Abstract
This in vitro study evaluated the bacterial reduction provided by the EndoActivator (EA), Easy Clean (EC), passive ultrasonic irrigation (PUI), and XP-Endo Finisher. Eight-four mesial roots of mandibular first molars were instrumented, inoculated with Enterococcus faecalis, and divided into four groups (n. 20). Bacterial reduction in the main canals and dentinal tubules were respectively determined by MTT assays and Live/Dead BackLight technique through confocal laser scanning microscopy (CLSM) at 50, 100, and 150 µm in-depth (n. 10 per group). Statistical analyses were conducted following a significance level of 95% (P < 0.05). A significant statistical difference was just identified between XPF and EC in the main canals. In the dentinal tubules from the main root canals, at 100 and 150 µm in-depths, significant statistical differences were only observed between XPF and EC (P = 0.027) for the former and between XPF and EC (P = 0.011) and XPF and PUI (P = 0.021) for the latter. In the dentinal tubules from the isthmus, at 100 µm in-depth, statistically relevant differences did occur between XPF and EC (P = 0.038) and EC and EA (P = 0.029). At 150 µm in-depth, these differences were only significant by comparing XPF and PUI (P = 0.025) and XPF and EC (P = 0.036). Although no irrigation method could thoroughly disinfect the RCS, bacterial reduction indexes were generally better after using XPF.
Similar content being viewed by others
Introduction
Chemomechanical preparation is the main responsible for the bacterial load reduction in the root canal system (RCS) to prevent the development or promote conditions for the healing of a perirradicular disease. It is primarily based on the associated use of endodontic instruments (mechanical cleaning) and auxiliary chemical solutions (chemical cleaning), further complemented by the physical cleaning performed by injection followed by aspiration of the irrigating solutions into the RCS1,2.
The metallurgical revolution occurred in the last years has positively impacted endodontic science, stemming from the development and improvement of instruments with different designs, objectives, kinematics, characteristics, and properties3. However, the difficulties imposed by the anatomical complexity and the infection of the RCS continue to be the pillars of endodontic failure4, thus encouraging the study of alternatives to enhance the chemical and disinfection processes5,6,7,8,9.
The EndoActivator system (EAS) (Dentsply-Maillefer, Ballaigues, Switzerland) is a sonically driven activation tool developed to promote a vigorous agitation of the irrigant or chelating solutions within the root canals. Using a needle and syringe, the system has raised irrigation efficiency better than conventional irrigation (CI). It comprises a portable handpiece and three disposable flexible polymer tips of distinct sizes (15/02, 25/04, and 35/04) that do not wear the root canal walls10,11.
Easy Clean (EC) is an acrylonitrile butadiene styrene plastic file (Easy Equipamentos Odontológicos, Belo Horizonte, Minas Gerais, Brazil). It has a size of 25/04 and an “aircraft wing” shaped cross-section that may be used in both reciprocating or rotary motion to enhance endodontic cleaning and disinfection12,13.
Passive ultrasonic irrigation (PUI) was initially described by Weller et al.14 and is based on the passive insertion of a metal tip/file attached to an ultrasonic device oscillating at a frequency of 30 kHz into a canal filled with irrigating or chelating solutions14. When the instrument is activated, it is surrounded by acoustic streaming to boost the performance of the agitation of the irrigating or chelating solutions, increasing the endodontic cleaning and disinfection15.
XP-Endo Finisher (XPF) (FKG Dentaire, La Chaux-de-Fonds, Switzerland) is an n. 25 non-tapered instrument produced with a special NiTi alloy called MaxWire (Martensite-Austenite Electropolish-Flex, FKG). The file is straight in its martensite phase (at room temperature and when cooled) and changes to its austenite phase when exposed to the body temperature. From this moment on, it takes on a unique spoon shape, 10 mm long from the tip and 1.5 mm deep, fashioned by its molecular memory. The instrument should be used at 800 rpm, after an apical enlargement of at least n. 25, and with the canal filled with irrigating or chelating solutions to increase the endodontic cleaning and disinfection processes12,16.
Only a few studies have been conducted to investigate the cleaning and disinfection of the RCS (including the dentinal tubules), comparing different active supplementary irrigation methods in teeth and/or roots with well-known anatomical complexity. Therefore, this research aimed to evaluate the performance of EA, EC, PUI, and XPF on the bacterial reduction in the main root canals and dentinal tubules from root canals and isthmus in the mesial roots of extracted human mandibular first molars17,18. The null hypothesis established was that there is no difference in the cleaning and disinfection potential among the methods investigated herein, regardless of the place (main root canals and dentinal tubules from the main root canals and isthmus).
Methods
Approval by the research ethics committee
For performing this scientific investigation, a research project was previously written, submitted, and duly approved by the Research Ethics Committee of the Pontifical Catholic University of Paraná–PUC/PR, Curitiba, Paraná, Brazil (CAAE. 61195016.5.0000.0020) on 03/05/2018. Nonetheless, all stages exposed below were strictly performed following the Declaration of Helsinki19.
