Abstract
Hypertension is a known risk factor for sudden cardiac arrest (SCA). However, the role of temporal changes in blood pressure on the risk of SCA is not fully understood. This study was conducted to determine whether a temporal increase or decrease in blood pressure is associated with the risk of SCA. This study was based on nationwide healthcare insurance data. Individuals who underwent nationwide health check-ups in 2009 and 2011 were analyzed. A total of 2,801,153 individuals were evaluated for 8100 SCA events during the 17, 740, 420 person-years of follow-up. In a multivariate analysis, there were linear association between the degree of temporal elevation of systolic blood pressure (SBP) and the risk of SCA: (i) adjusted-hazard ratio (HR) 1.11 (p = 0.001) in 10 ≤ ΔSBP < 20 (mmHg) group; (ii) adjusted-HR 1.40 (p < 0.001) in 20 ≤ ΔSBP < 40 group; and (iii) adjusted-HR 1.88 (p < 0.001) in 40 ≤ ΔSBP group as compared with the reference group (− 10 ≤ ΔSBP < 10). Temporal increase in diastolic blood pressure (DBP) also a showed significant association with SCA risk with the highest risk observed in ∆DBP ≥ 25 group (adjusted-HR 1.61; p < 0.001) as compared with the reference group (− 5 ≤ ΔDBP < 5). The association between SBP and SCA was not affected by age, sex, presence of diabetes mellitus, or baseline SBP. In conclusion, a temporal increase in blood pressure was significantly associated with the occurrence of SCA, and this association was consistent across all subgroups. However, a temporary decrease in blood pressure does not reduce the risk of SCA. Prevention of elevated blood pressure may play an important role in preventing SCA.
Similar content being viewed by others
Introduction
Sudden cardiac arrest (SCA) is one of the most emergent medical condition which require immediate therapeutic intervention to bring the victim back to life1,2,3. Various efforts intended to improve neurologically intact survival rate in SCA patients were attempted4,5,6,7,8,9. Although widespread supply of automatized defibrillators, high quality education of the citizens, and early initiation of bystander cardiopulmonary resuscitation have been demonstrated to improve survival of SCA patients7,8,9, it is uncertain whether other therapeutic interventions such as induced hypothermia, antiarrhythmic drugs, and coronary angiography can be beneficial to SCA victims4,5,6. Therefore, primary prevention of SCA, rather than treatment, can have a more profound impact on public health, preserving medical resources.
Hypertension is a known risk factor for SCA10. Our previous study demonstrated that hypertension and prehypertension were both associated with a significantly increased risk of SCA, and the risk can be even higher if combined with diabetes mellitus10. However, it is unclear whether temporal changes in blood pressure are associated with the risk of SCA. Elevated blood pressure during a certain period can aggravate atherosclerosis of the cerebral and coronary arteries, which can lead to cerebrovascular accidents and acute coronary syndrome, thereby increasing the risk of SCA11,12,13. In contrast, a temporary decrease in blood pressure may stabilize atherosclerotic cardiovascular disease and decrease the risk of SCA.
We aimed to evaluate whether temporal elevation in blood pressure is associated with an increased risk of SCA using nationwide population-based data from the Republic of Korea. We further analyzed whether maintaining a similar blood pressure or decreasing blood pressure is better for the prevention of SCA, as this issue has not been fully addressed in prior studies.
Material and methods
Database
The Korean National Health Insurance Service (K-NHIS) database was used in this study. All people in the Republic of Korea are mandatory subscribers of the K-NHIS; therefore, the entire population of the Republic of Korea is represented by data derived from the K-NHIS. The K-NHIS is distinguished from other claims data-based medical cohorts because it offers a regular health checkup program to its subscribers. During the nationwide health check-up, various laboratory tests, medical measurements, and self-questionnaires were provided and stored in a database. Therefore, medical researchers can analyze (i) blood test results, such as complete blood cell counts, liver and renal function tests, lipid profiles, fasting blood glucose (FBG), and gamma-glutamyl transferase levels; (ii) measurements of systolic and diastolic blood pressure (SBP and DBP), body weight, height, and waist circumference; and (iii) lifestyle factors, including smoking status, alcohol consumption, and exercise level. Importantly, a nationwide health check-up program is provided to subscribers once every 2 years which enables the evaluation of temporal changes in various parameters, including blood pressure. The K-NHIS database also contains reports of the International Classification of Disease, tenth edition (ICD-10) diagnostic codes, and prescription histories of all government-approved drugs available in the Republic of Korea.
