Abstract
Diabetes is associated with cognitive decline, but the underlying mechanisms are complex and their relationship with Alzheimer’s Disease biomarkers is not fully understood. We assessed the association of small vessel disease (SVD) and amyloid burden with cognitive functioning in 47 non-demented older adults with type-2 diabetes from the Israel Diabetes and Cognitive Decline Study (mean age 78Y, 64% females). FLAIR-MRI, Vizamyl amyloid-PET, and T1W-MRI quantified white matter hyperintensities as a measure of SVD, amyloid burden, and gray matter (GM) volume, respectively. Mean hemoglobin A1c levels and duration of type-2 diabetes were used as measures of diabetic control. Cholesterol level and blood pressure were used as measures of cardiovascular risk. A broad neuropsychological battery assessed cognition. Linear regression models revealed that both higher SVD and amyloid burden were associated with lower cognitive functioning. Additional adjustments for type-2 diabetes-related characteristics, GM volume, and cardiovascular risk did not alter the results. The association of amyloid with cognition remained unchanged after further adjustment for SVD, and the association of SVD with cognition remained unchanged after further adjustment for amyloid burden. Our findings suggest that SVD and amyloid pathology may independently contribute to lower cognitive functioning in non-demented older adults with type-2 diabetes, supporting a multimodal approach for diagnosing, preventing, and treating cognitive decline in this population.
Similar content being viewed by others
Introduction
Diabetes is consistently associated with cognitive decline and dementia1. Various factors have been suggested to contribute to this association, including altered insulin signaling, hyperglycemia, advanced glycation, chronic low-grade inflammation, small vessel disease (SVD), large vessel disease, and Alzheimer’s disease (AD) pathology2, but specific pathways and their relationship with AD biomarkers are complex and not fully understood. Brain imaging correlates for some of these pathological mechanisms include white matter hyperintensities (WMH) as a measure of SVD, total gray matter (GM) thickness/volume as a measure of atrophy, and quantified amyloid-beta (Aβ) load on amyloid-PET.
WMHs are thought to reflect demyelination and axonal loss as a consequence of chronic ischemia caused by cerebral SVD3. Diabetes-related abnormalities in small vessels, such as seen throughout the body in patients with diabetes, may be the cause of such microangiopathic brain changes. In agreement, WMHs have been shown to be more prevalent in patients with diabetes and linked to cognition and cognitive decline3,4, though were found insufficient to explain all the associations between diabetes and cognition4.
The association between diabetes and AD is controversial. Some studies have shown that diabetes is associated with increased risk for clinical AD5, though most clinico-pathological studies have failed to show such an association6. Other studies even found lower AD pathology in brains of patients with diabetes7,8. In line with the clinico-pathological studies, many AD biomarker-based studies encompassing PET imaging or cerebro-spinal fluid (CSF) found no association between diabetes and AD biomarkers9.
The association of amyloid pathology and cognitive functioning is also controversial with some studies showing that higher amyloid pathology is associated with lower cognitive functioning10, while others showing no such association11. There is scarce knowledge about the impact of amyloid deposition on cognition in patient with diabetes12.
In this work we aim to assess the association between SVD, amyloid burden measured by PET imaging, and GM volume with cognitive functioning in older adults with type-2 diabetes (T2D). We hypothesize that both pathological biomarkers are associated with cognition in patients with diabetes, contributing to the lower cognitive functioning and higher dementia rates seen in this population.
Results
Cohort characteristics
The cohort consistent of 47 IDCD participants that had both amyloid-PET and MRI (Fig. 1, mean age = 78Y, SD = 4; 64% men; mean education = 14Y, median MMSE = 28, Table 1). The median duration of type-2 diabetes at time of PET was 16 years (range 13–21) and participants’ mean glycemic control levels suggests a relatively well controlled sample (HbA1c 6.7% (SD = 1.1%)).
Amyloid SUVR and cognitive functioning
Higher Aβ-SUVR was associated with lower global cognitive functioning, adjusting for demographics and the time interval between PET and cognitive testing (Model 1: β = − 1.30, SD = 0.47, p = 0.01; Table 2). The association of Aβ-SUVR with global cognition was essentially unchanged with increasing degree of adjustments of covariates, type-2 diabetes characteristics (Model 2), GM volume (Model 3), and cardiovascular risk and depression (Model 4). Except for education (β = 0.12, SD = 0.03, p < 0.001), all covariates were not associated with global cognition (Table 2).
