Abstract
The global production of soybean, among other drought-susceptible crops, is reportedly affected by drought periods, putting more pressure on food production worldwide. Drought alters plants’ morphology, physiology and biochemistry. As a response to drought, reactive oxygen species (ROS) concentrations are elevated, causing cellular damage. However, lower concentrations of ROS were reported to have an alleviating role through up-regulating various defensive mechanisms on different levels in drought-stressed plants. This experiment was set up in a controlled environment to monitor the effects of exogenous spray of different (0, 1, 5 and 10 mM) concentrations of H2O2 on two soybean genotypes, i.e., Speeda (drought-tolerant), and Coraline (drought-susceptible) under severe drought stress conditions (induced by polyethylene glycol) during flowering stage. Furthermore, each treatment was further divided into two groups, the first group was kept under drought, whereas drought was terminated in the second group at the end of the flowering stage, and the plants were allowed to recover. After 3 days of application, drought stress significantly decreased chlorophyll-a and chlorophyll-b, total carotenoids, stomatal conductance, both optimal and actual photochemical efficiency of PSII (Fv/Fm and Df/Fm, respectively), relative water content, specific leaf area, shoot length and dry weight, and pod number and fresh weight, but significantly increased the leaf concentration of both proline and total soluble sugars, the root length, volume and dry weight of both genotypes. The foliar application of 1 mM and 5 mM H2O2 on Speeda and Coraline, respectively enhanced most of the decreased traits measurably, whereas the 10 mM concentration did not. The group of treatments where drought was maintained after flowering failed to produce pods, regardless of H2O2 application and concentration, and gradually deteriorated and died 16 and 19 days after drought application on Coraline and Speeda, respectively. Overall, Speeda showed better performance under drought conditions. Low concentrations of foliar H2O2 could help the experimented soybean genotypes better overcome the influence of severe drought during even sensitive stages, such as flowering. Furthermore, our findings suggest that chlorophyll fluorescence and the cellular content of proline and soluble sugars in the leaves can provide clear information on the influence of both drought imposition and H2O2 application on soybean plants.
Similar content being viewed by others
Introduction
Drought periods and intensities have reportedly increased during the past few decades, and are expected to further elevate along with globally rising temperature, with more devastating influence in the arid and semi-arid regions1. The increased global population is another factor imposing more pressure on both food production and water resources2. Drought stress is reported to be one of the most significant environmental factors affecting agricultural crop production and, hence, food security3,4. Decreased available water has serious consequences on the plants on several levels (morphological, physiological, molecular and biochemical), leading to hindered metabolic activities5,6.
To cope with drought conditions, plants undergo several changes on different levels, including reduced transpiration rate and stomatal conductance, decreased shoot growth and increased root growth7,8,9, elevated osmolyte production (e.g., soluble sugars, proline, etc.) which maintains cellular capacity of water retention through the osmolytes’ anti-dehydration characteristic10. Proline can also protect the enzymatic system during drought occasions with its protective ability for several enzymes, in addition to its role in redox regulation11. It was previously reported that proline accumulation under drought stress conditions in soybean was associated with better seed yield12. However, this defensive system can widely vary among plant species, and might differ depending on the species’ developmental stage.
In order for these changes to happen, chemical signals are initiated in the root system, including elevated abscisic acid levels, leading to reactive oxygen species (ROS) production in higher levels. If drought continues, excessive ROS production can lead to oxidative stress that can lead to massive damages on the cellular level13. It was reported earlier that ROS accumulation in the leaves can harm the photosynthetic pigments, leading to rapid leaf senescence14,15.
Low concentrations of ROS, however, were reported to potentially regulate the gene expression and the stress-responsive pathways, facilitate certain molecular and physiological alterations, cause a moderate accumulation of ROS which up-regulates the antioxidant system and, hence, partially alleviate the negative influence of several abiotic stresses, including drought16,17,18,19,20.
It was previously reported that the application of methyl viologent21, melatonin22, acetic acid23,24, abscisic acid25, salicylic acid26,27 and hydrogen peroxide28 positively helped in alleviating stress. Hydrogen peroxide is one of the most stable molecules among ROS that is naturally found in plant tissues, with several vital co-tasks on the cellular level including stomatal opening and cell growth and development29,30,31,32. The positive effects of exogenous H2O2 spray at different concentrations on several plant species are well documented; however, most of these studies focused on the seedlings of these species (e.g. 1.5 mM H2O2 on cucumber seedlings33, 0.5 mM H2O2 on tomato plants34, 1 MM H2O2 on soybean35, 10 mM H2O2 on maize seedlings36 or on the pre-treatment with H2O2 (e.g.37 on cucumber) rather than later developmental stages. Moreover, whether exogenous H2O2 spray can help the recovering plants after drought is finished and/or plants that are suffering from continuous drought is not well documented. It would therefore be of vital importance to address these questions properly.
Soybean is reported to be drought-susceptible crop38. Its susceptibility is widely different among its different varieties and, more importantly, depending on the developmental stage at which drought is imposed39. For example, it has been reported that drought during flowering stages40 and during the following stages35 massively reduces soybean yield by affecting both pod setting and seed filling41. Moreover, whether drought continues throughout several stages or is only occurring during certain developmental stage is another important issue to consider, and research is lacking on this particular issue. That said, it would be of considerable importance to understand the response of different soybean genotypes to either continuous or temporal drought at the more-sensitive reproductive stages. We hypothesized that low concentrations of H2O2 will have positive influence on the morpho-physiology and the biochemistry of soybean plants that suffer from drought stress during the sensitive flowering stage. We also hypothesized that the response of these drought-stressed plants to H2O2 would differ in case the drought was temporal as compared to continuous drought.This experiment aimed at evaluating the response of two soybean genotypes to short-term and continuous drought stress, in addition to evaluating the effects of exogenous application of H2O2 on the morpho-physiology and biochemistry of the drought-stressed soybeans.