Sample selection (first stage)
Informed consent was obtained from all subjects and/or their legal guardian(s) to use their extracted teeth for scientific purposes. Then, the Human Teeth Bank of the University provided three hundred and sixty human mandibular first molars extracted for reasons unrelated to the present study. After, they were digitally radiographed in two directions (buccolingual and mesiodistal) and then subjected to cone-beam computed tomography (CBCT) scanning process for the initial assessment of their internal anatomy using a Scanora 3D tomograph (Soredex, Tuusula, Finland) set with the following parameters: 120 kV, 12.5 mA, a field of view (FOV) measuring 75 × 100 mm, and a voxel size of 0.2 mm. This initial analysis permitted the preliminary selection of 180 specimens based on the subsequent criteria: no prior endodontic treatment, absence of pulp calcifications, and complete root formation without significant root curvatures (falling within 20° to 75°)20. During this initial phase of sample selection, exclusion criteria consisted of endodontically treated teeth, teeth with pulp calcifications, open apexes, or teeth displaying pronounced root curvatures (greater than or equal to 75°)20.
Sample selection (second stage)
The 180 specimens previously chosen underwent a micro-computed tomography (micro-CT) scanning process using the SkyScan 1174 system (Bruker micro-CT, Kontich, Belgium). This assessment aimed to confirm the absence of cracks, fractures, or root resorptions. Additionally, it provided more detailed information regarding the internal root canal anatomy, particularly in the mesial roots, which would have to present with two distinct canals that converged into a single foramen through a cervical isthmus, conforming to the Vertucci Type II classification (additional inclusion criterion). The technical parameters for the micro-CT scanning process were as follows: 800 μA, 50 kVp, rotation step of 0.7° spanning 360° around the vertical axis, pixel size of 16.82 μm, and 0.5-mm thick aluminum filter. The time for scanning each specimen was around 55 min. Then, three-dimensional images were generated, and volumetric analyses were carried out using the NRecon V.1.6.8.0 and CTAn v. 1.12 softwares (Bruker micro-CT)10. At − 1 mm from the apical foramen, the canals also would have the following values, represented as mean ± standard deviation (median)—area (mm2): 0.191 ± 0.13 (0.157); perimeter (mm): 2.687 ± 0.84 (2.245); roundness: 0.403 ± 0.20 (0.093); major diameter (mm): 0.857 ± 0.42 (0.291), and minor diameter: 0.325 ± 0.13 (0.269) (complementary inclusion criteria)21. During this second stage of sample selection, the additional exclusion criteria included teeth with a mesial configuration differing from the Vertucci Type II classification or teeth with mesial roots and canals at the 1 mm mark from the apical foramen displaying values of area, perimeter, roundness, major and minor diameters that differed from those previously specified21. As a result, 84 specimens presenting stringent anatomical features in the mesial root were definitively selected to constitute the sample of the current research.
Specimens’ preparation
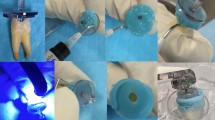
Initially, the teeth were submitted to hemi-sections using a low-speed steel cutting disc (Isomet-Buehler, Lake Bluff, Illinois, United States). After discarding the distal portion, each specimen's pulp cavity coronary distal wall was reconstructed with composite resin (Opallis, FGM, Joinville, Santa Catarina, Brazil) to create a reservoir for the auxiliary chemical solutions. Afterward, the crown was cut using a low-speed steel cutting disc (Isomet-Buehler) to obtain specimens with a standard length of 17 mm. The working length (WL) was established by inserting a n. 15K-file (Dentsply-Maillefer) until the apical foramen, subtracting 1 mm from this measurement to establish the apical limit around the apical constriction22. This procedure was performed with an operating microscope (DF Vasconcellos, Londrina, Paraná, Brazil) at 8× magnification.
Biomechanical preparation was performed using the Medium File (35/06) of the WaveOne Gold system (Dentsply-Maillefer) powered by an X-Smart Plus electric endodontic motor (Dentsply-Maillefer) according to the manufacturer's instructions. After each instrument use, the apical patency was checked with an n. 10K-file (Dentsply-Maillefer). Irrigation was carried out with 2.5 ml of 2.5% sodium hypochlorite (NaOCl) solution (Farmácia Precisão, Curitiba, Paraná, Brazil) using a 30-G NaviTip needle (Ultradent, South Jordan, UT, USA) calibrated at − 4 mm from the WL. After the chemomechanical preparation, the final irrigation was performed with 2.5 ml of 2.5% NaOCl (Farmácia Precisão), followed by the same amount of both 17% EDTA and 2.5% NaOCl (Farmácia Precisão). Thus, all root canals were irrigated with the same quantity of NaOCl (12.5 ml) and EDTA (2.5 ml). Complete drying of the canals was achieved using WaveOne Gold Medium absorbent paper points (Dentsply-Maillefer), and the roots were then covered by two layers of nail enamel, leaving only the foraminal region free to prevent the contamination of the canals. The teeth were autoclaved at 121 °C and immersed in a phosphate-buffered solution (PBS).
