Abstract
Core symptoms in patients with schizophrenia spectrum disorders (SSD), like hallucinations or ego-disturbances, have been associated with a failure of internal forward models to predict the sensory outcomes of self-generated actions. Importantly, forward model predictions must also be able to flexibly recalibrate to changing environmental conditions, for example to account for additional delays between action and outcome. We investigated whether transcranial direct current stimulation (tDCS) can be used to improve these sensorimotor temporal recalibration mechanisms in patients and healthy individuals. While receiving tDCS on the cerebellum, temporo-parietal junction, supplementary motor area, or sham stimulation, patients with SSD and healthy control participants were repeatedly exposed to delays between actively or passively elicited button presses and auditory outcomes. Effects of this procedure on temporal perception were assessed with a delay detection task. Similar recalibration outcomes and faciliatory effects of cerebellar tDCS on recalibration were observed in SSD and healthy individuals. Our findings indicate that sensorimotor recalibration mechanisms may be preserved in SSD and highlight the importance of the cerebellum in both patients and healthy individuals for this process. They further suggest that cerebellar tDCS could be a promising tool for addressing deficits in action-outcome monitoring and related adaptive sensorimotor processes in SSD.
Similar content being viewed by others
Introduction
Core symptoms in patients with schizophrenia and schizoaffective disorder (referred to as schizophrenia spectrum disorders, SSD) encompass hallucinations (e.g., perceiving the own inner speech as external voice) and ego-disturbances (e.g., perceiving own thoughts or actions as externally controlled). The emergence of these symptoms has been associated with a failure of adequately predicting the sensory outcomes of one´s own actions1. When performing an action, copies of the motor commands are thought to be used by an internal forward model to predict the action´s sensory outcomes. Re-afferent sensory input that is in line with the prediction is typically found to be associated with modulations in perceptual acuity and neural responses in multiple brain regions compared to input that deviates from the prediction, and is thus perceived as self-generated2,3,4. Dysfunctions in this predictive mechanism can result in the misattribution of self-generated sensory input as externally produced and lead to the symptoms in SSD described above5,6,7,8,9,10.
Importantly though, forward model predictions are not rigid, but need to be able to flexibly recalibrate to preserve adequate distinction between self- and externally generated input even under dynamically changing environmental conditions. For instance, the outcome of an action can be transiently delayed under certain circumstances, e.g., a mouse click can lead to delayed responses from a computer11. Studies with healthy participants have frequently shown that after repeated exposure to a delayed action-outcome, the predicted sensory outcome timing shifted toward that delay. As consequence, the delayed outcome was perceived as occurring in synchrony with the action, a phenomenon known as the sensorimotor temporal recalibration effect (TRE)12,13,14,15,16, and neural responses for the delayed outcome resembled the ones typically observed for undelayed outcomes17. To date, it remains unknown whether the dysfunctions in predictive mechanisms observed in SSD are due to a general failure of prediction generation or, more specifically, a failure of adequately recalibrating these predictions to the constantly changing requirements of the environment.
Neural correlates of the predictive processes based on the forward model have been identified in several brain regions. The cerebellum is most prominent in this regard since it has been suggested to play a vital role in the generation and updating of predictions about sensory action-outcomes2,4,18,19,20,21,22. Additionally, regions in parietal cortex, particularly the temporo-parietal junction (TPJ) or angular gyrus9,23,24,25,26, and the supplementary motor area (SMA)27,28 could be associated with the subjective feeling of control or agency over action-outcomes and the distinction between self- and externally generated stimuli. Interestingly, non-invasive brain stimulation techniques have demonstrated the potential to modulate these processes when applied to the respective brain regions. For instance, transcranial magnetic stimulation (TMS)29 or transcranial direct current stimulation (tDCS)30 of the cerebellum influenced the effect of sensorimotor temporal recalibration on perception in healthy individuals. Furthermore, tDCS on the angular gyrus31, the pre-supplementary motor area32,33, and frontal regions34,35 modulated measures for agency and action-outcome-related processing, even in patients with SSD36. Thus, tDCS may also be a promising tool to enhance sensorimotor temporal recalibration mechanisms and thereby improve action-outcome processing and self-other distinction in patients with SSD.
Therefore, the present study investigated for the first time (1) whether sensorimotor temporal recalibration mechanisms are impaired in patients with SSD compared to healthy control (HC) participants, and (2) whether tDCS on the bilateral cerebellum, right SMA, or right TPJ can enhance recalibration and thus reduce potential deficits. Participants were exposed to delayed or undelayed tones following either actively performed or passively elicited button press movements, and the effects of this procedure on auditory and visual temporal perception were assessed with a delay detection task. The undelayed tone should align with the natural prediction of undelayed action-outcomes, while exposure to the delayed tones was expected to induce a TRE in terms of reduced delay detection performance. While active movements were expected to trigger sensorimotor temporal recalibration based in the forward model, passive movements were applied to control for recalibration effects due to changes in the expected inter-sensory timing between the tactile sensations during the button movement and the auditory or visual outcome37,38,39,40. We expected patients with SSD to exhibit reduced temporal recalibration compared to HC, specifically in active movement conditions, due to impaired recalibration of forward model predictions. We expected tDCS applied on the mentioned brain regions to enhance temporal recalibration in both groups, particularly in active conditions, due to the presumed importance of these regions in the generation and updating of forward model predictions.
Materials and methods
Participants
Twenty-four patients with SSD and 20 HC with no psychiatric diagnosis (10 female, mean age: 36.90, SD = 10.37) participated in the study. Two patients had to be excluded (see Supplementary Material S1), resulting in a final sample of 22 patients (11 female, mean age: 35.80, SD = 10.37). Fifteen patients were diagnosed with an ICD-10 diagnosis of schizophrenia, six patients with schizoaffective disorder, and one patient with an acute and transient psychotic disorder (for further details on sample characteristics see Supplementary Material S1). All participants had normal or corrected-to-normal visual acuity, normal hearing, and no history of neurological disorders. No contraindications for tDCS (e.g., electric, or metallic implants) were reported. Participants gave written informed consent and were financially reimbursed for their participation. The study was conducted according to the Declaration of Helsinki and was approved by the local ethics commission (Study 06/19) of the medical faculty of University of Marburg, Germany. The study was pre-registered in the German Clinical Trials Register (DRKS-ID: DRKS00025885; https://drks.de; date of registration: July 23, 2021).