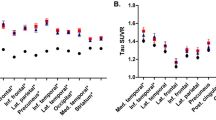
Similar models for the cognitive domains showed that higher Aβ-SUVR was significantly associated with worse executive and language functioning when adjusting for demographics (β = − 1.47, SD = 0.49, p = 0.004 and β = − 1.20, SD = 0.55, p = 0.04, respectively, Fig. 2 and supplementary Table 1). Additional adjustments for diabetes-related characteristics, and then also for total GM volume, did not alter the results.
The association between (A) amyloid burden, and (B) white matter hyperintensity volume with global and domain specific cognitive functioning. A dichotomized Aβ-SUVR index of 1.21 was used as threshold for global Aβ-SUVR positivity. SUVR standardized uptake value ratio, WMH white matter hyperintensities.
Amyloid in the frontal, parietal, cingulate, and temporal cortices was associated with cognition. The beta estimates of the models across the four regions were similar (Supplementary Table 2), suggesting that amyloid deposition affects the brain globally in a similar way. Further adjustment for total GM volume did not alter the results (Supplementary Table 2, model 2).
A secondary analysis showed no quadratic association between Aβ-SUVR and global cognition (β = 1.64, SD = 1.61, p = 0.32), indicating a linear association. Similar models for the cognitive domains showed quadratic associations only for attention (β = 4.22, SD = 1.96, p = 0.04 for attention; β = 1.47, SD = 1.67, p = 0.39 for executive functions, β = 0.45, SD = 1.70, p = 0.79 for language; and β = -1.47, SD = 1.86, p = 0.43 for memory).
White matter hyperintensities and cognitive functioning
Greater WMH volume was associated with lower global cognitive functioning, when adjusting for demographics, MRI-to-cognitive testing time interval, and ICV (β = − 0.02, SD = 0.01, p = 0.03). Results remained essentially unchanged when adding type-2 diabetes characteristics to the model (β = − 0.02, SD = 0.01, p = 0.04), and when adding cardiovascular risk and depression to the models (β = − 0.03, SD = 0.01, p = 0.04, Table 3).
Higher WMH volume was associated with lower attention (β = − 0.03, SD = 0.01, p = 0.03) and approached significance with lower executive functions and language functioning (β = − 0.02, SD = 0.01, p = 0.07; β = − 0.02, SD = 0.01, p = 0.07, respectively, for the full models, Fig. 2, and supplementary Table 1).
As Aβ-SUVR and WMH were both associated with cognition we sought to further assess the relationship between the two and GM volume. In a secondary analysis using Spearman's rank-order correlation, we found no associations between the three brain measures as shown in supplementary Table 3.
Finally, the association between Aβ-SUVR and global cognition, as well as with executive functions and language, remained unchanged when adding WMH as a covariate in addition to adjustment for type-2 diabetes-related characteristics and GM (β = − 1.17, SD = 0.44, p = 0.01 for global cognition, β = − 1.26, SD = 0.43, p = 0.01 for executive functions, and β = − 1.11, SD = 0.52, p = 0.04 for language, Table 4A). Similarly, the association between WMH and global cognition as well as attention remained, while the associations between WMH and executive function or language showed a trend toward significance when adding Aβ-SUVR as a covariate in addition to adjustment for type-2 diabetes-related characteristics and ICV (β = − 0.02, SD = 0.01, p = 0.03 for global cognition, β = − 0.03, SD = 0.01, p = 0.03 for attention, β = − 0.02, SD = 0.01, p = 0.06 for executive functions, and β = − 0.02, SD = 0.01, p = 0.06 for language, Table 4B).
No interaction was found between WMH and Aβ-SUVR on global cognition or on any of the four cognitive domains assessed (all p > 0.73).
Discussion
In this cross-sectional study of non-demented older adults with type-2 diabetes, we found that higher amyloid pathology and WMH volume were associated with lower cognitive functioning after adjusting for sociodemographic variables, type-2 diabetes related characteristics, GM volume, and cardiovascular risk. This association remained significant after further adjustment for WMH or Aβ-SUVR, respectively. Taken together, our findings suggest that amyloid and SVD are distinct pathological mechanisms that independently contribute to lower cognitive functioning in non-demented older adults with type-2 diabetes.