Canal inoculation with Enterococcus faecalis
A standard suspension of Enterococcus faecalis (E. faecalis) (ATCC 29212™) was created in a concentration of 1 × 108 cells/ml. This suspension was prepared from a 24-h bacterial culture in brain heart infusion broth (BHI). Using sterile 1 ml insulin syringes equipped with a 30-gauge needle, 40 specimens were filled up to the orifice level with the E. faecalis suspension. Subsequently, they were placed in 15 ml tubes containing 10 ml of BHI broth and incubated at 37 °C for 21 days in an environment with 100% humidity, favoring the bacterial growth and the colonization of the RCS (main root canal and dentinal tubules). Every three days, BHI broth was replaced17.
Supplementary disinfection methods
After this period, specimens were removed from the inoculation tubes, and the root apexes were sealed with composite resin in a clean environment with a laminar flow cabinet to prevent contamination. Then, they were randomly divided into four groups (n. 10) according to the active supplementary disinfection methods investigated (Table 1).
E. faecalis sampling
Following the 21 days of bacterial inoculation, samples of E. faecalis from the root canals of each specimen were procured before (S1) and after (S2) the disinfection procedures. All materials and instruments employed in the subsequent sample retrieval were thoroughly sterilized.
PBS replaced the BHI broth in the root canals for the collection of S1 samples. Then, a n. 25 Hedstroem instrument (Dentsply-Maillefer) was used to file the dentinal walls with 20 vigorous strokes. The canal contents were aspirated using a 1 ml insulin syringe with a 25-gauge needle and transferred into a microcentrifuge tube. This process was repeated three times for each canal, resulting in a final volume of bacteria collected in PBS of 100 ml, considering both canals of each specimen17.
After obtaining the S1 samples, specimens of each group were submitted to the respective supplementary disinfection method investigated. Following this, the root canals were flushed with 1 ml of a 10% sodium thiosulfate solution to neutralize the effects of NaOCl. Subsequently, each canal was prepared for the collection of S2 samples and carried out in the same manner described for S117.
MTT assay
Bacterial specimens (S1 and S2) retrieved from the canals underwent a standard MTT analysis to identify the viable bacteria. Ten microliters of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reagents (Sigma-Aldrich, Steinheim, Germany) were introduced into each of the microcentrifuge tubes containing the bacterial samples. The samples were agitated and subsequently kept at 37 °C for 4 h. Afterward, 110 ml of isopropanol/HCl was included in each tube to dissolve the formazan dye. The microcentrifuge tubes were centrifuged for 5 min at 6000 rpm, and 190 ml of the supernatant was transferred to a 96-well plate. The optical density was measured at 570 nm using a SpectraStar Nano spectrophotometer (BMGLabTech, Ortenberg, Germany) (BMGLabTech, Ortenberg, Germany)17.
CLSM
Confocal laser scanning microscopy (CLSM) was applied to assess the efficacy of the active supplementary disinfection methods in eradicating bacteria from the dentinal tubules of the main root canals and isthmus. For this phase, forty-four specimens (10 per group) were submitted to the same procedures described before. Four specimens were designated as controls. Two underwent autoclaving (negative control), while the others did not receive any disinfection method (positive control). After the same bacterial inoculation period, the specimens were decorated, and roots were longitudinally separated into two halves, following the procedure described by Al Shahrani et al.23 Specifically, a groove was created along the teeth's longitudinal axis without breaching the root canals and a chisel was employed to split each root.
Subsequently, all specimens were stained using the Live/Dead Back Light bacterial viability kit (Molecular Probes, Eugene, Orange, United States) for 15 min. Then, they were submitted to the CLSM process to obtain the respective images by using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany) programmed with excitation/emission wavelengths of 480/500 nm and 490/635 nm for SYTO 9 and propidium iodide, respectively. The inspection used a 20× magnification with an additional zoom of 2×. CLSM images were captured using Zen V. 2 software (Carl Zeiss) at a resolution of 1024 × 1024 pixels. Figure 1 illustrates the methodological flow diagram considering the specimens' selection and the study's phases.
CLSM analysis
For each specimen, 1 mm from each root canal third was scanned using a degree depth of 10 µm between each slice, resulting in 10 and 30 slices per root canal third and specimen, respectively. The ten slices from each root canal third were superposed to build up a single image from these three regions. Three distinct measurements were conducted for every superposed image, corresponding to three different depth levels (50, 100, and 150 µm into the dentinal tubules). The Zen 2 software (Zeiss) measured all the points along the dashed line within the specified depth level. At each depth level, the software quantified the intensities of red and green fluorescence (dead and live bacteria, respectively) (Fig. 2).
Illustrative figure showing microorganisms at different depths in dentinal tubules. Central image: cross-sectional microtomographic slice evidencing approximate locations for obtaining images of dentinal tubules from the main canals (yellow) and isthmuses (purple). CLSM images on the left and right (dentinal tubules from the main canals and isthmuses, respectively). Groups: A and E) EndoActivator. B and F) Easy Clean. C and G) Passive ultrasonic irrigation. D and H) XP-Endo Finisher.