Transcranial direct current stimulation
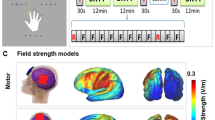
tDCS was applied using a DC stimulator (neuroConn GmbH, Ilmenau) and two rubber electrodes (5 × 7 cm) in saline-soaked sponges (0.9% NaCl). For all stimulation conditions, the anode was placed over the respective brain region since anodal tDCS has been shown to increase cortical excitability41. For stimulation of the bilateral cerebellum, the center of the anode was placed on the midline 2 cm below the inion. For tDCS on the right SMA and TPJ, electrodes were positioned according to the 10–20 EEG system. For stimulating the right SMA, the anode was placed on FC2 (10% of the distance between nasion and inion anteriorly to Cz; and 10% of the distance between the preauricular points to the right). tDCS on the right TPJ was applied by placing the anode between C4 and P4 (20% of the distance between nasion and inion posteriorly to Cz; and 20% of the distance between the preauricular points to the right). The right hemisphere was chosen based on previous findings indicating the involvement of right parietal and right supplementary motor regions in action-outcome processing in healthy individuals as well as in patients with SSD9,36. During the sham stimulation session, electrodes were attached similarly as for the cerebellar tDCS condition. In all conditions, the cathode was attached on the deltoid muscle of the right upper arm. A similar electrode montage with an extracephalic location for the return electrode has been successfully applied earlier in studies investigating effects of stimulating the cerebellum42, SMA32, and posterior parietal cortex43 and was not accompanied with reduced stimulation outcomes compared to cephalic locations when a current of 2 mA was applied44. This montage also ensured that the obtained stimulation effects cannot be attributed to confounding influences of the cathode on neural excitability44,45. All electrodes were attached with rubber bands. The stimulation was applied with a current of 2 mA for 20 min (+ 10 s fade in and fade out periods). Next to these three active stimulation conditions, a sham stimulation condition was implemented by using sinus (half wave) mode for 30 s. Here, the current gradually increased during the first 15 s and then decreased again to generate the same subjective sensations (like tingling) due to changes in current intensity as in active stimulation conditions while no actual stimulation was applied. The stimulation parameters were chosen in accordance with established tDCS safety guidelines46. Each participant experienced the four stimulation conditions (cerebellum, SMA, TPJ, sham) in four separate sessions. Sessions were performed at least 18 h apart to prevent residual effects from the previous stimulation. The stimulation conditions were applied in counterbalanced order, ensuring that in each group, across participants, each of the four stimulation conditions was applied approximately equally often during the first, second, third, or fourth session. Participants were sequentially assigned to one of the possible combinations of stimulation conditions and were unaware of the hypothesized effects of stimulating the respective brain region on task performance.
Equipment and stimuli
Participants performed the experiment in a dimly lit room in front of a computer screen. Button presses were executed with the right index finger using a custom-made electromagnetic passive button device. In active conditions, participants pressed the button actively by themselves. In passive conditions, the button was pulled down automatically by an electromagnet (max. force 4N). An elastic fabric band was used to attach the participants’ fingers to the button to ensure that it smoothly followed its movement in passive conditions. When the button reached the lowest position, the presentation of an auditory or visual stimulus was triggered. The visual stimulus was a Gabor patch (1° visual angle, spatial frequency: 2 cycles/degree) which was presented at the center of the screen. The auditory stimulus was a brief sine-wave tone (2000 Hz with 2 ms rise and fall) presented through headphones. Both stimuli appeared for a duration of 33.4 ms. All stimuli were created and presented using Octave and the Psychophysics Toolbox47. To prevent any influence of the direct visual or auditory feedback from the actual button presses on sensory outcome perception, the button device was covered by a black box and pink noise was applied through headphones during the experiment. The intensity of the pink noise was adjusted individually for each participant until they indicated that they could no longer hear the inherent noise of the button device.
Experimental design and task description
The experiment consisted of an established temporal recalibration paradigm22,30,48 in which participants were exposed to multiple pairs of adaptation and test phases. In adaptation phases, 18 consecutive button presses had to be executed each followed by the tone as auditory sensory outcome. The button presses were either performed actively or they were elicited passively (factor movement type). The tone occurred either immediately after the button press (undelayed, 0 ms delay) or was delayed by 200 ms (factor adaptation delay). Originally, we chose an adaptation delay of 150 ms as is had been used in previous studies with young healthy participants30,48. But given the lower delay detection performance observed among older participants, the adaptation delay was adjusted to 200 ms after collecting the data from the first four patients. Nonetheless, the data of all patients were included in the current analyses. Pairwise comparisons indicated that excluding the data of these four patients would not lead to differences in group-dependent effects on the overall effect of temporal recalibration (for details see Supplementary Material S3).
Each adaptation phase was followed by a test phase that assessed the impact of the adaptation delay on perception. A test phase consisted of six test trials for which the button had to be pressed once, either actively or passively. The movement type was the same as the one used in the previous adaptation phase. While the stimulus during adaptation phases was always auditory, in test phases, the button presses elicited either the auditory or the visual stimulus (factor test modality). This was done because the TRE has previously been shown to transfer between modalities, such that recalibration to a sensorimotor delay in one modality also affected temporal perception in another modality13,48,49,50. In a test phase, the sensory stimuli were either visual or auditory in all of the six test trials. In each test trial, the stimulus occurred with one of six test delay levels (0, 83, 167, 250, 333, 417 ms). Each of the test delays was used once in each test phase in counterbalanced order. Participants were instructed to report via keyboard presses after each trial whether they detected a delay between the button press and the stimulus. The TRE was defined as the difference in the proportion of detected delays after exposure to delayed vs. undelayed tones with worse performance for delayed tones reflecting a shift of the expected stimulus timing in the direction of the adapted delay, indicating temporal recalibration.
Procedure
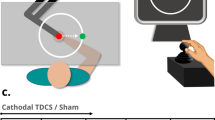
An adaptation phase started with instructions displayed for 2000 ms indicating the movement type of the button presses (see Fig. 1). After the instructions disappeared, participants could start pressing the button or it started to move passively. Each button press was followed by the tone, either undelayed or delayed by 200 ms. After nine button presses, a fixation cross appeared on the screen for a jittered duration (1000, 1500, 2000, or 2500 ms) indicating a short break. After the fixation cross disappeared, the remaining nine button presses could be performed.