Medical researchers were permitted to use the K-NHIS database with approval from relevant institutional review boards and the official review committee of the K-NHIS (https://nhiss.nhis.or.kr/). This study was approved by the institutional review board of the Korea University Medicine Anam Hospital and the K-NHIS review committee. The requirement for written informed consent was waived by the Institutional Review Board of Korea University Medicine Anam Hospital owing to the retrospective nature of the study. The legal regulations of the Republic of Korea and the ethical guidelines of the 2013 Declaration of Helsinki were followed throughout this study.
Definitions
In this study, ∆SBP and ∆DBP were defined as temporal changes in SBP and DBP during 2009 and 2011 nationwide health check-ups. Based on a self-questionnaire during the 2009 health check-up, smoking status was defined as follows: (i) current smokers: those who smoked at least 100 cigarettes in their lifetime and continued smoking within 1 month of the 2009 health check-up; (ii) ex-smokers: those who smoked at least 100 cigarettes in their lifetime but had not smoked within 1 month of the 2009 health check-up; and (iii) never smokers: those who smoked < 100 cigarettes in their lifetime. Participants were classified into three groups according to the total amount of alcohol consumed per week: (i) non-drinkers: 0 g per week, (ii) mild drinkers: less than 210 g but more than 0 g per week, and (iii) heavy drinkers: 210 g or more per week. Diabetes mellitus was diagnosed if an individual had a measured fasting blood glucose (FBG) ≥ 126 mg/dL during a 2009 health check-up or a prior history of physician-diagnosed diabetes. The diagnosis of hypertension was based on ICD-10 codes for hypertension, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg during the 2009 health checkup. Dyslipidemia was defined based on a prior diagnosis of dyslipidemia by a physician. The estimated glomerular filtration rate was calculated based on creatinine levels measured during the 2009 health check-up. Heart failure was identified based on ICD-10 (I50 and its sub-codes) codes for heart failure reported during in-hospital admissions. Cardiovascular disease was defined as prior reports of ICD-10 codes for myocardial infarction (I21 and I22) or ischemic stroke (I63 and I64) at either inpatient or outpatient setting. Our previous studies demonstrated the robustness of these definitions14,15,16,17.
Participants
All patients who underwent serial nationwide health check-ups between 2009 and 2011 were screened for eligibility. The exclusion criteria were (i) prior diagnosis of SCA prior to the 2009 health check-up, (ii) age < 20 years, and (iii) SCA or death that occurred within 1 year after the 2011 health screening. Data obtained from January 2002 to December 2008 were used to identify baseline medical history such as hypertension, diabetes mellitus, heart failure, or dyslipidemia. Medical follow-up data until December 2018 were available.
Study participants were classified into six groups based on ∆SBP between 2009 and 2011: (i) ∆SBP < − 20 mmHg, (ii) − 20 mmHg ≤ ∆SBP < − 10 mmHg, (iii) − 10 mmHg ≤ ∆SBP < 10 mmHg, (iv) 10 mmHg ≤ ∆SBP < 20 mmHg, (v) 20 mmHg ≤ ∆SBP < 40 mmHg, and (vi) ∆SBP ≥ 40 mmHg. For ∆DBP, study participants were classified as follows: (i) ∆DBP < − 15 mmHg, (ii) − 15 mmHg ≤ ∆DBP < − 5 mmHg, (iii) − 5 mmHg ≤ ∆DBP < 5 mmHg, (iv) 5 mmHg ≤ ∆DBP < 15 mmHg, (v) 15 mmHg ≤ ∆DBP < 25 mmHg, and (vi) ∆DBP ≥ 25 mmHg.
Primary outcome endpoint
The main outcome of this study was the occurrence of SCA which was identified through the reporting of ICD-10 codes during visits to the emergency department, excluding incidents in inpatient or outpatient settings: I46.0 (cardiac arrest with successful resuscitation), I46.1 (sudden cardiac arrest), I46.9 (cardiac arrest, cause unspecified), I49.0 (ventricular fibrillation and flutter), R96.0 (instantaneous death), and R96.1 (death occurring less than 24 h from symptom onset). If a prior diagnosis of sepsis (A40 or A41), cerebral hemorrhage (I60–I62), ischemic stroke (I63 or I64), asphyxia (R09.0), gastrointestinal bleeding (K25.0, K25.2, K25.4, K26.0, K26.2, K26.4, K26.6, K27.0, K27.2, K27.6, K28.0, K28.4, K28.6, K29.0, K92.0, K92.1, or K92.2), anaphylaxis (T78), trauma (S00–S99), or consequences of external causes such as poisoning, suffocation, drowning, hit by lightning, electric shock, or burn (T00–T98) was made within 6 months of the occurrence of SCA, that event was not counted as the primary outcome endpoint. To prevent errors arising from repeated reports of ICD-10 codes for SCA, reports of SCA or death that occurred within 1 year after the 2011 health screening were not counted as the main outcome. For example, a report of SCA codes immediately after health screening can be an actual SCA event after the 2011 health screening or just a repeated report of aborted SCA events that occurred before the 2011 health screening. Both aborted and non-aborted SCA were included as primary outcomes. The incidence of SCA was defined as the numbers per 1000 person-years of follow-up.