Statistical analysis
All data were tabulated and subjected to statistical analysis using IBM Statistics SPSS 25.0 software (IBM, Chicago, IL, USA) with a significance level set at 0.0524. For the MTT assay, each group's approximated percentage of bacterial reduction was calculated using the following formula: S2 − S1/100%. For CLSM analyses, the percentage of red to red-green combined was calculated for each group through the following formula: intensity of red/intensity of red + intensity of green × 100. In both assessments, the data distribution did not show normality, according to the Kolmogorov–Smirnov normality test. Therefore, the non-parametric Kruskal–Wallis’s test was used, followed by the non-parametric pairwise Dunn multiple comparisons test. The power analysis of the test indicated that the sample size was adequate for both MTT (90%) and CLSM analyses (99%)25.
Results
Considering the median of the data provided by the MTT assay, the highest percentage of bacterial reduction was obtained by XPF (94.73%), followed by PUI (82,01%) and EA (69.47%) without statistically significative differences among them (P > 0.05). A significative statistical difference was identified only when XPF (94.73%) was compared with EC (49.59%) (P = 0.017) (Table 2).
Considering the percentage of the bacterial reduction provided by the CLSM analysis at 50 µm in-depth, no relevant statistical differences were identified among the groups, regardless of the place (dentinal tubules from the main canals or isthmus) (P > 0.05). In deeper depths, XPF showed better performances. In the dentinal tubules from the main root canals, at 100 and 150 µm in-depths, significant statistical differences were only observed between XPF and EC (P = 0.027) for the former and between XPF and EC (P = 0.011) and XPF and PUI (P = 0.021) for the latter. In the dentinal tubules from the isthmus, at 100 µm in-depth, statistically relevant differences did occur between XPF and EC (P = 0.038) and EC and EA (P = 0.029). At 150 µm in-depth, these differences were only significant by comparing XPF and PUI (P = 0.025) and XPF and EC (P = 0.036) (Table 3).
No difference was found in the performance of the active supplementary irrigation methods compared to the results observed in the main root canals and isthmus, regardless of the depth level (P > 0.05).
Discussion
Significant advances in metallurgical science in the last decades have impacted Endodontics by developing many instruments with different designs, purposes, manufacturing methods/surface treatments, and characteristics. However, deficiencies in the (mechanical) disinfection process have been frequently demonstrated, mainly by the relevant amounts of untouched walls in the root canals26,27. Hence, an increasing number of studies have been conducted to enhance the effectiveness of irrigation, primarily through different alternatives capable of promoting the agitation of the irrigating and chelating solutions due to the well-known limitations of CI28. This study sought to evaluate the effects of four active supplementary irrigation methods (EA, EC, PUI, and XPF) on the bacterial reduction in the main root canals by MTT assay and dentinal tubules from the main root canals and isthmus in mesial roots of extracted human mandibular first molars by CLSM. The null hypothesis was rejected because (i) XPF presented better results than EC considering the percentage of bacterial reduction provided by the MTT assay, and (ii) CLSM analyses showed that in the dentinal tubules from the main root canals, at 100 and 150 µm in-depths, significant statistical differences were observed between XPF and EC for the former and between XPF and EC, and XPF and PUI for the latter. In the dentinal tubules from the isthmus, at 100 µm in-depth, statistically relevant differences occurred between XPF, EC, and EC and EA. At 150 µm in-depth, these differences were only significant by comparing XPF, PUI, and XPF and EC.
Although endodontic infections are polymicrobial29, a single species culture of E. faecalis was selected to carry out the present research due to its following features: frequent involvement in treatment-resistant cases30 and in assessing materials' antimicrobial properties against endodontic infections30, high capacity of penetration into dentinal tubules and biofilm formation making it more resistant to disinfection processes31, adherence to dentin collagen32 and resistance to centrifugation33. Furthermore, E. faecalis can achieve a viable but unculturable state by activating a starvation response under stress conditions, which allows its regrowth34.
Data provided by the MTT assay showed the highest percentage of bacterial reduction was obtained by XPF (94.73%), followed by PUI (82.01%) and EA (69.47%) without statistically significant differences among them. However, XPF performed significantly better than EC (49.59%). This finding may be correlated to the design of the instruments. While EC is a straight plastic tip, XPF is a file that expands at body temperature, allowing it to provide a better debridement of the RCS.
At 50 µm in-depth, CLSM analyses showed no differences among the groups in the percentage of bacterial reduction regardless of the place (dentinal tubules from the main canals and isthmus). Nonetheless, XPR showed better performances in deeper depths. In the dentinal tubules from main canals, at 100 and 150 µm in-depth, significant statistical differences were only observed between XPF and EC for the former and between XPF and EC, and XPF and PUI for the latter. In the dentinal tubules from the isthmus, at 100 µm in-depth, statistically relevant differences occurred between XPF, EC, and EC and EA. At 150 µm in-depth, these differences were only significant by comparing XPF, PUI, and XPF and EC.