Trial sequence and timing of events. The experiment consisted of multiple pairs of adaptation and test phases. During adaptation phases, 18 button presses had to be performed either actively by participants or they were executed passively. A button press was followed by a delayed (200 ms) or undelayed tone. Adaptation phases were divided into two parts separated by a fixation cross presentation. In test phases, the button was pressed once in each test trial, either actively or passively. Here, the outcome occurred after one of six delays (0–417 ms) and participants had to report in each trial whether they detected a delay. The outcome modality was always auditory during adaptation, but it could be visual or auditory during test.
A test phase was initiated by instructions (2000 ms) about the movement type and stimulus modality of the following test trials. The cue “Ready” was presented for 1000 ms before each test trial. After the cue disappeared, participants had 2000 ms in active conditions to press the button. But they were instructed to wait for approx. another 700 ms to ensure that the movement was voluntary and not reflexive upon cue disappearance36,51. Passive movements were initiated after a jittered interval of 0, 500, 1000 ms. Each button press triggered the auditory or visual outcome with one of the six test delays. Afterwards, the question “Delay?” was presented and participants had 2000 ms to respond via keyboard press whether they detected a delay. After a pause of 500 ms the “Ready” cue initiated the next test trial. After the last trial of a test phase a jittered inter-trial interval (1000, 1500, 2000, 2500 ms) was inserted before the start of the next adaptation-test pair.
Each of the eight experimental conditions was presented with eight adaptation-test pairs per session. Within each session, conditions with the same adaptation delay were blocked to prevent spill-over effects due to rapid switching of delays. The first block of conditions with one of the adaptation delays took place while the stimulation was applied. After this first task block, electrodes were detached. The conditions with the other adaptation delay were presented in a second task block without stimulation. Whether the undelayed or delayed tones were presented first as well as the order of conditions within blocks was counterbalanced across participants.
After each session, side effects due to the tDCS stimulation (e.g., itching sensations, headache, changes in visual perception, difficulties in concentration) had to be rated on a scale from one (no side effect) to five (strong side effect) using a custom-designed questionnaire of 28 items. During the first session, participants additionally went through a training procedure to familiarize with the task (see Supplementary Material S2).
Data analyses
Test trials for which no button press, or response were registered were excluded from the analyses. For SSD, 1.858% of all trials were excluded due to missing button presses, and 5.617% of trials due to missing responses. For HC, the button press was missing in 0.781% of all trials and the response in 3.503% of trials. The percentage of detected delays served as a measure for delay detection performances and was calculated for each participant and experimental condition. These data were forwarded into a mixed ANOVA with the between-participants factor group and the within-participants factors stimulation, test modality, movement type, and adaptation delay. We examined main and interaction effects including the factors adaptation delay and group, to test for the impact of the adaptation delay on temporal perception and the modulatory influence of the other experimental factors as well as group-dependent effects. For significant interaction effects with the adaptation delay, we calculated the TRE defined as the difference in the percentage of detected delays in conditions with the 200 ms compared to 0 ms adaptation delay. Positive values indicate worse detection performance after exposure to the 200 ms delay, reflecting a TRE into the expected direction. If indicated, post-hoc one-sided one-sample t-tests were used to assess whether the TRE was significantly greater than zero in the individual conditions of an interaction effect. Furthermore, two-samples t-tests were used to determine the difference in the TRE between the relevant conditions or groups. Since we had clear hypotheses regarding the direction of difference in TRE between conditions (i.e., a stronger TRE in HC vs. SSD, active vs. passive, auditory vs. visual conditions, and in active tDCS conditions vs. sham stimulation) one-sided t-tests were used. All post-hoc tests were Bonferroni-corrected if indicated. For significant interaction effects without the group factor, post-hoc tests were exploratorily performed not only across but also individually for both groups to identify similarities in temporal recalibration effects for SSD and HC. Since the SSD group in our study was heterogeneous and comprised different diagnoses of the schizophrenia spectrum, the same analysis was also exploratorily performed for the subgroup of patients diagnosed with schizophrenia (F20; N = 15). Results of this exploratory analysis closely resembled the ones obtained with the entire SSD group and are reported in Supplementary Material S7. Finally, we tested for differences in perceived stimulation side effects between the groups and stimulation conditions by a mixed ANOVA with the factors group and stimulation (results are reported the Supplementary Material S4). All analyses were performed in JASP (Version 0.14.1)52.
Results
A mixed ANOVA with the between-participants factor group (SSD, HC) and the within-participants factors stimulation (cerebellum, SMA, TPJ, sham), test modality (auditory, visual), movement type (active, passive), and adaptation delay (0 ms, 200 ms) was conducted on the percentage of detected delays. Results revealed no significant main effect of group [F(1, 40) = 0.239, p = 0.628, ηp2 = 0.006], indicating that patients did not differ from HC in general delay detection abilities [HC: Mean = 37.138, SD = 8.845; SSD: Mean = 41.758, SD = 17.393]. Furthermore, none of the interaction effects including the factors group and adaptation delay were significant (all p > 0.139), thus providing no evidence for impairments in temporal recalibration and differences in the effectiveness of tDCS in patients with SSD (see Supplementary Material S3 for a summary of all effects).
However, across groups and conditions, there was a significant main effect of the adaptation delay [F(1, 40) = 14.033, p < 0.001, ηp2 = 0.260]. Thus, participants’ perception recalibrated to the 200 ms delay between button press and auditory outcome, leading to a significant TRE in terms of reduced delay detection performance [Mean TRE = 3.197, SD = 5.439; see Table 1 for an overview of effects computed individually for both groups and Fig. 2 for an illustration of effects]. Additionally, the interaction of movement type and adaptation delay was significant [F(1, 40) = 8.762, p = 0.005, ηp2 = 0.180].