Previous studies assessed the relationship between amyloid pathology, WMH, and cognition in population-based or memory clinic cohorts, regardless of diabetes status. An association was found between baseline WMH volume and amyloid levels with worsening cognitive performance in individuals with normal aging, MCI, or AD dementia13,14,15 in agreement with our findings. Another study found an association between amyloid deposition and poorer episodic memory with an additive contribution of WMH burden on episodic memory and language. Generally, these results are in line with ours, though with different cognitive domains involved. A synergistic association between WMH and amyloid burden on executive functions and attention was found in cognitively normal older adults (n = 104)16, a finding that we did not find, possibly due to the relatively small cohort size or differences in cohort characteristics. Recent work from MEMENTO12, a clinic-based cohort that recruits non-demented older adults, showed that SVD, neurodegeneration, and amyloid pathology are independently associated with lower cognition and that the association between diabetes and cognition is mainly mediated by greater neurodegeneration. Our work is in line with these findings, expands them to a population-based cohort, accounts for HbA1c, and adds the aspect of non-atrophy-dependent contribution of amyloid pathology to cognition.
Longitudinal studies indicate that cognitive decline is faster in amyloid positive cognitively normal adults17 but cross-sectional studies show mixed results. Our findings are in line with previous studies demonstrating an association between higher amyloid and cognitive impairment10,18, though other studies did not find such an association11,19. This complex relationship between amyloid deposition and cognitive functioning may depend on the specific cohort characteristics and disease stage. This is consistent with the robust association we observed between amyloid and executive functions, the cognitive domain most affected in diabetes20.
The rate of amyloid positivity in our cohort (11/47, 23%) were lower than the accepted rates for this age range, ~ 35% for 80Y with normal cognition21. Such lower amyloid positivity rates have been suggested before in patients with type-2 diabetes22 and warrant further investigation in larger cohorts. Our results suggest that the association of amyloid with cognition does not depend on frank amyloid positivity since the best model fit was linear, rather than quadratic for global cognition and most cognitive domains assessed. A quadratic association was found for amyloid load and attention, which may suggest a J-shaped relationship potentially due to a deceleration or plateauing in the rate of cognitive decline at higher amyloid burden. Nonetheless, the limited number of participants with high Aβ-SUVR in our cohort prohibits drawing definitive conclusions. Our results also suggest that the association of amyloid with cognition does not depend on the specific localization of amyloid deposition. Taken together, our findings suggest that though lower rates of amyloid pathology may be seen in patients with type-2 diabetes, the presence of amyloid may be clinically important as it contributes to lower cognition independent of glycemic control level, SVD or brain atrophy.
Although data from cross-sectional studies have been ambiguous3, prospective studies demonstrate that SVD as indicated by WMHs is associated with, and directly contributes to, cognitive decline in the general population3. It has also been shown that type-2 diabetes is associated with higher levels of SVD4. Consistent with our findings, some studies demonstrate an association between higher degree of SVD and lower cognitive functioning in patients with diabetes12.
Strengths of our study include measurable criteria (rather than self-reported) for type-2 diabetes diagnosis, a broad cognitive battery, amyloid-PET and MRI performed on the same patients with quantifiable measurements for both. Limitations include the cross-sectional design, relatively small number of patients, relatively small number of amyloid-positive scans, namely, with high Aβ-SUVR, risk for selection bias, an average of 3.5 years interval between the MRI that was acquired first and amyloid-PET, though no difference in the number of cognitive tests performed before the two scans. Previous studies have shown that both amyloid accumulation and WMH progression rates are slow when baseline values are low23,24 and that diabetes is not a predictor for lesion progression24. Therefore, the time interval between the scans likely did not significantly affect the results. In addition, diabetes was an inclusion criterion in our cohort thus, our work cannot inform about the relationship between amyloid, WMH and cognition in patients without diabetes.
In conclusion, we found that higher amyloid and SVD burden are independently associated with lower cognitive functioning after adjusting for glycemic control. Our findings suggest that multiple factors may independently contribute to cognitive decline in non-demented older adults with type-2 diabetes, indicating a multimodal and individualized approach for the prevention, diagnosis, and treatment of cognitive decline in this population.
Methods
Participants
This is a cross-sectional study utilizing the subset of subjects from the Israel Diabetes and Cognitive Decline Study (IDCD) cohort25 that were randomly selected to have both amyloid-PET and brain MRI (scanned between 2013 and 2019). The IDCD is a longitudinal community-based cohort study that recruits cognitively normal, older adults (> 65Y), with type-2 diabetes, from the Maccabi Healthcare Services, the second largest HMO in Israel, providing detailed medical information on each participant, including diagnoses, medications, and blood exams.
The research was conducted in accordance with the relevant ethical guidelines and regulations. Signed informed consent was obtained from all participants. The Research Ethics Committee of Icahn School of Medicine at Mount Sinai, Sheba Medical Center, and Maccabi Healthcare Services approved the study.
Glycemic control measurements
Hemoglobin A1c (HbA1c) data was provided by Maccabi. All HbA1c levels available in Maccabi up to PET-date for amyloid analysis and MRI-date for WMH analysis were averaged for each participant and used as a measure of glycemic control.