Poor results reached by EC in dentinal tubules (regardless of its location—main canals or isthmus) may be explained by the same reasons previously exposed to justify its limited performance in the main root canals. However, deficient results generally showed by PUI compared with XPF in dentinal tubules from both places analyzed (main canals and isthmus) must be discussed. While some papers showed better results of PUI over several other irrigation methods35,36,37, others found the contrary38,39,40, considering several types of analyses. The primary mechanism of action promoted by PUI to increase the cleaning and disinfection processes is the transmission of acoustic energy through the irrigating and chelating solutions, resulting in cavitation and microstreaming. This allows the solutions to move dynamically and thoroughly within the RCS. Acoustic waves generate cavitation bubbles, and the energy released upon bubble collapse is transferred to the root canal walls, initially dislodging the debris. Then, microstreaming promotes its removal from the root canal. The efficient impact of PUI is attributed to the generation of nodes along activated files, leading to the production of a strong current along the activated instrument. Multiple nodes along the instrument prevent a reduction in acoustic streaming when the file comes into contact with the canal wall. However, while microstreaming is a biophysical force strongly linked to endodontic files, the role of cavitation in vivo is controversial. The combination of acoustic streaming and cavitation can be considered a critical element in the most effective utilization of PUI15,28,41,42.
Even considering there is "common sense" regarding the importance of PUI to enhance the cleaning and disinfection of the RCS, recently published meta-analyses and systematic reviews have raised severe questions on the matter43,44. According to Moreira et al.43, the levels of evidence comparing PUI and CI are fragile since, in all studies researched, some bias was observed, which may interfere with the respective results and conclusions. Still, following Silva et al.44, there is no robust scientific evidence to effectively prove that PUI can provide better disinfection processes or increase the endodontic prognosis compared to non-activated irrigation techniques.
Considering that no other antimicrobial strategy was employed in the current research, the disinfection of the dentinal tubules could only be achieved by the penetration of the irrigating solution. In the Akcay et al.45 study, PUI reached a significantly larger penetration than CI, considering the sum of the penetration areas of 5.25% NaOCl at 2, 5, and 8 mm from the apex. On the other hand, Gu et al.46 reported no significant difference between the same techniques and areas by using 5% NaOCl. From the antimicrobial point of view, Neelakantan et al.47 observed no significant differences between PUI and CI in reducing E. faecalis biofilms from dentinal tubules at 200 and 400 µm in-depth, using 3% NaOCl followed by 17% EDTA. Azim et al.17 investigated the bacterial reduction from root canals and dentinal tubules provided by four irrigation methods (CI, EA, XPF, and erbium: yttrium aluminum garnet laser [PIPS]) by using the same methodological analyses employed herein. In the main root canal, MTT assays showed that bacterial reduction ranged from 89.6 to 98.2% among the groups, but XPF presented better results. In the dentinal tubules, CLSM showed that XPF promoted the more significant bacterial reduction in the three root canal thirds at 50 µm in-depth. At 150 µm in-depth, the better results were reached by PIPS. Considering PIPS was not used in the present research, in a way, our findings are in line with those obtained by Azim et al.17. According to Alves et al.48, the design and helical movement of XPF may favor the instrument to reach previously untouched areas and disrupt bacterial biofilms. However, distinct findings of the studies mentioned above may be attributed to several factors, such as different methodological designs, teeth used (anatomical complexity), microbial features (single or polymicrobial bacterial cultures; incubation/inoculation times), chemomechanical protocols, and so forth49.
One of the most critical advantages of performing in vitro studies is the possibility of controlling the variables to carry out more accurate investigations and thus obtain more reliable findings. Anatomical complexity plays a crucial role in endodontic research because their impacts cannot be entirely controlled but only limited. Micro‑CT is a nondestructive approach to reconstructing samples on a micrometric and real scale50. It is considered the gold standard research device in Endodontics for studying several matters, such as the quality of shaping and cleaning processes51, the performance of different obturation techniques52, the removal of root canal filling materials53, and the root canal anatomy54. Therefore, the main strength of the present research was the methodology developed to select the sample that has used both CBCT and micro-CT scanning processes to limit (even if partially) the impacts of the anatomical complexity on the variables studied. Even so, the standardization of dentin morphology (quantity, diameter, and depth of tubules, as well as the presence and quantity of intertubular dentin) could not be established, being, therefore, the main limitation of this scientific investigation and the most important reason to justify that its findings must be carefully transposed to the clinical practice.
The mechanical, chemical, and physical actions performed by the instrumentation and irrigation of the RCS present limitations, mainly imposed by the anatomic complexity and the microbial survivability that make it impossible to eliminate the endodontic infection55. The results of the present research confirm this fact since none of the irrigation protocols investigated provided total microbial elimination from both sites studied (main root canal and dentinal tubules). Therefore, new and more effective methods to disinfect the RCS should be sought, as the presence of microorganisms at the filling time does not necessarily cause failure; however, its absence certainly favors the success of endodontic treatment55.
Conclusions
Regarding the limitations of the present research, no irrigation method could render the RCS thoroughly disinfected. Nonetheless, XPF presented better results than the other systems in both analyses (MTT assay and CLSM).