Statistical analysis
Continuous variables were compared using Student’s t-test. We used the Kolmogorov–Smirnov test to confirm the normal distribution of continuous variables and they were all normally distributed. The chi-squared test or Fisher’s exact test was used to compare categorical variables. The Cox proportional hazards model was used to calculate the hazard ratios (HR) and 95% confidence intervals (CI). In the multivariable Cox proportional hazard model, age, sex, body mass index (BMI), income level, smoking status, alcohol consumption status, regular physical activity, baseline SBP (or DBP for ∆DBP analysis), diabetes mellitus, heart failure, cardiovascular disease, and prescription of antihypertensive drugs were adjusted. All tests were two-tailed, with p values ≤ 0.05 considered to indicate statistical significance. SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all the statistical analyses.
Ethics approval
The current study was approved by the Institutional Review Board of Korea University Medicine Anam Hospital and the official review committee of the K-NHIS. Considering the retrospective nature of this study, the requirement for written informed consent was waived. The ethical guidelines of the 2013 Declaration of Helsinki and the legal medical regulations of the Republic of Korea were strictly undertaken throughout the study.
Results
Participants
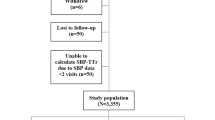
We identified 10 million people aged > 20 years who underwent a nationwide health checkup in 2009, 40% of whom were randomly selected for this analysis (n = 4,234,341). Among the 4,234,341 people, 2,884,135 underwent follow-up health screening in 2011. Individuals with a prior diagnosis of SCA (n = 408), missing data (n = 76,105), and occurrence of death or SCA within 1 year after the 2011 health checkups (n = 6469) were excluded from the analysis. Since blood pressure was measured in both 2009 and 2011 health check-up, ∆SBP and ∆DBP were calculated and their impact on SCA risk was examined in this study. The flow of this study is summarized in Fig. 1. During 17,740,420 person-years of follow-up, 8100 SCA events were observed. The incidence per 1000 person*years was 0.457. Differences in the baseline characteristics of people who experienced SCA during follow-up are summarized in Table 1: they were older, likely to be male and current smokers, and had a higher prevalence of hypertension, diabetes mellitus, chronic kidney disease, and dyslipidemia17. People who had hypertension in 2009 were older, had higher BMI and waist circumference, and had a higher prevalence of diabetes mellitus, chronic kidney disease, and dyslipidemia (Table 2).
Temporal changes in blood pressure
Participants of this study were stratified into six groups according to ∆SBP (difference between 2009 and 2011) and baseline characteristics of each group are summarized in Table 3. Major differences were observed in sex, age, diabetes mellitus, and baseline blood pressure (measured in 2009). Although statistically significant differences were observed in low-density lipoprotein levels and eGFR, the degree of absolute difference was not clinically significant.
In univariate analysis, ∆SBP showed a significant association with the risk of SCA (Supplementary Table S1) with people having extreme changes being associated with the highest risk (∆SBP within 10 mmHg as a reference group): HR 2.07 (95% CI 1.92–2.23; p < 0.001) and 3.38 (95% CI 2.90–3.93; p < 0.001) for ∆SBP < –20 and ≥ 40 group, respectively. In the multivariate model, baseline SBP in 2009 was adjusted for, in addition to other covariates, as baseline SBP showed the most prominent difference across the six groups. Compared with the reference group, people with ∆SBP < − 20 (adjusted HR 1.06; 95% CI 0.98–1.15) and − 20 ≤ ∆SBP < − 10 (adjusted HR 0.98; 95% CI 0.91–1.05) demonstrated no significant difference regarding SCA risk. However, positive ∆SBP (10 ≤ ∆SBP < 20) was associated with a significantly increased risk of SCA (adjusted HR 1.11; 95% CI 1.04–1.19; p = 0.001). The degree of increased SCA risk showed a linear association with ∆SBP with 20 ≤ ∆SBP < 40 group (adjusted HR 1.40; 95% CI 1.30–1.50; p < 0.001) and ∆SBP ≥ 40 group (adjusted HR 1.88; 95% CI 1.62–2.19; p < 0.001) having greater risk of SCA. Further adjustment for heart failure, cardiovascular disease, and prescription of antihypertensive medications showed similar results with ∆SBP ≥ 40 group having 67.4% increased risk of SCA (adjusted HR 1.67; 95% CI 1.44–1.95; p < 0.001; Supplementary Table S1).