Materials and methods
This experiment was conducted through the hydroponic system in the controlled-climate chamber of the department of applied plant biology, University of Debrecen in 2022 to investigate the effects of exogenous application of different concentrations of hydrogen peroxide on soybean morpho-physiology and biochemistry under severe drought stress during the flowering (R1 and R2) stages42. In addition, this experiment aimed at monitoring the recovery path of soybean plants post-drought relative to continuous drought during reproductive stages (R1 onwards), and whether exogenously applied H2O2 might have a protective role. During the whole experimental period, the day/night temperature was kept at 26/19 °C with 65% relative humidity and light intensity of 300 µmol m−2 s−1 during the light period.
In a big field experiment, a total of 25 soybean genotypes were subjected to drought stress during 2017, 2018 and 2019 cropping years43. Based on their performance, two genotypes; Coraline (drought-susceptible) and Speeda (drought-tolerant) were chosen for this study. Severe drought stress was applied using polyethylene glycol (PEG 6000) (VWR International bvba Geldenaaksebaan, Leuven, Belgium) at a concentration of 10% (w/v) (equivalent to an osmotic potential of -0.19 MPa44) dissolved properly and completely in the nutrient solution of each pot (except control treatment). PEG is a widely used aqueous substance to conduct experiments on drought stress's effects on plants. It unites with water molecules, but can't enter the cells due to its high molecular weight. Drought stress was applied starting from R1 stage, and then either lifted at the end of R2 stage, or kept in place afterwards. Three H2O2 concentrations; 1, 5 and 10 mM were exogenously sprayed each other day throughout the flowering stages and a control treatment was alternatively sprayed with distilled water (DW). At the end of the flowering stage, the pots of each genotype were further divided into two groups; the first group was allowed to recover from drought stress by terminating PEG application, whereas the second group was kept under continuous drought stress conditions. Thus, there was 9 treatments for each genotype; 4 treatments sprayed with either 0, 1, 5 or 10 mM H2O2 (D, D1, D5 and D10, respectively) under drought stress imposed between R1 and R2 stages, 4 treatments sprayed with either 0, 1, 5 or 10 mM H2O2 (CD, CD1, CD5 and CD10, respectively) under continuous drought stress imposed from R1 stage onwards, in addition to a control treatment, where the plants were kept under optimum conditions and were sprayed with DW whenever the other treatments were sprayed with any concentration of H2O2.
To monitor the response of the different treatments, sampling for the different traits was made at 3 different occasions; 3 days after drought stress application (3 days after the beginning of R1 stage, equivalent to 51 and 59 days after sowing (DAS) in Coraline and Speeda, respectively), 3 days after terminating the drought stress as mentioned earlier (3 days after the ending of R2 stage and the beginning of R3 stage, equivalent to 64 and 73 DAS in Coraline and Speeda, respectively) and at the end of the experiment (at the end of R4 stage, equivalent to 89 and 101 DAS in Coraline and Speeda, respectively). At each sampling occasion, the second-most developed leaf was selected.The experiment was set in a randomized complete block design with 3 replications, so the final pot number was 54 (2 genotypes * 9 treatments * 3 replications).
Seeds of both genotypes were surface sterilized using 6% (v/v) H2O2 for 20 min, rinsed extensively with deionized water and germinated geotropically between moisten filter papers at 22 °C. After germination, 10 homogenous seedlings with good vigor were transferred into 3-L pots, and the number of seedlings was reduced later to 7 homogenous seedlings per pot. Each pot received 300 ml of dicot nutrient solution of the following: 0.7 mM K2SO4, 2.0 mM Ca(NO3)2, 0.1 mM KH2PO4, 0.5 mM MgSO4, 0.5 μM MnSO4, 0.1 mM KCl, 10 μM H3BO3, 0.2 μM CuSO4, 0.5 μM ZnSO4. Iron was supplied in the form of 10–4 M Fe-EDTA45, in addition to corresponding PEG solution. The nutrient solution was renewed every 3 days.
Stomatal conductance (gs) was measured with AP4 porometer (Delta-t devices, UK). Chlorophyll fluorescence was measured for dark-adapted leaves (20 min of dark adaptation) by attaching light exclusion clips to the central region of each leaf. Chlorophyll fluorescence parameters were measured with a portable chlorophyll fluorometer-PAM-2100 (WALZ, Germany) as described by46. chlorophyll-a, chlorophyll-b and total carotenoids were calculated as described by47. The extract content of the pigment was measured with UV–VIS spectrophotometry (Metertech SP-830 PLUS, Taiwan) at three wavelengths; 480, 647 and 664 nm, and chlorophyll-a and chlorophyll-b, in addition to total carotenoid contents were determined according to47.
The specific leaf area (SLA) was measured as described by48.
Root and shoot dry weights were calculated after freeze-drying the samples (Christ Gefriertrocknungsanlagen Freeze Dryer, Type 101,041, Germany). Root and shoot lengths were measured using a standard ruler. Root volume was measured by placing the root in a suitable, graded tube containing a known volume of DW and then calculating the increase in the overall volume. The flower number was counted for each plant in each pot at R2 stage. Pod number and weight were calculated by harvesting the pods of 3 plants from each pot. Proline content was calculated as described by49. Total soluble sugar content was calculated as described by50.