Data availability
The datasets and the complete statistical analysis of the present research are available and can be requested from the corresponding author.
References
Arias, A. & Peters, O. A. Present status and future directions: Canal shaping. Int. Endod. J. 55(Suppl 3), 637–655. https://doi.org/10.1111/iej.13698 (2022).
Boutsioukis, C. & Arias-Moliz, M. T. Present status and future directions—irrigants and irrigation methods. Int. Endod. J. 55(Suppl 3), 588–612. https://doi.org/10.1111/iej.13739 (2022).
Ounsi, H. F. et al. Evolution of nickel-titanium alloys in Endodontics. J. Contemp. Dent. Pract. 18, 1090–1096. https://doi.org/10.5005/jp-journals-10024-2181 (2017).
Nardello, L. C. L. et al. Nature and prevalence of bacterial taxa persisting after root canal chemomechanical preparation in permanent teeth: A systematic review and meta-analysis. J. Endod. 48, 572–596. https://doi.org/10.1016/j.joen.2022.01.016 (2022).
Matoso, F. B. et al. Effect of different disinfection protocols in bacterial viability of an intraradicular biofilm formed in situ. Braz. Dent. J. 34, 42–49. https://doi.org/10.1590/0103-6440202305244 (2023).
Loyola-Fonseca, S. C. et al. Disinfection and shaping of vertucci class II root canals after preparation with two instrument systems and supplementary ultrasonic activation of sodium hypochlorite. J. Endod. 49, 1183–1190. https://doi.org/10.1016/j.joen.2023.06.017 (2023).
Kotecha, N. et al. Microbiological effectiveness of sodium hypochlorite gel and aqueous solution when implemented for root canal disinfection in multirooted teeth: A randomized clinical study. J. Funct. Biomater. https://doi.org/10.3390/jfb14050240 (2023).
Dede, M. et al. Efficacy of endodontic disinfection protocols in an E. faecalis biofilm model-using DAPI staining and SEM. J. Funct. Biomater. https://doi.org/10.3390/jfb14040176 (2023).
Alquria, T. A. et al. Comparison of conventional and contemporary root canal disinfection protocols against bacteria, lipoteichoic acid (LTA), and lipopolysaccharide (LPS). Sci. Rep. 13, 1206. https://doi.org/10.1038/s41598-022-26855-y (2023).
Baumeier, N. C. et al. Passive ultrasonic irrigation, EndoActivator system and XP-endo Finisher R as additional cleaning techniques to remove residual filling materials from flattened root canals. J. Conserv. Dent. 25, 385–391. https://doi.org/10.4103/jcd.jcd_117_22 (2022).
Alakshar, A., Saleh, A. R. M. & Gorduysus, M. O. Debris and smear layer removal from oval root canals comparing XP-endo finisher, endoactivator, and manual irrigation: A SEM evaluation. Eur. J. Dent. 14, 626–633. https://doi.org/10.1055/s-0040-1714762 (2020).
Machado, R. et al. Smear layer removal by passive ultrasonic irrigation and 2 new mechanical methods for activation of the chelating solution. Restor. Dent. Endod. 46, e11. https://doi.org/10.5395/rde.2021.46.e11 (2021).
Silva, E. et al. Micro-CT evaluation of different final irrigation protocols on the removal of hard-tissue debris from isthmus-containing mesial root of mandibular molars. Clin. Oral. Investig. 23, 681–687. https://doi.org/10.1007/s00784-018-2483-1 (2019).
Weller, R. N., Brady, J. M. & Bernier, W. E. Efficacy of ultrasonic cleaning. J. Endod. 6, 740–743. https://doi.org/10.1016/S0099-2399(80)80185-3 (1980).
Mozo, S., Llena, C. & Forner, L. Review of ultrasonic irrigation in Endodontics: Increasing action of irrigating solutions. Med. Oral. Patol. Oral. Cir. Bucal. 17, e512-516. https://doi.org/10.4317/medoral.17621 (2012).
De-Deus, G. et al. Micro-CT comparison of XP-endo Finisher and passive ultrasonic irrigation as final irrigation protocols on the removal of accumulated hard-tissue debris from oval shaped-canals. Clin. Oral. Investig. 23, 3087–3093. https://doi.org/10.1007/s00784-018-2729-y (2019).
Azim, A. A. et al. Efficacy of 4 irrigation protocols in killing bacteria colonized in dentinal tubules examined by a novel confocal laser scanning microscope analysis. J. Endod. 42, 928–934. https://doi.org/10.1016/j.joen.2016.03.009 (2016).
de Mattos de Araujo, B. M. et al. Micro-CT evaluation of four final irrigation protocols on hard-tissue debris removal from mesial roots of mandibular molars containing isthmus. Clin. Oral. Investig. 26, 6121–6128. https://doi.org/10.1007/s00784-022-04561-3 (2022).
General Assembly of the World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 81, 14–18 (2014).
Schneider, S. W. A comparison of canal preparations in straight and curved root canals. Oral. Surg. Oral. Med. Oral. Pathol. 32, 271–275. https://doi.org/10.1016/0030-4220(71)90230-1 (1971).