Temporal recalibration effects. (A) The TRE, defined as the difference in the percentage of detected delays between conditions with the 200 ms vs. 0 ms delay during preceding adaptation phases, is displayed for each experimental condition (i.e., for both test modalities and movement types), across groups, as well as separately for both groups. In both groups, for auditory (unimodal) conditions, the TRE was significantly larger in active compared to passive movement conditions. (B) The TRE is displayed for each of the four stimulation conditions, again across groups and separately for both groups. Across groups and for HC alone, cerebellar tDCS significantly facilitated the TRE compared to sham stimulation. For SSD, cerebellar tDCS induced a significant TRE which was absent during sham stimulation. Error bars indicate standard errors of the mean. *p > .05, **p < .01, ***p < .001.
Post-hoc tests revealed that the TRE was significantly greater than zero in both active [Mean TRE = 4.103, SD = 4.586, t(41) = 5.799, p < 0.001, d = 0.895, ɑcorr = 0.025] and passive conditions [Mean TRE = 2.291, SD = 6.830, t(41) = 2.174, p = 0.018, d = 0.335, ɑcorr = 0.025], but was significantly stronger in active ones [Mean difference = 1.812, SD = 4.124, t(41) = 2.848, p = 0.003, d = 0.439]. Furthermore, there was a significant test modality and adaptation delay interaction [F(1, 40) = 9.229, p = 0.004, ηp2 = 0.187]. While the TRE was significantly greater than zero for both, audition [Mean TRE = 4.279, SD = 5.939, t(41) = 4.669, p < 0.001, d = 0.720, ɑcorr = 0.025] and vision [Mean TRE = 2.115, SD = 5.871, t(41) = 2.335, p = 0.012, d = 0.360, ɑcorr = 0.025], indicating a modality transfer of the TRE, it remained significantly larger in auditory (unimodal) than in visual (cross-modal) conditions [Mean difference = 2.164, SD = 4.599, t(41) = 3.050, p = 0.002, d = 0.471]. The interaction of the three factors movement type, test modality, and adaptation delay [F(1, 40) = 7.781, p = 0.008, ηp2 = 0.163] further indicated that the active–passive difference in the TRE was specific to auditory outcomes [Mean difference = 4.187, SD = 5.872, t(41) = 4.622, p < 0.001, d = 0.713, ɑcorr = 0.025; Active: Mean TRE = 6.373, SD = 6.255, t(41) = 6.603, p < 0.001, d = 1.019, ɑcorr = 0.025; Passive: Mean TRE = 2.185, SD = 6.976, t(41) = 2.030, p = 0.024, d = 0.313, ɑcorr = 0.025] but did not transfer to the visual modality [Mean difference = − 0.563, SD = 7.625, t(41) = − 0.478, p = 0.682, d = − 0.074, ɑcorr = 0.025; Active: Mean TRE = 1.834, SD = 4.662, t(41) = 2.549, p = 0.007, d = 0.393, ɑcorr = 0.025; Passive: Mean TRE = 2.396, SD = 8.733, t(41) = 1.778, p = 0.041, d = 0.274, ɑcorr = 0.025].
Regarding the influence of tDCS on temporal recalibration, according to the interaction of stimulation and adaptation delay [F(3, 120) = 2.800, p = 0.043, ηp2 = 0.065] and subsequent post-hoc tests, across groups, the TRE was significantly stronger after cerebellar tDCS compared to sham stimulation [Mean difference = 3.071, SD = 8.050, t(41) = 2.472, p = 0.009, d = 0.381, ɑcorr = 0.016; Sham: Mean TRE = 2.028, SD = 6.250, t(41) = 2.103, p = 0.021, d = 0.324, ɑcorr = 0.025; Cerebellum: Mean TRE = 5.099, SD = 8.183, t(41) = 4.038, p < 0.001, d = 0.623, ɑcorr = 0.025], but not after tDCS on the right SMA [Mean difference = 0.640, SD = 7.643, t(41) = 0.542, p = 0.295, d = 0.084, ɑcorr = 0.016] or the right TPJ [Mean difference = 0.967, SD = 6.257, t(41) = 1.001, p = 0.161, d = 0.155, ɑcorr = 0.016]. Finally, the significant four-way interaction of stimulation, movement type, test modality, and adaptation delay [F(3, 120) = 3.343, p = 0.022, ηp2 = 0.077] further revealed that, across groups, the faciliatory influence of cerebellar tDCS on the TRE occurred specifically for active and auditory conditions [Mean difference = 5.188, SD = 14.052, t(41) = 2.393, p = 0.011, d = 0.369, ɑcorr = 0.0125; Sham: Mean TRE = 4.518, SD = 9.580, t(41) = 3.057, p = 0.002, d = 0.472, ɑcorr = 0.025; Cerebellum: Mean TRE = 9.707, SD = 12.633, t(41) = 4.979, p < 0.001, d = 0.768, ɑcorr = 0.025], but was absent in passive/auditory [Mean difference = 3.109, SD = 16.246, t(41) = 1.240, p = 0.111, d = 0.191, ɑcorr = 0.0125], active/visual [Mean difference = 1.405, SD = 9.725, t(41) = 0.937, p = 0.177, d = 0.145, ɑcorr = 0.0125], and in passive/visual conditions [Mean difference = 2.581, SD = 10.710, t(41) = 1.562, p = 0.063, d = 0.241, ɑcorr = 0.0125].
Discussion
In this study, we investigated for the first time the commonalities and differences in sensorimotor temporal recalibration mechanisms between HC and SSD and whether tDCS on relevant regions could facilitate recalibration effects. We found similar effects of sensorimotor temporal recalibration in both groups indicating that recalibration mechanisms may be preserved in SSD. Furthermore, the faciliatory impact of cerebellar tDCS on these effects in both groups highlights the importance of the cerebellum for recalibrating forward model predictions in response to environmental changes.
Regardless of the tDCS stimulation, both HC and SSD showed a significant TRE across conditions, and specifically so for active movements. Furthermore, no group differences in the TRE were observed depending on the movement type, test modality, or stimulation condition. Thus, our study does not provide evidence for a fundamental impairment in sensorimotor temporal recalibration in SSD, but rather suggests commonalities in recalibration mechanisms between SSD and HC. Predictable action-outcomes, i.e., outcomes in active conditions in our study, are typically associated with perceptual differences compared to externally generated sensory input2,3,4. However, this difference is often found to be reduced in SSD which is usually considered as an indicator of impairments of the forward model in predicting the sensory outcomes of self-generated actions5,7,8,9. In our study, overall group differences in delay detection performances between actively and passively generated stimuli appeared to have been too small or associated with too much variance to manifest in a significant interaction effect (see also Supplementary Material S8 for study limitations). Nevertheless, according to supplementary analyses (see Supplementary Material S5), patients with SSD exhibited a reduced difference between active and passive delay detection rates for a specific test delay level, indicating that the previously reported deficit5,7,8,9,36 also weakly manifested in our data. Importantly, due to the absence of differences in temporal recalibration between the groups, our findings suggest that the aberrant processing associated with self-generated action-outcomes in SSD cannot be attributed to dysfunctions in flexibly recalibrating forward model predictions in response to changes in environmental conditions, such as varying action-outcome delays. Instead, they point to a more general failure in the prediction generation process in SSD.