GenStat 20th edition (VSN International Ltd, UK) software was used to conduct the Analysis of Variance test, followed by Duncan’s Multiple Range Test51 to identify the statistically different treatments. All values are the means of 3 replicates (indicated by columns within each figure) ± standard errors (indicated by vertical whiskers on each respective column).
Plant material
The collection of plant material comply with relevant institutional, national, and international guidelines and legislation.
Results
The group of treatments, where drought stress was continuously imposed starting from R1 stage (i.e., CD, CD1, CD5 and CD10), gradually deteriorated until complete death 16 and 19 days after drought stress application (i.e., 5 and 7 days after the beginning of R3 stage) on Coraline and Speeda, respectively.
Root dry weight
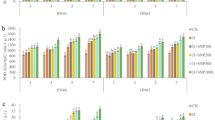
The root dry weight of both genotypes was higher in treatments that were subjected to drought stress and received no foliar spray as compared to control treatments. The difference was more obvious and distinct in Coraline, where the root dry weight was even significantly higher (by 64.5%, 47.1% and 41.3% after 3 days of drought stress application, after 3 days from the beginning of R3 stage and at the end of R4 stage, respectively) than in the control treatment (Fig. 1). The application of H2O2 foliar spray decreased the dry weight of the roots of both genotypes as compared to the non-sprayed counterparts. However, the 10 mM concentration increased the root dry weight of Coraline as compared to both the 1 mM and the 5 mM concentrations but decreased it in Speeda. At podding stages, Coraline plants that were sprayed with 10 mM H2O2 had significantly higher root dry weight as compared to the 1 mM or 5 mM H2O2 concentrations; however, the non-sprayed plants still had significantly higher root dry weight. In Speeda, the 1 mM concentration resulted in significantly higher dry root weight as compared to the other concentrations. Similar results were recorded at the end of the podding stage of both genotypes (Fig. 1).
Root dry weight (g) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Root length
After 3 days of drought stress application, the root length of both genotypes was significantly higher in all drought-stressed treatments than that of control treatment. In Coraline, the treatment which was sprayed with 10 mM H2O2 had significantly shorter roots (by 3.5%) compared to the treatment that was not sprayed. On the other hand, Speeda plants that received 1 mM H2O2 foliar spray had significantly longer roots (by 3.6%) compared to the non-sprayed counterpart. Within the group of treatments that was allowed to recover from drought, Coraline plants that were sprayed with either 1 mM or 5 mM H2O2 and Speeda plants that were sprayed with 1 mM H2O2 had significantly higher root lengths compared to their counterpart treatments that did not receive foliar spray. Within the group of treatments that were continuously under drought stress conditions, the root length of Coraline plants that were sprayed with either 1 mM or 5 mM H2O2 was significantly higher than the non-sprayed counterpart, whereas the foliar spray did not enhance this trait for Speeda plants at this point. At the end of the podding stage, the root length of all the treatments that received any concentration of H2O2 foliar spray was higher than the control treatments of both genotypes (Fig. 2).
Root length (cm) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
After 3 days of drought stress application, the root length was not measurably different between the studied genotypes, regardless of H2O2 treatment and concentration. Later however, Coraline had longer roots in most treatments, especially within the group of treatments that were allowed to recover from drought. This last observation was more obvious at the end of the podding stage, where the root length of all treatments of Coraline was significantly higher than the counterpart treatments of Speeda (Fig. 2).
Root volume
Significant increase in the root volume of all sprayed plants from both genotypes was recorded 3 days after drought stress application as compared to control counterparts. In Coraline, H2O2 foliar spray application at any concentration significantly reduced the root volume as compared to the non-sprayed treatment (by 15.5%, 14.2% and 16.4% in the treatments that received 1, 5 and 10 mM, respectively), whereas a 1 mM H2O2 foliar spray significantly increased the root volume of Speeda plants. In Coraline, the root volume was significantly higher for the recovering plants that were sprayed with 5 mM H2O2 foliar spray compared to the other concentrations, whereas the foliar spray at all concentrations resulted in significant root volume decrease in the treatments that were continuously subjected to drought. In Speeda, the root volume of the treatment sprayed with 1 mM H2O2 was significantly higher than the other sprayed treatments for both groups (recovered, unrecovered). Similar conclusion was obtained on the recovered groups of both genotypes at the end of the podding stage (Fig. 3).
Root volume (cm3) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Shoot dry weight
Drought stress significantly decreased the shoot dry weight of both genotypes 3 days after application, and the foliar spray of H2O2, regardless of concentration, did not enhance this trait. After removing drought, the shoot dry weight of the plants that were sprayed with any concentration of H2O2 foliar spray was significantly higher compared to the plants that were allowed to recover without receiving any concentration of foliar spray. Under continuous drought stress conditions, the shoot dry weight of both genotypes was significantly less than that of the recovering plants. However, the foliar spray of H2O2 at any concentration significantly enhanced the dry shoot weight in Coraline (by 13.6%, 22.4% and 17.7% in CD1, CD5 and CD10 treatments, respectively as compared to CD treatment), but not in Speeda. By the end of the podding stage, the effect of the foliar spray on the shoot dry weight was more measurable in Coraline than in Speeda.
After drought application, Coraline plants had higher shoot dry weight; however, Speeda plants showed measurably higher values in most treatments during the following stages (Fig. 4).