Cardiovascular risk and depression
Mean cholesterol levels and systolic and diastolic blood pressure measurements were provided by Maccabi and added to the statistical models. The score of the geriatric depression scale (GDS) at baseline was used as a measure of depression.
Imaging
Acquisition
Imaging was performed at Sheba Medical Center. MRI on a 3T scanner (GE, Sigma HDxt, v16VO2) included high-resolution (1 mm3) 3-dimensional spoiled gradient recalled echo (SPGR) T1-weighted sequence and T2-weighted fluid attenuated inversion recovery (FLAIR) sequence26. Amyloid-PET scans were performed on a Philips-Vereos PET/CT scanner in 3D acquisition mode with a low-dose CT scan for attenuation correction. Acquisition began 90 min post-injection 4–5 millicurie of [F18]Flutemetamol Vizamyl (GE Healthcare) and took 20 min.
Preprocessing
T1-weighted images were processed using voxel-based morphometry (VBM http://www.fil.ion.ucl.ac.uk/spm/ext/#VBMtools)27 and implemented in Statistical Parametric Mapping (SPM8) as previously described28. Total GM volume adjusted to total intracranial volume (ICV) was used for analysis. WMH volume was extracted from FLAIR images using SPM8 and its Lesion Segmentation Toolbox (LST) with k = 0.15 as previously described26. Fazekas scale score for deep white matter lesions29 was assigned by a neuro-radiologist (OLS) based on FLAIR images.
Standardized uptake value ratio (SUVR) maps were created using whole cerebellum as reference region as previously described30. Global cortical uptake value was extracted in native space based on the weighted uptake in the frontal, temporal, parietal and cingulate cortex using Freesurfer31. This value is referred to as Aβ-SUVR and is used as a measure of Aβ “cortical burden.
Neuropsychological assessment
The IDCD study administers a broad neuropsychological battery using 12 neuropsychological tests covering four cognitive domains—(1) Episodic Memory: word list immediate/delay recall and word list recognition from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery; (2) Attention/Working Memory: diamond cancellation and Digit Span forward and backwards from the Wechsler Memory Scale-Revised (WMS-R); (3) Language/Semantic Categorization: similarities subscale of Wechsler Adult Intelligence Scale Revised (WAIS-R), animal fluency, and Boston Naming Test; (4) Executive Function: Digit Symbol Substitution Test from the WAIS-R and Trail Making Tests part A and part B25. Each test score was converted to z-score, normalized based on the corresponding baseline mean and standard deviation of the whole IDCD study population. The z-scores of the tests within a domain were averaged and then normalized again by its mean and standard deviation to create a domain specific composite score. A global cognition z-score was obtained by averaging the domains z-score. The cognitive assessment closest to PET or MRI, were used.
Statistical analysis
Linear regression models were tested to assess the associations of amyloid and WMH burden with cognition. Covariates were added in a sequential manner with demographics (age, sex, and years of education), the time interval between PET/MRI and cognitive testing (and intracranial volume for WMH) first, then type-2-diabetes related characteristics (mean HbA1C, and duration of type-2-diabetes), and then cardiovascular risk (cholesterol level, systolic and diastolic blood pressure) and depression. For the analyses of amyloid, total GM volume (adjusted for total intracranial volume) was further added. Finally, WMH was added as a secondary analysis to evaluate the independent contributions of WMH and Aβ-SUVR to the cognitive outcomes. Spearman rank-order correlation coefficient were used to assess the correlations between WMH, amyloid and GM volume. Statistical significance was defined by p < 0.05. Complete-case analysis was performed with SAS software, Version [9.4] (Cary, NC). Global Aβ-SUVR was used as the quantitative measure of amyloid burden. Secondary analyses tested associations of SUVR in specific brain regions with cognition,
Data availability
The data collected for the current study is available from the corresponding author on reasonable request.
References
Xue, M. et al. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 55, 100944 (2019).
Srikanth, V., Sinclair, A. J., Hill-Briggs, F., Moran, C. & Biessels, G. J. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 8, 535–545 (2020).
Prins, N. D. & Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 11, 157–165 (2015).
Gerstein, H. C. et al. Diabetes, brain infarcts, cognition, and small vessels in the Canadian alliance for healthy hearts and minds study. J. Clin. Endocrinol. Metab. 106, e891–e898 (2021).
Arvanitakis, Z., Wilson, R. S., Bienias, J. L., Evans, D. A. & Bennett, D. A. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 61, 661–666 (2004).
Abner, E. L. et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 12, 882–889 (2016).