The risk of SCA was also associated with temporal changes in DBP. Compared with the reference group (− 5 ≤ ΔDBP < 5), people with increased ∆DBP showed significantly higher risk of SCA: (i) 5 ≤ ΔDBP < 15 (adjusted HR 1.10; 95% CI 1.04–1.17; p = 0.001; Table 4); (ii) 15 ≤ ΔDBP < 25 (adjusted HR 1.28; 95% CI 1.17–1.40; p < 0.001; Table 4); (iii) ∆DBP ≥ 25 group (adjusted HR 1.61; 95% CI 1.37–1.89; p < 0.001; Table 4). In ∆DBP ≥ 25 group, the risk of SCA was 51.6% higher after further adjusting for heart failure, cardiovascular disease, and prescription of antihypertensive medications (adjusted HR 1.52; 95% CI 1.33–1.73; p < 0.001; Supplementary Table S1).
Subgroup analysis
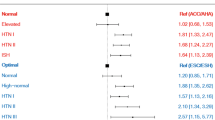
The association between ∆SBP and SCA was evaluated according to age, sex category, presence of diabetes mellitus, and baseline SBP (measured in 2009), and no significant interactions were observed (Fig. 2 and Supplementary Table S2). Adjusted HR was numerically higher in people with diabetes mellitus and ∆SBP ≥ 40 as compared with non-diabetes and ∆SBP ≥ 40 (adjusted HR 2.18 vs. 1.68) but the p-value for interaction was insignificant (p = 0.102). In people with baseline SBP < 140, ∆SBP < 20 was associated with 12.7% increased risk of SCA compared with − 10 ≤ ∆SBP < 10 group (adjusted HR 1.13; 95% CI 1.03–1.24; p = 0.011; Fig. 2 and Supplementary Table S2).
Impact of ∆SBP on SCA in various subgroups. The association between ∆SBP and SCA risk was not affected by sex category (A), age (B), diabetes mellitus (C), and baseline SBP (D). Hazard ratios (HRs) were adjusted for age, sex, body mass index, smoking status, alcohol consumption, regular physical activity, income level, and baseline blood pressure (measured in 2009). DM diabetes mellitus, SBP systolic blood pressure, SCA sudden cardiac arrest.
Discussion
This study revealed that (i) a temporal increase in blood pressure was significantly associated with the occurrence of SCA; (ii) the association was consistent for both SBP and DBP; (iii) the greater the increase in blood pressure, the higher the risk of SCA regardless of the patient subgroup; and (iv) a temporal decrease in SBP or DBP did not show a protective effect against SCA. Despite the challenges of low incidence and the need for long-term follow-up, we were able to analyze the association between temporal changes in blood pressure and SCA risk using nationwide healthcare insurance data. The strengths of this study are the sequential measurement of blood pressure in a large population-based cohort and the long-term clinical follow-up.
Blood pressure and SCA
Our previous study demonstrated that hypertension and prehypertension were significantly associated with an increased risk of SCA10,17. This study revealed that temporal elevation in blood pressure is also associated with an increased risk of SCA. In this study, HRs were adjusted for baseline blood pressure, and temporal elevation of blood pressure was associated with SCA risk in both people with SBP < 140 and ≥ 140 suggesting not only baseline blood pressure but also temporal elevation in blood pressure can contribute to SCA risk (Fig. 2 and Supplementary Table S2). The association between ∆SBP and the risk of SCA was not affected by age, sex, presence of diabetes mellitus, and baseline SBP, and other confounders such as BMI, smoking status, alcohol consumption, regular physical activity, and income level, were adjusted for in the multivariate model. Our study indicates that a temporal elevation in blood pressure can be associated with an increased risk of SCA.