Shoot dry weight (g) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Shoot length
The soot length of both genotypes significantly decreased as a result of drought stress application. However, the H2O2 foliar spray application, of any concentration in Coraline and of 1 mM in Speeda, could significantly enhance this trait. When plants of both genotypes were relieved from drought and allowed to recover, the groups of treatments that received any concentration of H2O2 foliar spray showed significantly higher shoot length compared to the non-sprayed counterparts. Under continuous drought conditions, only the concentrations of 5 mM and 1 mM H2O2 foliar spray could result in better shoot lengths in Coraline and Speeda, respectively. At the end of podding stage, all Coraline plants that received H2O2 foliar spray showed significantly better shoot length (better even than the control), whereas that positive effect was noticed only on the treatment of Speeda that received 1 mM H2O2 foliar spray, where the shoot length was 9.1% higher than that of the treatment that was not sprayed (D) and 4.8% higher than that of the control treatment (Fig. 5).
Shoot length (cm) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Regardless of drought and H2O2 application, the shoot length of Speeda was significantly higher than that of Coraline through the whole experimental period.
Specific leaf area
Drought stress application resulted in significant reductions in the specific leaf area (SLA) of both genotypes as compared to control counterparts. However, the application of H2O2 foliar spray significantly increased SLA, regardless of its concentration. Compared to control plants, the application of 5 mM and 10 mM H2O2 foliar spray on Coraline resulted in significantly higher SLA (by 26.7% and 12.3%, respectively) after 3 days of drought application, whereas only the 1 mM H2O2 concentration could enhance this trait in Speeda. Under continuous drought stress conditions, the application of H2O2 foliar spray could significantly increase SLA in both genotypes as compared to the non-sprayed counterparts. Recovering Coraline plants sprayed with either 5 mM or 10 mM H2O2 could maintain higher SLA values as compared to control plants, whereas Speeda plants could not. Similar findings were observed at the end of the podding stge (Fig. 6).
Specific leaf area (mm2 mg-1) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
At all sampling dates, SLA of Speeda was higher in control and drought-stressed treatments that did not receive any foliar spray; however, Coraline plants that were sprayed with either 5 mM or 10 mM H2O2 had higher SLA.
Optimal photochemical efficiency of PSII
Drought stress caused a significant reduction in this trait after 3 days of application on both genotypes (by 26% and 21.1% in Coraline and Speeda, respectively). However, applying the foliar spray of all concentrations on Coraline and of 1 mM and 5 mM on Speeda led to significant enhancement in Fv/Fm. Compared to the treatments that were subjected to continuous drought, the group of treatments where the drought was relieved was able to maintain higher Fv/Fm values, with and without the application of H2O2 foliar spray and regardless of its concentration. Fv/Fm was significantly higher (by 8.8%) when 5 mM H2O2 foliar spray was applied to the recovering Coraline plants after 3 days of recovery compared to the non-sprayed recovering plants, whereas the foliar spray had no significant enhancement in Speeda recovering plants. Similar results were recorded at the end of the podding stage, where Fv/Fm values were not affected by the foliar spray on Speeda plants but were significantly better when 1 mM or 5 mM H2O2 was applied on Coraline plants (Fig. 7).
Optimal photochemical efficiency of PSII of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
After drought application, Speeda plants had significantly higher Fv/Fm values in all treatments, except for the treatments where 10 mM H2O2 was applied (where the Fv/Fm values of Speeda was still higher, yet not significantly). However, there were no measurable differences between the two genotypes after the recovery process, i.e. Coraline plants were able to retain comparable Fv/Fm values when the plants had the chance to recover with and without foliar spray application (Fig. 7).
Actual photochemical efficiency of PSII (Yield)
The application of drought stress significantly reduced the actual photochemical efficiency of PSII of both genotypes 3 days after its application, regardless of H2O2 application and concentration. However, the exogenous application of H2O2 at any concentration significantly enhanced this trait in both genotypes as compared to the non-sprayed, drought-stressed treatment. After terminating the drought, the treatments that were sprayed with any concentration of H2O2 were still significantly higher in terms of yield as compared to the non-sprayed treatment. The group of treatments that were kept under drought stress conditions had significantly lower values of this trait. Within this group, the plants that received either 1 or 5, but not 10 mM H2O2, had significantly higher values than the treatment that did not receive any foliar H2O2. At the end of the podding stage, the actual photochemical efficiency of PSII of the treatments that received any concentration of H2O2 was still significantly higher than that of the treatment that did not receive H2O2 spray (Fig. 8).
Actual photochemical efficiency of PSII of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Chlorophyll-a
After 3 days of drought stress application, the chlorophyll-a content significantly decreased in both genotypes, regardless of H2O2 application and concentration. In the drought-susceptible genotype Coraline, the treatments which were allowed to recover from drought stress had significantly higher chla content compared to the treatments which suffered from continuous drought stress. Among these treatments, the foliar application of H2O2, regardless of its concentration, led to significantly higher chla compared to the treatment where the plants were allowed to recover without H2O2 foliar spray. The foliar spray, however, did not have measurable effects on the chla content of the treatments which suffered from continuous drought. The foliar spray had no significant effect on the chla content in the drought-tolerant genotype Speeda; however, the group of treatments which was allowed to recover from drought stress had significantly higher chla content compared to the group which suffered from continuous drought. At the end of the podding stage, the control treatment had significantly higher chla content than the drought-stressed treatments (by 54% and 36% in Coraline and Speeda, respectively), and the foliar spray had no measurable effect (Fig. 9).