Beeri, M. S. et al. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J. Gerontol. A Biol. Sci. Med. Sci. 60, 471–475 (2005).
Nelson, P. T. et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim. Biophys. Acta 1792, 454–469 (2009).
Gottesman, R. F. et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317, 1443–1450 (2017).
Svenningsson, A. L. et al. β-amyloid pathology and hippocampal atrophy are independently associated with memory function in cognitively healthy elderly. Sci. Rep. 9, 11180 (2019).
Aizenstein, H. J. et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 65, 1509–1517 (2008).
Frison, E. et al. Diabetes mellitus and cognition: A pathway analysis in the MEMENTO cohort. Neurology https://doi.org/10.1212/WNL.0000000000012440 (2021).
Dadar, M., Camicioli, R., Duchesne, S., Collins, D. L., Alzheimer’s Disease Neuroimaging Initiative. The temporal relationships between white matter hyperintensities, neurodegeneration, amyloid beta, and cognition. Alzheimers Dement. 12, e12091 (2020).
Ortega, G. et al. Combination of white matter hyperintensities and Aβ burden is related to cognitive composites domain scores in subjective cognitive decline: The FACEHBI cohort. Alzheimers Res. Ther. 13, 141 (2021).
DeCarli, C. et al. Vascular burden score impacts cognition independent of amyloid PET and MRI measures of Alzheimer’s disease and vascular brain injury. J. Alzheimers Dis. 68, 187–196 (2019).
Dupont, P. S. et al. Amyloid burden and white matter hyperintensities mediate age-related cognitive differences. Neurobiol. Aging 86, 16–26 (2020).
Donohue, M. C. et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 317, 2305–2316 (2017).
Petersen, R. C. et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 73, 85–92 (2016).
Marchant, N. L. et al. The aging brain and cognition: Contribution of vascular injury and aβ to mild cognitive dysfunction. JAMA Neurol. 70, 488–495 (2013).
Gao, Y. et al. The characteristic of cognitive function in Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 109, 299–305 (2015).
Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 313, 1924–1938 (2015).
Beeri, M. S. et al. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology 71, 750–757 (2008).
Jack, C. R. et al. Brain β-amyloid load approaches a plateau. Neurology 80, 890–896 (2013).
Brown, R., Low, A. & Markus, H. S. Rate of, and risk factors for, white matter hyperintensity growth: A systematic review and meta-analysis with implications for clinical trial design. J. Neurol. Neurosurg. Psychiatry 92, 1271–1277 (2021).
Beeri, M. S. et al. The Israel Diabetes and Cognitive Decline (IDCD) study: Design and baseline characteristics. Alzheimers Dement. 10, 769–778 (2014).
Livny, A. et al. Long-term variability in glycemic control is associated with white matter hyperintensities in APOE4 genotype carriers with type 2 diabetes. Diabetes Care 39, 1056–1059 (2016).
Ashburner, J. & Friston, K. J. Voxel-based morphometry—The methods. Neuroimage 11, 805–821 (2000).
Livny, A. et al. Haptoglobin 1–1 genotype modulates the association of glycemic control with hippocampal volume in elderly individuals with type 2 diabetes. Diabetes 66, 2927–2932 (2017).
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I. & Zimmerman, R. A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356 (1987).
La Joie, R. et al. Associations between [18F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology 90, e282–e290 (2018).
Villeneuve, S. et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: Statistical and pathological evaluation. Brain 138, 2020–2033 (2015).
Funding
This work was supported by NIH grants R01AG034087, AG053446, AG051545, and AG043878 to Dr. Beeri, P50 AG05138 to Dr. Sano, and AACSF-21-850735 to Dr. Lesman-Segev. We are also grateful for the generosity of the Katzin Foundation, the LeRoy Schecter Foundation and to Dr. Marina Nissim.
Author information
Authors and Affiliations
Contributions
O.L.S. and M.S.B. designed the study. S.G. helped with amyloid PET processing and SUVR calculations. A.L. helped with WMH volume and GM extraction. R.R.S., M.Z., I.G., A.H., C.H., and L.D. helped with collecting the data. H.M.L. and O.Y. constructed the statistical analysis. O.L.S. analyzed the data. O.L.S. and M.S.B. interpreted the data and wrote the manuscript. All the authors reviewed the manuscript. O.L.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lesman-Segev, O.H., Golan Shekhtman, S., Springer, R.R. et al. Amyloid deposition and small vessel disease are associated with cognitive function in older adults with type 2 diabetes. Sci Rep 14, 2741 (2024). https://doi.org/10.1038/s41598-024-53043-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53043-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.