Importantly, cerebellar tDCS facilitated the TRE in both groups. In HC, the TRE increased significantly with cerebellar tDCS compared to sham stimulation. In SSD, cerebellar tDCS was able to induce a significant TRE which was absent with sham stimulation. The cerebellum has frequently been suggested as the site of internal forward models2,4,18,19,20,21. The adaptation of these predictions when required due to changing environmental conditions could also be associated with processes in the cerebellum19,22,29,30,53,54. Thus, the faciliatory impact of cerebellar tDCS on the TRE suggests that the recalibration of these predictive processes in the cerebellum was amplified by the stimulation, which is in line with previous studies demonstrating a faciliatory influence of cerebellar stimulation on sensorimotor temporal recalibration mechanisms in healthy participants29,30. This is further supported by the fact that the TRE was generally larger in active than in passive conditions for both groups in our study. In both active and passive conditions, the TRE can be associated with the recalibration of the expected inter-sensory timing between the tactile sensation of the button movement and the visual or auditory outcome. A stronger TRE for active movements thus suggests that, next to inter-sensory recalibration mechanisms, the recalibration of forward model predictions additionally contributed to the TRE in this condition48,55. Moreover, the fact that the faciliatory impact of cerebellar tDCS was specific to active conditions further indicates that it specifically amplified the recalibration of forward model predictions in this region.
Furthermore, according to supplementary analyses, across groups, the TRE in active and auditory conditions appeared to be larger after cerebellar tDCS compared to tDCS applied to the TPJ or SMA (see Supplementary Material S6). Although both TPJ and SMA have often been associated with processes closely related to forward model-based predictive mechanisms, such as the sense of agency9,23,24,25,26,27,28, they do not to appear as strongly linked to the recalibration of action-outcome predictions as the cerebellum. This suggests that these regions are more likely to play a role at a different processing stage, such as in the generation of efference copy signals56 or the comparison of predictions and outcomes25,26. This emphasizes once again the central role of the cerebellum in generating forward model predictions and in adapting them to additional action-outcome delays19,22,29,30, and that this adaptability can consequently be most effectively amplified by means of cerebellar tDCS in patients and healthy individuals.
Beyond that, the faciliatory impact of cerebellar tDCS on the TRE appeared to be specific to auditory conditions for both groups. Since the adaptation delay was always inserted between the button press and the auditory outcome, a transfer of the TRE to vision, especially for active conditions, would suggest that forward model predictions are generated and recalibrated simultaneously for sensory outcomes of different modalities. This would indicate that recalibration results in changes in the general predicted timing for sensory action-outcomes rather than in modality-specific changes13,48,49,50. Although the adaptation procedure had an impact on temporal perception in the visual domain in our study, leading to a visual TRE across the groups, cerebellar tDCS did not affect the size of this modality-transfer effect. Furthermore, the transfer of the TRE to vision was not stronger in active than in passive conditions. Thus, these findings do not speak for the presence of supra-modal predictive mechanisms in the cerebellum. Instead, the modality-transfer can rather be explained by the supra-modal recalibration of inter-sensory matching mechanisms, assumed to be involved in both active and passive conditions, leading to changes in the expected timing between tactile, auditory, and visual outcomes57. Importantly though, the TRE across active and passive conditions was larger for auditory than for visual stimuli. Thus, there was only a partial transfer of the effect to vision. For sensorimotor, i.e., active conditions, a general superiority of the TRE due to recalibration to auditory compared to visual action-outcome delays has also been reported previously15. And while a transfer of the TRE from audition to vision has been found in a few studies49,50, others failed to replicate this finding13,22,48. This may be related to the fact that temporal perception is less precise for vision than for audition and the temporal predictability of visual signals is therefore thought to be worse compared to auditory ones58,59. Hence, it may be assumed that due to the lower temporal predictability or higher levels of noise associated with visual sensory stimuli, visual perception is generally less prone to small changes in inter-sensory or in action-outcome delays.
Overall, the fact that cerebellar tDCS had a similar impact on the TRE for both groups highlights the importance of cerebellum-based predictive processes, which play a vital role in the adaptation to action-outcome delays in both healthy individuals and patients with SSD. This could also imply the potential of cerebellar tDCS to facilitate related adaptive processes in SSD, which are tightly connected to the cerebellum as well and have frequently been reported to be impaired in these patients. Among them is the process of sensorimotor adaptation, i.e., the adaptation of movements in response to a discrepancy between predicted and observed sensory outcomes of these movements60,61,62. For instance, compared to healthy individuals, patients exhibited impaired sensorimotor adaptation abilities in tasks where movements had to adapt to shifted or rotated visual feedback63,64,65, and reduced saccade adaptation66,67. These adaptation deficits in SSD may also be explained by dysfunctions in accurately building and updating internal forward models in the cerebellum to minimize the error between predicted and perceived sensory action-outcomes65,67. In healthy individuals, tDCS on the cerebellum already showed the potential to improve sensorimotor adaptation performances in similar tasks68,69,70. Initial evidence in non-clinical psychosis further demonstrated the effectiveness of cerebellar tDCS in ameliorating sensorimotor learning deficits71. Since cerebellar tDCS had a faciliatory impact on sensorimotor temporal recalibration in both groups in our study, these findings emphasize the potential of this technique to also improve these related adaptive processes in SSD. Furthermore, intact sensorimotor recalibration mechanisms which can be further amplified by cerebellar tDCS could also represent a valuable resource of patients with SSD. For instance, it might be conceivable to train sensorimotor adaptation abilities or the general ability of the forward model to generate appropriate action-outcome predictions and thereby to improve self-other distinction, action-outcome monitoring, and ultimately related clinical symptoms in SSD. Concurrent stimulation of the cerebellum via tDCS may be able to enhance respective training outcomes. However, it is important to note that while our study suggests similar behavioral temporal recalibration effects between patients and healthy individuals, as well as similar effects of cerebellar tDCS on recalibration, further neural correlates of sensorimotor temporal recalibration mechanisms have not yet been investigated in SSD. Future fMRI or EEG studies (for example see22,72,73) could prove useful in this regard to determine whether the similar behavioral recalibration effects observed between the groups are also accompanied by similar neural processing during recalibration, or whether differences emerge at the neural level, indicating dysfunctional recalibration-related neural processes.