Temporal decrease in blood pressure
Preventing elevated blood pressure may contribute to the primary prevention of SCA, according to the current analysis. However, a temporal decrease in blood pressure was not associated with a decreased risk of SCA in our study. The degree of decrease in both SBP and DBP also showed no association with the risk of SCA. However, the mechanisms underlying this lack of association are not fully understood. There may be an irreversible portion of target organ damage during periods of hypertension, emphasizing the importance of the early detection of hypertension and continuous, not temporary, blood pressure control. Our study is in accordance with the ACCORD blood pressure trial, which found no benefit of targeting SBP less than 120 mmHg compared to < 140 mmHg in patients with type 2 diabetes mellitus18. Maintaining optimal blood pressure throughout the clinical course can be more beneficial than aggressively decreasing blood pressure within a short period of time.
However, the optimal level of blood pressure control remains controversial. The 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA hypertension guidelines presented a strict standard for blood pressure control with a target of 130/80 mmHg compared with 140/90 mmHg in the previous guidelines and the 2018 European guideline19,20,21. In terms of SCA risk, our study revealed no benefit of blood pressure reduction compared with blood pressure maintenance. Furthermore, ∆SBP < − 20 in people with baseline SBP < 140 showed a statistically significant increase in the risk of SCA (adjusted HR 1.13, 95% CI 1.03–1.24; Supplementary Table S2). In contrast to this study, the SPRINT trial demonstrated that in patients at high risk of cardiovascular events, targeting SBP less than 120 mmHg as compared with SBP < 140 mmHg resulted in lower rates of fatal and nonfatal major cardiovascular events and death from any cause22. Identification the ideal degree of blood pressure control and specific subgroups to prevent SCA and other cardiovascular complications remain to be elucidated in future studies.
Limitations
This study had several limitations. First, owing to the retrospective nature and utilization of nationwide healthcare insurance data, coding inaccuracies may exist. However, our coding strategy for various medical conditions has been validated in previous publications14,15,16,23. Second, our analysis focused on the occurrence of SCA; the clinical course of SCA events was not available, and the type of treatment performed for SCA events could not be analyzed. Third, the current study was exclusively based on East Asians, and the extrapolation of our results to different ethnic groups requires caution. Fourth, our results suggest an association between the temporal elevation of blood pressure and SCA risk. Whether medical treatments for hypertension will reduce the risk of SCA requires further evaluation. Our sequential blood pressure measurements were separated by 2 years. Long-term changes in blood pressure can have different influences on the risk of SCA. Multiple BP measurements (for example, more than twice) can also have additional clinical implications. Fifth, the blood pressure measurement process was not standardized, because the measurements were performed in multiple primary care centers and hospitals across the nation.
Conclusions
A temporal increase in blood pressure was significantly associated with the occurrence of SCA, which was consistent among various subgroups. In contrast, a temporal decrease in blood pressure failed to demonstrate any significant association with a reduced risk of SCA.
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding authors. Raw data cannot be shared due to the legal regulations of the Republic of Korea and the policy of the K-NHIS.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DBP:
-
Diastolic blood pressure
- FBG:
-
Fasting blood glucose
- HR:
-
Hazard ratio
- ICD:
-
International classification of disease
- K-NHIS:
-
Korean National Health Insurance Service
- SBP:
-
Systolic blood pressure
- SCA:
-
Sudden cardiac arrest
References
Kragholm, K. et al. Bystander efforts and 1-year outcomes in out-of-hospital cardiac arrest. N. Engl. J. Med. 376, 1737–1747. https://doi.org/10.1056/NEJMoa1601891 (2017).
Brady, W. J., Mattu, A. & Slovis, C. M. Lay responder care for an adult with out-of-hospital cardiac arrest. N. Engl. J. Med. 381, 2242–2251. https://doi.org/10.1056/NEJMra1802529 (2019).
Ricceri, S. et al. Factors predisposing to survival after resuscitation for sudden cardiac arrest. J. Am. Coll. Cardiol. 77, 2353–2362. https://doi.org/10.1016/j.jacc.2021.03.299 (2021).
Desch, S. et al. Angiography after out-of-hospital cardiac arrest without ST-segment elevation. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2101909 (2021).
Dankiewicz, J. et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 384, 2283–2294. https://doi.org/10.1056/NEJMoa2100591 (2021).
Kudenchuk, P. J. et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N. Engl. J. Med. 374, 1711–1722. https://doi.org/10.1056/NEJMoa1514204 (2016).
Cummins, R. O., Ornato, J. P., Thies, W. H. & Pepe, P. E. Improving survival from sudden cardiac arrest: The “chain of survival” concept. A statement for health professionals from the Advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation 83, 1832–1847. https://doi.org/10.1161/01.cir.83.5.1832 (1991).