Chlorophyll-a content (µg g-1) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Under drought stress conditions, the chla content in Speeda was significantly higher than in Coraline at all 3 sampling dates, regardless of H2O2 application and concentration.
Chlorophyll-b
The application of drought stress resulted in significant reduction in the chlb content in both genotypes. The foliar H2O2 spray could not alleviate that effect. However, the recovered plants of both genotypes had significantly higher content of chlb as compared to the plants where drought was continuous. Moreover, the application of H2O2 spray on the recovering plants of both genotypes increased the chlb content; that increase was significant when 1 mM or 5 mM of H2O2 foliar spray was applied. The foliar spray, on the other hand, did not result in significant enhancements in this trait under continuous drought stress conditions. At the end of the podding stage, the chlb content was significantly higher in Coraline (by 14.7%) and Speeda plants (by 7.9%) which were sprayed with 5 mM and 1 mM of H2O2, respectively compared to the non-sprayed counterparts (Fig. 10).
Chlorophyll-b content (µg g-1) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Speeda plants had significantly higher chlb content under drought stress conditions at all sampling dates.
Total carotenoids
Significant decrease in the total carotenoid (chlxc) content was recorded in both genotypes (by 28% and 35.5% in Coraline and Speeda, respectively) as a consequence of drought stress application. Except for the application of 10 mM H2O2 on Coraline plants, the chlxc content was significantly increased by the H2O2 foliar spray on both genotypes. The recovered plants of both genotypes had significantly higher chlxc content compared to the continuously drought-stressed counterparts. In Coraline plants, 1 mM H2O2 foliar spray on the recovering plants of Coraline significantly increased chlxc compared to the non-sprayed recovering plants, whereas the chlxc of recovering Speeda plants was significantly increased by the application of any concentration of H2O2. On the other hand, the foliar spray had no measurable effects on the chlxc of the plants that were not allowed to recover from both genotypes. Interestingly, the chlxc at the end of the podding stage was significantly higher in Speeda plants that were sprayed with 1 mM H2O2 as compared to the recovering plants that were not sprayed, whereas this trait did not have measurable differences in Coraline at the same period (Fig. 11).
Total carotenoid content (µg g-1) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Regardless of the drought application and the H2O2 application and concentration, the chlxc content was significantly higher in Speeda than in Coraline plants at all sampling dates.
Stomatal conductance
The stomatal conductance significantly decreased in both genotypes when subjected to drought stress. The foliar application of H2O2 at any concentration could significantly elevate the stomatal conductance of both Coraline and Speeda plants. The stomatal conductance of both genotypes dramatically degraded when drought stress was kept; however, the foliar spray with H2O2 at all concentrations could significantly increase the stomatal conductance of Coraline (by an average 22%), but not that of Speeda plants. On the other hand, the recovered plants of both genotypes had significantly higher stomatal conductance when foliar spray was applied at any concentration as compared to the recovering plants that was not sprayed. Interestingly, Coraline and Speeda plants that were sprayed with 5 mM and 1 mM H2O2, respectively had significantly higher stomatal conductance values (by 5.3% and 3.7%) compared to the control counterparts that were kept under optimum conditions throughout the whole experimental period (Fig. 12).
Stomatal conductance (mmol m-2 s-1) of two soybean genotypes (Coraline and Speeda) at 3 different sampling dates (A: 3 days after drought stress application at R1 stage, B: 3 days after R3 stage started, C: at the end of R4 stage) as affected by hydrogen peroxide foliar spray application under drought stress conditions (D: drought from R1 till R2 stage, D1: drought from R1 till R2 stage + 1 mM hydrogen peroxide, D5: drought from R1 till R2 stage + 5 mM hydrogen peroxide, D10: drought from R1 till R2 stage + 10 mM hydrogen peroxide, CD: continuous drought starting from R1 stage, CD1: continuous drought starting from R1 stage + 1 mM hydrogen peroxide, CD5: continuous drought starting from R1 stage + 5 mM hydrogen peroxide, CD10: continuous drought starting from R1 stage + 10 mM hydrogen peroxide). All values are the means of 3 replicates (columns) ± standard errors (vertical whiskers). In each genotype, different letters indicate significant differences at .05 level as indicated by Duncan’s multiple range test.
Speeda plants could maintain higher stomatal throughout the experimental period as compared to Coraline plants.
Relative water content
The relative water content of all drought-stressed treatments of both genotypes significantly decreased as compared to control treatment, regardless of H2O2 application and concentration. On the other hand, the exogenous application of either 1 or 5 mM H2O2 on Coraline, and 1 mM H2O2 on Speeda significantly enhanced the RWC. After terminating drought, a very similar result was obtained, where these concentrations helped in elevating the RWC of both genotypes to reach nearly similar values to those of control plants. On the other hand, all the treatments where the plants of both genotypes were kept under drought stress conditions had significantly lower RWC, yet all treatments that received any concentration of H2O2 (except for 10 mM on Speeda) were able to keep significantly better RWC as compared to the non-sprayed counterpart. At the end of the podding stage, the RWC of (D1) and (D5) treatments in Coraline, and of (D1) treatment in Speeda was significantly higher than that of (D) treatment (Table 1).
At all sampling dates, the RWC of Speeda was significantly higher than that of Coraline in all drought treatments except for the D5 treatment.