In conclusion, our study points to similar sensorimotor temporal recalibration mechanisms in HC and SSD and highlights the importance of the cerebellum in both groups for this process. Our results suggest that cerebellar tDCS may constitute a promising tool for addressing deficits in related predictive or adaptive processes based on the forward model in the cerebellum, and potentially linked symptomatology in SSD.
Data availability
The data that support the findings of this study are openly available in Zenodo at: https://doi.org/10.5281/zenodo.10047376.
References
Pynn, L. K. & DeSouza, J. F. X. The function of efference copy signals: Implications for symptoms of schizophrenia. Vision. Res. 76, 124–133. https://doi.org/10.1016/j.visres.2012.10.019 (2013).
Arikan, B. E. et al. Perceiving your hand moving: BOLD suppression in sensory cortices and the role of the cerebellum in the detection of feedback delays. J. Vis. 19(14), 4. https://doi.org/10.1167/19.14.4 (2019).
Blakemore, S. J., Wolpert, D. M. & Frith, C. D. Central cancellation of self-produced tickle sensation. Nat. Neurosci. 1(7), 635–640. https://doi.org/10.1038/2870 (1998).
Straube, B. et al. Predicting the multisensory consequences of one’s own action: BOLD suppression in auditory and visual cortices. PLoS ONE 12(1), e0169131. https://doi.org/10.1371/journal.pone.0169131 (2017).
Ford, J. M. et al. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am. J. Psychiatry 158(12), 2069–2071. https://doi.org/10.1176/appi.ajp.158.12.2069 (2001).
Lindner, A., Thier, P., Kircher, T. T. J., Haarmeier, T. & Leube, D. T. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr. Biol. 15(12), 1119–1124. https://doi.org/10.1016/j.cub.2005.05.049 (2005).
Martinelli, C., Rigoli, F. & Shergill, S. S. Aberrant force processing in schizophrenia. Schizophr. Bull. 2, 417–424. https://doi.org/10.1093/schbul/sbw092 (2016).
Shergill, S. S. et al. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiat. 71(1), 28. https://doi.org/10.1001/jamapsychiatry.2013.2974 (2014).
Uhlmann, L., Pazen, M., van Kemenade, B. M., Kircher, T. & Straube, B. Neural correlates of self-other distinction in patients with schizophrenia spectrum disorders: The roles of agency and hand identity. Schizophr. Bull. 47(5), 1399–1408. https://doi.org/10.1093/schbul/sbaa186 (2021).
Frith, C. D., Blakemore, S. J. & Wolpert, D. M. Abnormalities in the awareness and control of action. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355(1404), 1771–1788. https://doi.org/10.1098/rstb.2000.0734 (2000).
Cai, C., Ogawa, K., Kochiyama, T., Tanaka, H. & Imamizu, H. Temporal recalibration of motor and visual potentials in lag adaptation in voluntary movement. Neuroimage 172, 654–662. https://doi.org/10.1016/j.neuroimage.2018.02.015 (2018).
Keetels, M. & Vroomen, J. Exposure to delayed visual feedback of the hand changes motor-sensory synchrony perception. Exp. Brain Res. 219(4), 431–440. https://doi.org/10.1007/s00221-012-3081-0 (2012).
Sugano, Y., Keetels, M. & Vroomen, J. The build-up and transfer of sensorimotor temporal recalibration measured via a synchronization task. Front. Psychol. https://doi.org/10.3389/fpsyg.2012.00246 (2012).
Sugano, Y., Keetels, M. & Vroomen, J. Auditory dominance in motor-sensory temporal recalibration. Exp. Brain Res. 234(5), 1249–1262. https://doi.org/10.1007/s00221-015-4497-0 (2016).
Sugano, Y., Keetels, M. & Vroomen, J. Audio-motor but not visuo-motor temporal recalibration speeds up sensory processing. PLOS ONE. 12(12), e0189242. https://doi.org/10.1371/journal.pone.0189242 (2017).
Yamamoto, K. & Kawabata, H. Adaptation to delayed auditory feedback induces the temporal recalibration effect in both speech perception and production. Exp. Brain Res. 232(12), 3707–3718. https://doi.org/10.1007/s00221-014-4055-1 (2014).
Elijah, R. B., Le Pelley, M. E. & Whitford, T. J. Modifying temporal expectations: Changing cortical responsivity to delayed self-initiated sensations with training. Biol. Psychol. 120, 88–95. https://doi.org/10.1016/j.biopsycho.2016.09.001 (2016).
Blakemore, S. J., Frith, C. D. & Wolpert, D. M. The cerebellum is involved in predicting the sensory consequences of action. NeuroReport 12(9), 1879–1884. https://doi.org/10.1097/00001756-200107030-00023 (2001).
Tanaka, H., Ishikawa, T., Lee, J. & Kakei, S. The cerebro-cerebellum as a locus of forward model: A review. Front. Syst. Neurosci. 14, 19. https://doi.org/10.3389/fnsys.2020.00019 (2020).
van Kemenade, B. M. et al. Distinct roles for the cerebellum, angular gyrus, and middle temporal gyrus in action-feedback monitoring. Cereb. Cortex 29(4), 1520–1531. https://doi.org/10.1093/cercor/bhy048 (2018).
Welniarz, Q., Worbe, Y. & Gallea, C. The forward model: A unifying theory for the role of the cerebellum in motor control and sense of agency. Front. Syst. Neurosci. 15, 644059. https://doi.org/10.3389/fnsys.2021.644059 (2021).
