel computerized analysis program and visual interpretation of cardiotocography. PloS One. 9(12), e112296 (2014).
WHO. Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2. Scientific brief. 7 February 2021, COVID-19: Scientific briefs, Geneva: World Health Organization, 2021. www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1 (last accessed 01/07/21) WHO reference number: WHO/2019-nCoV/mother-to-child_transmission/2021.1.
Timi, P. et al. Placental injury and antibody transfer after coronavirus disease 2019 in pregnancy. J. Infect. Dis. 227(7), 850–854 (2023).
Beharier, O. et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest https://doi.org/10.1172/JCI150319 (2021).
Atyeo, C. G. et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat Commun. 13(1), 3571 (2022).
Marshall, N. E. et al. SARS-CoV-2 vaccine booster elicits robust prolonged maternal antibody responses and passive transfer to the offspring via the placenta and breastmilk. Am J Obstet Gynecol MFM. 5(2), 100830 (2023).
Lubrano, C. et al. Immune response and transplacental antibody transfer in pregnant women after COVID-19 vaccination. J. Pers. Med. 13(4), 689 (2023).
Yang, Y., Xing, H. & Zhao, Y. Transplacental transmission of SARS-CoV-2 immunoglobulin G antibody to infants from maternal COVID-19 vaccine immunization before pregnancy. J Med Virol 95(1), e28296 (2023).
Zilver, S. J. M. et al. Vaccination from the early second trimester onwards gives a robust SARS-CoV-2 antibody response throughout pregnancy and provides antibodies for the neonate. Int. J. Infect. Dis. 130, 126–135 (2023).
Adhikari, E. H. et al. Diverging maternal and cord antibody functions from SARS-CoV-2 infection and vaccination in pregnancy. J. Infect. Dis. https://doi.org/10.1093/infdis/jiad421 (2023).
Funding
Direction Générale de l’Offre de Soins PHRC Interrégional 2020-Covid 19. PHRC Interrégional 2020 N° DGOS SERI-COVID-IV. Centre Hospitalier Universitaire de Besançon.
Author information
Authors and Affiliations
Contributions
Pr.N.M. conceived of the presented idea and developed the theory. M.P., Dr F.M. and Dr C.N. verified the analytical methods. Pr.N.M. encouraged L. L-R. to investigate and supervised the findings of this work. Dr C.B., Dr S.M-Q., Dr J-P.B., Dr C.D., Dr M.C., Dr C.C., Dr M-L.E., Dr Y.M., Dr S.R., Pr.D.R., Dr E.R., Dr M.R. and Pr E.S. were associated investigators and responsible of patient recruitment. L.L-R. wrote the manuscript with support from Pr.N.M., Dr M.V. and Dr F.M. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lucot-Royer, L., Nallet, C., Vouga, M. et al. Analysis of the transplacental transmission of SARS CoV-2 virus and antibody transfer according to the gestational age at maternal infection. Sci Rep 14, 3458 (2024). https://doi.org/10.1038/s41598-024-53580-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53580-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.