Keles, A. & Keskin, C. Apical root canal morphology of mesial roots of mandibular first molar teeth with vertucci type II configuration by means of micro-computed tomography. J. Endod. 43, 481–485. https://doi.org/10.1016/j.joen.2016.10.045 (2017).
Wolgin, M., Grundmann, M. J., Tchorz, J. P., Frank, W. & Kielbassa, A. M. Ex vivo investigation on the postoperative integrity of the apical constriction after the sole use of electronic working length determination. J. Dent. 64, 52–57. https://doi.org/10.1016/j.jdent.2017.06.005 (2017).
Al Shahrani, M., DiVito, E., Hughes, C. V., Nathanson, D. & Huang, G. T. Enhanced removal of Enterococcus faecalis biofilms in the root canal using sodium hypochlorite plus photon-induced photoacoustic streaming: An in vitro study. Photomed. Laser. Surg. 32, 260–266. https://doi.org/10.1089/pho.2014.3714 (2014).
George, D. & Mallery, P. IBM SPSS Statistics 25 Step by Step: A Simple Guide and Reference 15th edn. (Routledge, Taylor & Francis Group, 2019).
Sullivan, L. M. Essentials of Biostatistics in Public Health 4th edn. (Jones & Bartlett Learning, 2023).
Siqueira Junior, J. F., Rocas, I. D. N., Marceliano-Alves, M. F., Perez, A. R. & Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral. Res. 32, e65. https://doi.org/10.1590/1807-3107bor-2018.vol32.0065 (2018).
Siqueira, J. F. Jr. et al. What happens to unprepared root canal walls: A correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int. Endod. J. 51, 501–508. https://doi.org/10.1111/iej.12753 (2018).
Cheung, A. W. T., Lee, A. H. C. & Cheung, G. S. P. Clinical efficacy of activated irrigation in Endodontics: A focused review. Restor. Dent. Endod. 46, e10. https://doi.org/10.5395/rde.2021.46.e10 (2021).
Lee, E. S., de Josselin de Jong, E., Kim, E. & Kim, B. I. Real-time optical detection of endodontic infection using bacterial autofluorescence. J. Dent. 136, 104600. https://doi.org/10.1016/j.jdent.2023.104600 (2023).
Deng, Z., Lin, B., Liu, F. & Zhao, W. Role of Enterococcus faecalis in refractory apical periodontitis: From pathogenicity to host cell response. J. Oral. Microbiol. 15, 2184924. https://doi.org/10.1080/20002297.2023.2184924 (2023).
Liu, H., Nio, S. & Shen, Y. Sodium hypochlorite against Enterococcus faecalis biofilm in dentinal tubules: Effect of concentration, temperature, and exposure time. Odontology. https://doi.org/10.1007/s10266-023-00850-9 (2023).
Ferrer-Luque, C. M., Bejarano, I., Ruiz-Linares, M. & Baca, P. Reduction in Enteroccocus faecalis counts—A comparison between rotary and reciprocating systems. Int. Endod. J. 47, 380–386. https://doi.org/10.1111/iej.12158 (2014).
Morago, A. et al. Dentine tubule disinfection by different irrigation protocols. Microsc. Res. Tech. 82, 558–563. https://doi.org/10.1002/jemt.23200 (2019).
Pereira, T. C. et al. Chemical and mechanical influence of root canal irrigation on biofilm removal from lateral morphological features of simulated root canals, dentine discs and dentinal tubules. Int. Endod. J. 54, 112–129. https://doi.org/10.1111/iej.13399 (2021).
Salas, H., Castrejon, A., Fuentes, D., Luque, A. & Luque, E. Evaluation of the penetration of CHX 2% on dentinal tubules using Conventional Irrigation, Sonic Irrigation (EDDY) and Passive Ultrasonic Irrigation (PUI) techniques: An in vitro study. J. Clin. Exp. Dent. 13, e37–e42. https://doi.org/10.4317/jced.57065 (2021).
Seghayer, I., Lee, A. H. C., Cheung, G. S. P. & Zhang, C. Effect of passive ultrasonic irrigation, Er, Cr:YSGG laser, and photon-induced photoacoustic streaming against Enterococcus faecalis biofilms in the apical third of root canals. Bioengineering https://doi.org/10.3390/bioengineering10040490 (2023).
Teves, A. et al. Multispecies biofilm removal by XP-endo Finisher and passive ultrasonic irrigation: A scanning electron microscopy study. Aust. Endod. J. 48, 91–97. https://doi.org/10.1111/aej.12549 (2022).
Velardi, J. P. et al. Efficacy of GentleWave system and passive ultrasonic irrigation with minimally invasive and conventional instrumentation technique against Enterococcus faecalis lipoteichoic acid in infected root canals. J. Endod. 48, 768–774. https://doi.org/10.1016/j.joen.2022.01.021 (2022).
Velardi, J. P. et al. Comparison of GentleWave system and passive ultrasonic irrigation with minimally invasive and conventional instrumentation against LPS in infected root canals. Sci. Rep. 12, 4894. https://doi.org/10.1038/s41598-022-08835-4 (2022).