Schmitter, C. V. et al. Neural correlates of temporal recalibration to delayed auditory feedback of active and passive movements. Hum. Brain Mapp. https://doi.org/10.1002/hbm.26508 (2023).
Farrer, C. et al. The angular gyrus computes action awareness representations. Cereb. Cortex 18(2), 254–261. https://doi.org/10.1093/cercor/bhm050 (2008).
Farrer, C. & Frith, C. D. Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage 15(3), 596–603. https://doi.org/10.1006/nimg.2001.1009 (2002).
Nahab, F. B. et al. The neural processes underlying self-agency. Cereb. Cortex 21(1), 48–55. https://doi.org/10.1093/cercor/bhq059 (2011).
Zito, G. A., Wiest, R. & Aybek, S. Neural correlates of sense of agency in motor control: A neuroimaging meta-analysis. PLoS ONE. 15(6), e0234321. https://doi.org/10.1371/journal.pone.0234321 (2020).
Jo, H. G., Wittmann, M., Hinterberger, T. & Schmidt, S. The readiness potential reflects intentional binding. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2014.00421 (2014).
Kühn, S., Brass, M. & Haggard, P. Feeling in control: Neural correlates of experience of agency. Cortex. 49(7), 1935–1942. https://doi.org/10.1016/j.cortex.2012.09.002 (2013).
Cao, L., Veniero, D., Thut, G. & Gross, J. Role of the cerebellum in adaptation to delayed action effects. Curr. Biol. 27(16), 2442-2451.e3. https://doi.org/10.1016/j.cub.2017.06.074 (2017).
Schmitter, C. V. & Straube, B. The impact of cerebellar transcranial direct current stimulation (tDCS) on sensorimotor and inter-sensory temporal recalibration. Front Hum. Neurosci. 16, 998843. https://doi.org/10.3389/fnhum.2022.998843 (2022).
Khalighinejad, N. & Haggard, P. Modulating human sense of agency with non-invasive brain stimulation. Cortex. 69, 93–103. https://doi.org/10.1016/j.cortex.2015.04.015 (2015).
Cavazzana, A., Penolazzi, B., Begliomini, C. & Bisiacchi, P. S. Neural underpinnings of the “agent brain”: New evidence from transcranial direct current stimulation. Eur. J. Neurosci. 42(3), 1889–1894. https://doi.org/10.1111/ejn.12937 (2015).
Moore, J. W., Ruge, D., Wenke, D., Rothwell, J. & Haggard, P. Disrupting the experience of control in the human brain: Pre-supplementary motor area contributes to the sense of agency. Proc. Biol. Sci. 277(1693), 2503–2509 (2010).
Khalighinejad, N., Di Costa, S. & Haggard, P. Endogenous action selection processes in dorsolateral prefrontal cortex contribute to sense of agency: A meta-analysis of tDCS studies of ‘Intentional Binding’. Brain Stimul. 9(3), 372–379. https://doi.org/10.1016/j.brs.2016.01.005 (2016).
Straube, B., Schülke, R., Drewing, K., Kircher, T. & van Kemenade, B. M. Hemispheric differences in the processing of visual consequences of active vs passive movements: A transcranial direct current stimulation study. Exp. Brain. Res 235(10), 3207–3216. https://doi.org/10.1007/s00221-017-5053-x (2017).
Straube, B., van Kemenade, B. M., Kircher, T. & Schülke, R. Transcranial direct current stimulation improves action-outcome monitoring in schizophrenia spectrum disorder. Brain Commun. 2(2), 151. https://doi.org/10.1093/braincomms/fcaa151 (2020).
Fujisaki, W., Shimojo, S., Kashino, M. & Nishida, S. Recalibration of audiovisual simultaneity. Nat. Neurosci. 7(7), 773–778. https://doi.org/10.1038/nn1268 (2004).
Harrar, V. & Harris, L. R. The effect of exposure to asynchronous audio, visual, and tactile stimulus combinations on the perception of simultaneity. Exp. Brain Res. 186(4), 517–524. https://doi.org/10.1007/s00221-007-1253-0 (2008).
Van der Burg, E., Alais, D. & Cass, J. Rapid recalibration to audiovisual asynchrony. J. Neurosci. 33(37), 14633–14637. https://doi.org/10.1523/JNEUROSCI.1182-13.2013 (2013).
Vroomen, J., Keetels, M., de Gelder, B. & Bertelson, P. Recalibration of temporal order perception by exposure to audio-visual asynchrony. Cognit. Brain Res. 22(1), 32–35. https://doi.org/10.1016/j.cogbrainres.2004.07.003 (2004).
Nitsche, M. A. & Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527(3), 633–639. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x (2000).
van Dun, K., Bodranghien, F. C. A. A., Mariën, P. & Manto, M. U. tDCS of the cerebellum: Where do we stand in 2016? Technical issues and critical review of the literature. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2016.00199 (2016).
Bolognini, N., Olgiati, E., Rossetti, A. & Maravita, A. Enhancing multisensory spatial orienting by brain polarization of the parietal cortex. Eur. J. Neurosci. 31(10), 1800–1806. https://doi.org/10.1111/j.1460-9568.2010.07211.x (2010).
Moliadze, V., Antal, A. & Paulus, W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin. Neurophysiol. 121(12), 2165–2171. https://doi.org/10.1016/j.clinph.2010.04.033 (2010).
Cogiamanian, F., Marceglia, S., Ardolino, G., Barbieri, S. & Priori, A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur. J. Neurosci. 26(1), 242–249. https://doi.org/10.1111/j.1460-9568.2007.05633.x (2007).
Bikson, M. et al. Safety of transcraniel direct current stimulation: Evidence based update 2016. Brain Stimul. 9(5), 641–661. https://doi.org/10.1016/j.brs.2016.06.004 (2017).
Brainard, D. The psychophysics toolbox. Spat Vis. 10, 433–436 (1997).
Arikan, B. E. et al. Different contributions of efferent and reafferent feedback to sensorimotor temporal recalibration. Sci. Rep. 11(1), 22631. https://doi.org/10.1038/s41598-021-02016-5 (2021).
Heron, J., Hanson, J. V. M. & Whitaker, D. Effect before cause: Supramodal recalibration of sensorimotor timing. PLoS ONE. 4(11), e7681. https://doi.org/10.1371/journal.pone.0007681 (2009).