Flower number
At the end of the flowering stage, the treatments of both genotypes that were subjected to drought stress without any foliar spray produced significantly lower number of flowers (by 19.7% and 26.9% in Coraline and Speeda, respectively) as compared to control counterparts. The foliar spray enhanced this trait in Coraline, where both 1 mM and 5 mM H2O2 concentrations resulted in significantly higher flower number as compared to the non-sprayed treatments. However, the foliar spray did not enhance this trait in Speeda; it even decreased the flower number when 5 mM or 10 mM H2O2 was applied (Table 2).
Speeda had significantly higher flower number than Coraline in all treatments except for the treatments that were sprayed with 5 mM H2O2 where the flower numbers were very similar.
Pod number
The number of pods of both genotypes decreased due to drought stress application; the reduction was more measurable and significant in Speeda (37%). The pod number significantly increased in Coraline when 1 mM or 5 mM H2O2 foliar spray was applied, and the same result was obtained when 1 mM H2O2 foliar spray was applied on Speeda (Table 3).
The number of pods was higher for Speeda in all treatments as compared to Coraline.
Pod fresh weight
Significant decrease in the pod fresh weight under drought stress conditions was recorded in both genotypes. However, the application of 5 mM and 1 mM H2O2 foliar spray on Coraline and Speeda, respectively has led to significant increase (by 18.1% and 14.1%, respectively) in the pod fresh weight (Table 4).
The pod fresh weight of Speeda was significantly higher than that of Coraline, regardless of drought and H2O2 foliar spray application.
Proline content
The leaf proline content of both genotypes significantly increased under drought stress conditions. Furthermore, the foliar application of H2O2 at any concentration significantly increased the leaf proline content as compared to the treatment where the drought-stressed plants were sprayed with DW. When drought was terminated, proline content measurably decreased in both genotypes, yet it was still higher than that of control treatment. Under continuous drought conditions, proline continued to accumulate, and its levels were significantly higher than those of the drought-relieved counterparts. The foliar H2O2 spray had no measurable effect at this point in any of the two genotypes. The leaf proline content was still higher in the drought-relieved treatments at the end of the podding stage as compared to the control counterparts (Table 5).
The leaf proline content was always higher in Speeda than in Coraline, and the differences were significant in all drought-stressed treatments of both groups.
Total soluble sugars
Drought stress significantly increased the total soluble sugars in the leaves of both genotypes. Compared to the drought-stressed treatment, the application of H2O2 foliar spray at any concentration in Coraline, and at 1 or 5 mM in Speeda significantly induced the accumulation of soluble sugars. When drought was eliminated after the flowering stage, the total soluble sugars in Coraline noticeably decreased and reached levels that were insignificant as compared to the control plants, whereas these levels were still significantly higher in Speeda. Furthermore, the sprayed plants of both genotypes had very close levels of soluble sugars as compared to the non-sprayed counterparts. On the other hand, the group of treatments that had continuous drought accumulated significant levels of soluble sugars as compared to the drought-relieved group of both genotypes. In Coraline, the soluble sugar contents were not significantly different in the treatments sprayed with any concentration of H2O2 from the treatment that was not sprayed, whereas they were in Speeda. At the end of the podding stage, the content of the total soluble sugars was still significantly higher in the treatments that suffered from drought, regardless of H2O2 application and concentration, as compared to the control treatments that were sprayed with DW (Table 6).
Although Speeda plants had higher contents of the total soluble sugars, yet the differences were more announced after drought stress application, whereas these differences were much less in the group of treatments that was relieved from drought, and also at the end of the podding stage.
Discussion
Osmotic stress can limit energy transport from photosystem II to photosystem I, and parallelly constitute spongy, thin tissues in the leaves, leading to elevated chlorophyll-a fluorescence and, consequently, reduced photosynthetic activity52. ROS accumulation negatively affects the sensitive chlorophyll molecules53. In our experiment, both chlorophyll-a and chlorophyll-b of both soybean genotypes significantly decreased under PEG-induced drought stress conditions, leading to damaged photosynthesis machinery54. Similar conclusion was reported by55, who also reported that total carotenoids significantly decreased under drought stress conditions, which was the case in our experiment as well. The exogenous application of either 1 or 5 mM H2O2 could measurably enhance chlorophyll-a content in the drought-tolerant genotype Speeda, but not of the drought-susceptible genotype Coraline. However, no influence on Chl b content in both genotypes was detected. On the other hand, the total carotenoid content in both genotypes was significantly enhanced by H2O2 application at any concentration (except for 10 mM on Coraline). Low concentrations of H2O2 can induce certain enzymes and/or proteins related to photosynthesis process56. H2O2 foliar spray can protect the chloroplast under drought stress conditions, resulting in enhanced chlorophyll content37,57. Similar conclusions were also reported on soybean in the case of exogenous melatonin58 and ethanol59. Ethanol application can elevate the synthesis and/or reduce the degradation of the photosynthetic pigments55.