Hassan, R. & Elzahar, S. Cleaning efficiency of XP Finisher, XP Finisher R and passive ultrasonic irrigation following retreatment of teeth obturated with TotalFill HiFlow bioceramic sealer. Eur. Endod. J. 7, 143–149. https://doi.org/10.14744/eej.2022.39358 (2022).
Tonini, R. et al. Irrigating solutions and activation methods used in clinical Endodontics: A systematic review. Front. Oral. Health. 3, 838043. https://doi.org/10.3389/froh.2022.838043 (2022).
van der Sluis, L. W., Versluis, M., Wu, M. K. & Wesselink, P. R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 40, 415–426. https://doi.org/10.1111/j.1365-2591.2007.01243.x (2007).
Moreira, R. N., Pinto, E. B., Galo, R., Falci, S. G. M. & Mesquita, A. T. Passive ultrasonic irrigation in root canal: Systematic review and meta-analysis. Acta. Odontol. Scand. 77, 55–60. https://doi.org/10.1080/00016357.2018.1499960 (2019).
Silva, E. et al. Effectiveness of passive ultrasonic irrigation on periapical healing and root canal disinfection: A systematic review. Br. Dent. J. 227, 228–234. https://doi.org/10.1038/s41415-019-0532-z (2019).
Akcay, M., Arslan, H., Mese, M., Durmus, N. & Capar, I. D. Effect of photon-initiated photoacoustic streaming, passive ultrasonic, and sonic irrigation techniques on dentinal tubule penetration of irrigation solution: A confocal microscopic study. Clin. Oral. Investig. 21, 2205–2212. https://doi.org/10.1007/s00784-016-2013-y (2017).
Gu, Y. et al. Effect of different agitation techniques on the penetration of irrigant and sealer into dentinal tubules. Photomed. Laser. Surg. 35, 71–77. https://doi.org/10.1089/pho.2016.4125 (2017).
Neelakantan, P. et al. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er:YAG laser in vitro. Int. Endod. J. 48, 602–610. https://doi.org/10.1111/iej.12354 (2015).
Alves, F. R. et al. Adjunctive steps for disinfection of the mandibular molar root canal system: A correlative bacteriologic, micro-computed tomography, and cryopulverization approach. J. Endod. 42, 1667–1672. https://doi.org/10.1016/j.joen.2016.08.003 (2016).
Bao, P., Shen, Y., Lin, J. & Haapasalo, M. In vitro efficacy of XP-endo Finisher with 2 different protocols on biofilm removal from apical root canals. J. Endod. 43, 321–325. https://doi.org/10.1016/j.joen.2016.09.021 (2017).
Swain, M. V. & Xue, J. State of the art of micro-CT applications in dental research. Int. J. Oral. Sci. 1, 177–188. https://doi.org/10.4248/IJOS09031 (2009).
YeniceriOzata, M., Falakaloglu, S., Keles, A., Adiguzel, O. & Gundogar, M. Evaluation of shaping ability of different glide path instruments: A micro-computed tomography study. BMC. Oral. Health. 23, 780. https://doi.org/10.1186/s12903-023-03529-3 (2023).
Suassuna, F. C. M. et al. Thermal and volumetric assessment of endodontic filling techniques using infrared thermography and micro-CT. J. Oral. Sci. 65, 34–39. https://doi.org/10.2334/josnusd.22-0285 (2023).
Madarati, A. A., Sammani, A. M. N., Alnazzawi, A. A. & Alrahlah, A. Efficiency of the new reciprocating and rotary systems with or without ultrasonics in removing root-canals filling with calcium silicate-based sealer (MTA). BMC. Oral. Health. 23, 5. https://doi.org/10.1186/s12903-022-02684-3 (2023).
Saber, S. M., Elashiry, M. M., Sadat, S. & Nawar, N. N. A microcomputed tomographic analysis of the morphological variabilities and incidence of extra canals in mandibular first molar teeth in an Egyptian subpopulation. Sci. Rep. 13, 8985. https://doi.org/10.1038/s41598-023-36005-7 (2023).
Siqueira, J. F. Jr. & Rocas, I. N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 34, 1291-1301.e1293. https://doi.org/10.1016/j.joen.2008.07.028 (2008).
Author information
Authors and Affiliations
Contributions
A.T.G.C.: conceptualization, methodology, data curation and writing (original draft preparation). A.A.K.: methodology, data curation and writing (original draf preparation). E.A.R.R.: methodology and data curation. F.S.G.: methodology and data curation. B.M.: methodology, data curation and writing (original draf preparation). L.P.: writing (review and editing). R.M.: writing (review and editing). S.A.I.: statistical analysis. U.X.S.N.: supervision and writing (review and editing). All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Timponi Goes Cruz, A., Antoniw Klemz, A., Ribeiro Rosa, E.A. et al. Cleaning and disinfection of the root canal system provided by four active supplementary irrigation methods. Sci Rep 14, 3795 (2024). https://doi.org/10.1038/s41598-024-53375-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53375-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.