Ong, M. E. H., Perkins, G. D. & Cariou, A. Out-of-hospital cardiac arrest: Prehospital management. Lancet 391, 980–988. https://doi.org/10.1016/S0140-6736(18)30316-7 (2018).
Stiell, I. G. et al. Modifiable factors associated with improved cardiac arrest survival in a multicenter basic life support/defibrillation system: OPALS study phase I results. Ann. Emerg. Med. 33, 44–50. https://doi.org/10.1016/S0196-0644(99)70415-4 (1999).
Kim, Y. G. et al. Hypertension and diabetes including their earlier stage are associated with increased risk of sudden cardiac arrest. Sci. Rep. 12, 12307 (2022).
Flint, A. C. et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N. Engl. J. Med. 381, 243–251. https://doi.org/10.1056/NEJMoa1803180 (2019).
Itoga, N. K., Tawfik, D. S., Montez-Rath, M. E. & Chang, T. I. Contributions of systolic and diastolic blood pressures to cardiovascular outcomes in the ALLHAT study. J. Am. Coll. Cardiol. 78, 1671–1678. https://doi.org/10.1016/j.jacc.2021.08.035 (2021).
Ma, Y. et al. Blood pressure variation and subclinical brain disease. J. Am. Coll. Cardiol. 75, 2387–2399. https://doi.org/10.1016/j.jacc.2020.03.043 (2020).
Kim, Y. G. et al. Non-genetic risk factors for atrial fibrillation are equally important in both young and old age: A nationwide population-based study. Eur. J. Prev. Cardiol. 28, 666. https://doi.org/10.1177/2047487320915664 (2020).
Kim, Y. G. et al. The impact of body weight and diabetes on new-onset atrial fibrillation: A nationwide population based study. Cardiovasc. Diabetol. 18, 128. https://doi.org/10.1186/s12933-019-0932-z (2019).
Kim, Y. G. et al. Impact of the duration and degree of hypertension and body weight on new-onset atrial fibrillation: A nationwide population-based study. Hypertension 74, e45–e51. https://doi.org/10.1161/HYPERTENSIONAHA.119.13672 (2019).
Kim, Y. G. et al. Metabolic syndrome, gamma-glutamyl transferase, and risk of sudden cardiac death. J. Clin. Med. 11, 1781. https://doi.org/10.3390/jcm11071781 (2022).
Group, A. S. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1575–1585 (2010).
Whelton, P. K. et al. A guideline for the prevention, detection, evaluation and management of high blood pressure. A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 71, e13–e115 (2018).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 39, 3021–3104 (2018).
James, P., Oparil, S. & Carter, B. 20I4 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eight Joint National Committee (JNC 8) [published correction appears in JAMA. 20I4; 3II (I7): I809]. JAMA 311, 507–520 (2014).
Group, S. R. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 373, 2103–2116 (2015).
Kim, Y. G. et al. Different influence of blood pressure on new-onset atrial fibrillation in pre- and postmenopausal women: A nationwide population-based study. Hypertension 77, 1500–1509. https://doi.org/10.1161/HYPERTENSIONAHA.120.16513 (2021).
Funding
This work was supported by a Korea University Grant (J-I.C), a grant from Korea University Anam Hospital, Seoul, Republic of Korea (J-I.C), and grants from the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science and ICT (2021R1A2C2011325 to J-I.C). The funders had no role in the data collection, analysis, interpretation, trial design, patient recruitment, or any aspect pertinent to the study.
Author information
Authors and Affiliations
Contributions
J.I.C. had full access to all data in this study and took responsibility for the data integrity and analytical accuracy. The concept and design of the study were developed by Y.G.K., K.D.H., J.I.C., and Y.H.K. Data analysis and interpretation were performed by Y.G.K., J.H.J., S.Y.R., K.D.H., K.J.M., and J.I.C. The manuscript was drafted by Y.G.K., K.J.M., K.D.H., S.Y.R., and J.I.C. Statistical analysis was performed by Y.G.K., J.H.J., K.D.H., and J.I.C. Data collection was performed by Y.G.K., K.J.M., K.D.H., J.S., and J.I.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.G., Min, K., Jeong, J.H. et al. Temporal elevation of blood pressure is associated with increased risk of sudden cardiac arrest. Sci Rep 14, 2289 (2024). https://doi.org/10.1038/s41598-024-52859-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52859-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.