The drought-stressed plants of both soybean genotypes in our experiment had significantly higher proline and soluble sugar concentrations 3 days after drought stress application. Proline is an important amino acid that is engaged in many processes on the cellular level60. Under drought stress conditions, the concentrations of proline and soluble sugars, among other osmolytes, increase without disturbing the usual biochemical activities in the cells61. Thus, these osmolytes play a defensive role against drought by decreasing the permeability of the cellular membranes, leading to stabilized water balance62,63,64,65,66,67,68. In their experiment58, reported that drought-stressed soybean seedlings had 30, 125 and 334% higher proline concentration after 5, 10 and 15 days of drought stress application. Such conclusion on proline and soluble sugar accumulation was also reported on soybean by other studies (e.g.69,70,71) and on other species like hot pepper72, barley73, cotton74 and rice75. According to76, there is another important role of the elevated soluble sugar levels under drought stress conditions; that is, sustaining adequate metabolic C/N ratios. The concentrations of both proline and soluble sugars were measurably higher in Speeda than in Coraline at the 3 sampling dates. It was previously reported that the levels of proline accumulations are genotype-dependent and varies among the different stages of soybean development when the drought stress is taking place12,77, which is also confirmed by our findings, as the group of treatments of both genotypes that was relieved from drought stress after flowering stage had significantly higher concentrations of both proline and soluble sugars as compared to the other group, where the drought was continuously kept in place. On the other hand, the exogenous application of H2O2 noticeably increased both proline and soluble sugar concentrations in the drought-stressed plants of both genotypes 3 days after drought stress application. It was reported by36 that H2O2 foliar spray resulted in elevated proline and soluble sugar concentrations in drought-stressed maize plants, leading to enhanced drought tolerance. Similar conclusion was also reported when other osmo-regulators such as ethanol55 were exogenously applied on soybean plants.
Under unfavorable water availability conditions, sustaining water status within plants is vital to overcome these conditions, and leaf relative water content is considered as one of the most indicative traits for plant drought tolerance78. In our experiment, significant reduction in RWC under drought stress conditions was recorded in both soybean genotypes. It is well documented that drought stress results in reduced stomatal conductance by increasing stomatal closure ratio79 in order to maintain the water content of the drought-stressed plants. In their experiment80, concluded that RWC of both experimented soybean genotypes decreased under drought stress conditions, and55,81 reported that gs significantly decreased in drought-stressed soybean plants, which was also supported by our results. However, Significant enhancement in gs was recorded when any concentration of H2O2 was exogenously applied on both soybean genotypes. Simultaneously, the RWC was significantly better 3 days after drought stress imposition when 1 or 5 mM H2O2 was applied on Coraline plants and 1 mM H2O2 was applied on Speeda plants. Previously35,36,82. reported significant enhancement in both RWC and gs in drought-stressed soybean as a consequence of H2O2 foliar application. This conclusion was also reported when drought-stressed soybean plants were exogenously treated with melatonin, and when rice plants were exogenously sprayed with either H2O2 or SA58,75.
Both the optimal (Fv/Fm) and the actual photochemical efficiency of PSII (yield) significantly decreased in both genotypes 3 days after drought stress imposition. However, the exogenous application of any concentration of H2O2 significantly enhanced these traits in both genotypes (except for the Fv/Fm of Speeda plants sprayed with 10 mM H2O2). It was previously reported that a key factor of plant’s response to variations in the surrounding environment is the chlorophyll fluorescence83. Under drought stress conditions, leaves absorb and translocate less energy to PSII, leading to inhibited plant development84. Similar to our findings81, reported that Fv/Fm significantly decreased under PEG-induced drought stress.
The capacity of light intercepted by plants, and thus of photosynthetic rate is measurably affected by the plant’s leaf area85,86. It is well documented that drought stress results in reduced leaf area (e.g.87,88). Our results also are in agreement with this conclusion. Furthermore, we found out that plants of both genotypes that were sprayed with any concentration of H2O2 could noticeably maintain higher SLA at all 3 sampling dates. It was also found out earlier that the exogenous spray of H2O289, ethanol55 and SA90,91 could enhance the leaf area of soybean plants.
Drought stress can alter plant’s growth and development through several changes in the root92 and shoot89 levels. We found out that the root DW, root volume and root length increased 3 days after drought stress imposition as compared to control plants of both genotypes, with more announced increase in Coraline, the drought-susceptible genotype. Increased root length under drought stress conditions can help the plants acquire more water and nutrients from deeper soil levels93,94. On the other hand, shoot length and DW noticeably decreased under drought stress conditions; similar conclusion was also reported by95. The application of H2O2 enhanced both root and shoot length of Speeda, but not Coraline, whereas did not enhance the DW96 concluded that H2O2 enhances root growth by increasing ABA levels. It was previously reported that the roots and the seedlings of sweet potato responded positively to the application of H2O2 at levels between 0.5 and 2.5 mM, but negatively at levels of 5 mM or more97.
Conclusions
Drought stress affecting soybeans during flowering stages impairs several important morpho-physiological traits, leading to massive losses in reproductive organs and, consequently, potential yields. A post-drought recovery can still enable the plants survive and reach maturity stages. However, the continuous severe drought occurring for longer periods during reproductive stages can lead to even complete deterioration of plants. The exogenous application of H2O2 in low concentrations can help soybean plants overcome most of these impairments and enhance the root morphology, enabling for more water and nutrient uptake potentials under this unfavorable condition. This treatment was more effective on the drought-susceptible than on the drought-tolerant soybean genotype, with more announced enhancements of the stomatal conductance, relative water content, specific leaf area, root and shoot lengths and leaf proline and soluble sugar concentrations, where the plants had eventually similar levels of these traits as compared to the control treatments. A higher concentration of 10 mM H2O2 had little to no effect on the majority of the studied traits.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Harrison, M. T., Tardieu, F., Dong, Z., Messina, C. D. & Hammer, G. L. Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob. Change Biol. 20(3), 867–878 (2014).
Bradford, J. B., Schlaepfer, D. R., Lauenroth, W. K. & Palmquist, K. A. Robust ecological drought projections for drylands in the 21st century. Glob. Change Biol. 26(7), 3906–3919 (2020).
Zhao, M. & Running, S. W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329(5994), 940–943 (2010).