Sugano, Y., Keetels, M. & Vroomen, J. Adaptation to motor-visual and motor-auditory temporal lags transfer across modalities. Exp. Brain Res. 201(3), 393–399. https://doi.org/10.1007/s00221-009-2047-3 (2010).
Rohde, M. & Ernst, M. O. To lead and to lag—Forward and backward recalibration of perceived visuo-motor simultaneity. Front. Psychol. 3, 599. https://doi.org/10.3389/fpsyg.2012.00599 (2013).
JASP Team. JASP (Version 0.14.1)[Computer software].
Synofzik, M., Lindner, A. & Thier, P. The cerebellum updates predictions about the visual consequences of one’s behavior. Curr. Biol. 18(11), 814–818. https://doi.org/10.1016/j.cub.2008.04.071 (2008).
Sokolov, A. A., Miall, R. C. & Ivry, R. B. The cerebellum: Adaptive prediction for movement and cognition. Trends Cogn. Sci. 21(5), 313–332. https://doi.org/10.1016/j.tics.2017.02.005 (2017).
Stetson, C., Cui, X., Montague, P. R. & Eagleman, D. M. Motor-sensory recalibration leads to an illusory reversal of action and sensation. Neuron. 51(5), 651–659. https://doi.org/10.1016/j.neuron.2006.08.006 (2006).
Haggard, P. & Whitford, B. Supplementary motor area provides an efferent signal for sensory suppression. Cognit. Brain Res. 19(1), 52–58. https://doi.org/10.1016/j.cogbrainres.2003.10.018 (2004).
Di Luca, M., Machulla, T. K. & Ernst, M. O. Recalibration of multisensory simultaneity: Cross-modal transfer coincides with a change in perceptual latency. J. Vis. 9(12), 1–16. https://doi.org/10.1167/9.12.7 (2009).
Grahn, J. A. See what I hear? Beat perception in auditory and visual rhythms. Exp. Brain Res. 220(1), 51–61. https://doi.org/10.1007/s00221-012-3114-8 (2012).
Grondin, S. Timing and time perception: A review of recent behavioral and neuroscience findings and theoretical directions. Attent. Percept. Psychophys. 72(3), 561–582. https://doi.org/10.3758/APP.72.3.561 (2010).
Bernard, J. A. & Seidler, R. D. Cerebellar contributions to visuomotor adaptation and motor sequence learning: An ALE meta-analysis. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2013.00027 (2013).
Block, H. & Bastian, A. J. Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia. 50(8), 1766–1775. https://doi.org/10.1016/j.neuropsychologia.2012.03.034 (2012).
Izawa, J., Criscimagna-Hemminger, S. E. & Shadmehr, R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 32(12), 4230–4239. https://doi.org/10.1523/JNEUROSCI.6353-11.2012 (2012).
Bartolomeo, L. A. et al. Prism adaptation deficits in schizophrenia. Schizophrenia Bull. 46(5), 1202–1209. https://doi.org/10.1093/schbul/sbaa019 (2020).
Bigelow, N. O. et al. Prism adaptation in schizophrenia. Brain Cognit. 61(3), 235–242. https://doi.org/10.1016/j.bandc.2006.01.004 (2006).
Cornelis, C. et al. Impaired sensorimotor adaption in schizophrenia in comparison to age-matched and elderly controls. Neuropsychobiology. 81(2), 127–140. https://doi.org/10.1159/000518867 (2022).
Coesmans, M. et al. Cerebellar motor learning deficits in medicated and medication-free men with recent-onset schizophrenia. J Psychiatry Neurosci. 39(1), E3–E11. https://doi.org/10.1503/jpn.120205 (2014).
Picard, H. et al. Impaired saccadic adaptation in schizophrenic patients with high neurological soft sign scores. Psychiatry Res. 199(1), 12–18. https://doi.org/10.1016/j.psychres.2012.04.039 (2012).
Block, H. & Celnik, P. Stimulating the cerebellum affects visuomotor adaptation but not intermanual transfer of learning. Cerebellum. 12(6), 781–793. https://doi.org/10.1007/s12311-013-0486-7 (2013).
Galea, J. M., Vazquez, A., Pasricha, N., Orban de Xivry, J. J. & Celnik, P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cerebral Cortex. 21(8), 1761–1770. https://doi.org/10.1093/cercor/bhq246 (2011).
Weightman, M., Brittain, J. S., Punt, D., Miall, R. C. & Jenkinson, N. Targeted tDCS selectively improves motor adaptation with the proximal and distal upper limb. Brain Stimul. 13(3), 707–716. https://doi.org/10.1016/j.brs.2020.02.013 (2020).
Gupta, T. et al. Cerebellar transcranial direct current stimulation improves procedural learning in nonclinical psychosis: A double-blind crossover study. Schizophr Bull. 44(6), 1373–1380. https://doi.org/10.1093/schbul/sbx179 (2018).
Ody, E., Straube, B., He, Y. & Kircher, T. Perception of self-generated and externally-generated visual stimuli: Evidence from EEG and behavior. Psychophysiology https://doi.org/10.1111/psyp.14295 (2023).
Ody, E., Kircher, T., Straube, B. & He, Y. Pre-movement event-related potentials and multivariate pattern of EEG encode action outcome prediction. Human Brain Mapp. https://doi.org/10.1002/hbm.26506 (2023).
Acknowledgements
We thank Jens Sommer for technical support and Michelle Achenbach, Trâm Ðỗ, Luca Grolms, Lisa Herberstein, and Sabrina Nasri-Roudsari for assistance in data collection and patient recruitment.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research project was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): STR1146/15-1 (Project Number: 429442932), STR1146/9-2 (Project Number: 286893149) and by “The Adaptive Mind,” funded by the Excellence Program of the Hessian Ministry of Higher Education, Science, Research and Art.
Author information
Authors and Affiliations
Contributions
BS and CS conceived and designed the study, and discussed and interpreted the data. CS was responsible for data acquisition and analysis and wrote the manuscript. Both authors revised and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmitter, C.V., Straube, B. Facilitation of sensorimotor temporal recalibration mechanisms by cerebellar tDCS in patients with schizophrenia spectrum disorders and healthy individuals. Sci Rep 14, 2627 (2024). https://doi.org/10.1038/s41598-024-53148-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53148-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.