Kogan, F., Guo, W. & Yang, W. Drought and food security prediction from NOAA new generation of operational satellites. Geomatics Nat. Hazards Risk. 10(1), 651–666 (2019).
Jaleel, C. A. et al. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 11(1), 100–105 (2009).
Castro, P., Puertolas, J. & Dodd, I. C. Stem girdling uncouples soybean stomatal conductance from leaf water potential by enhancing leaf xylem ABA concentration. Environ. Exp. Bot. 159, 149–156 (2019).
Kang, S. & Zhang, J. Controlled alternate partial root-zone irrigation: Its physiological consequences and impact on water use efficiency. J. Exp. Bot. 55(407), 2437–2446 (2004).
Seleiman, M. F. et al. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10(2), 259 (2021).
Ilyas, M. et al. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 40, 926–944 (2021).
Farooq, M., Wahid, A., Kobayashi, N. S. M. A., Fujita, D. B. S. M. A., & Basra, S. M. A. Plant drought stress: Effects, mechanisms and management. Sustain. Agric., 153–188 (2009).
Lee, B. R. et al. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 157, 1–10 (2019).
Mwenye, O. J. Root properties and proline as possible indicators for drought tolerance in soybean. Doctoral dissertation, University of the Free State (2017).
Beis, A. & Patakas, A. Differential physiological and biochemical responses to drought in grapevines subjected to partial root drying and deficit irrigation. Eur. J. Agron. 62, 90–97 (2015).
Hussain, H. A. et al. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 9, 393 (2018).
Noctor, G., Reichheld, J. P. & Foyer, C. H. ROS-related redox regulation and signaling in plants. Sem. Cell Dev. Biol. (Academic Press) 80, 3–12 (2018).
Lee, K. W. et al. Nitric oxide-induced proteomic analysis in rice leaves. Plant Biotechnol. Rep. 13, 375–387 (2019).
Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I. & Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28(11), 1091–1101 (2006).
Hasanuzzaman, M. et al. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 8, 115 (2017).
Banerjee, A., & Roychoudhury, A. Abiotic stress tolerance in plants by priming and pretreatment with hydrogen peroxide. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants, 417–426 (2019).
Abdel Latef, A. A. H., Kordrostami, M., Zakir, A., Zaki, H. & Saleh, O. M. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 8(9), 303 (2019).
Vranová, E., Inzé, D. & Van Breusegem, F. Signal transduction during oxidative stress. J. Exp. Bot. 53(372), 1227–1236 (2002).
Imran, M. et al. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 13(4), plab026 (2021).
Sun, T. et al. Exogenous application of acetic acid enhances drought tolerance by influencing the MAPK signaling pathway induced by ABA and JA in apple plants. Tree Physiol. 42(9), 1827–1840 (2022).
Utsumi, Y. et al. Acetic acid treatment enhances drought avoidance in cassava (Manihot esculenta Crantz). Front. Plant Sci. 10, 521 (2019).
Zhang, A. et al. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 175(1), 36–50 (2007).
Kang, G., Wang, C., Sun, G. & Wang, Z. Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ. Exp. Bot. 50(1), 9–15 (2003).
Maruri-López, I., Aviles-Baltazar, N. Y., Buchala, A. & Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 10, 423 (2019).
Gechev, T. S. et al. Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell. Mol. Life Sci. 59, 708–714 (2002).
Niu, L. & Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 7, 230 (2016).
Wojtyla, Ł, Lechowska, K., Kubala, S. & Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 7, 66 (2016).
Huang, H., Ullah, F., Zhou, D. X., Yi, M. & Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10, 800 (2019).
Smirnoff, N. & Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 221(3), 1197–1214 (2019).
Sun, Y., Wang, H., Liu, S. & Peng, X. Exogenous application of hydrogen peroxide alleviates drought stress in cucumber seedlings. S. Afr. J. Bot. 106, 23–28 (2016).
Khan, T. A., Qazi, F. & Mohammad, Y. Effect of exogenously sourced hydrogen peroxide treatments on growth, photosynthesis and antioxidant traits in two contrasting cultivars of tomato: A mode and concentration dependent study. Acad. J. Agric. Res. 6(1), 19–29 (2018).
Ishibashi, Y. et al. Hydrogen peroxide spraying alleviates drought stress in soybean plants. J. Plant Physiol. 168(13), 1562–1567 (2011).
Terzi, R., Kadioglu, A., Kalaycioglu, E. & Saglam, A. Hydrogen peroxide pretreatment induces osmotic stress tolerance by influencing osmolyte and abscisic acid levels in maize leaves. J. Plant Interact. 9(1), 559–565 (2014).
Liu, Z. J., Guo, Y. K. & Bai, J. G. Exogenous hydrogen peroxide changes antioxidant enzyme activity and protects ultrastructure in leaves of two cucumber ecotypes under osmotic stress. J. Plant Growth Regul. 29, 171–183 (2010).
Nadeem, M. et al. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 20(10), 2541 (2019).
Buezo, J. et al. Drought tolerance response of high-yielding soybean varieties to mild drought: Physiological and photochemical adjustments. Physiol. Plant. 166(1), 88–104 (2019).
Cui, W., Chang, Z. & Li, N. Effect of drought stress on physiology ecology and yield of soybean. J. Water Resources Water Eng. 24, 20–24 (2013).
Liu, F., Andersen, M. N. & Jensen, C. R. Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Funct. Plant Biol. 30(3), 271–280 (2003).
Fehr, W. R., Caviness, C. E., Burmood, D. T. & Pennington, J. S. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill 1. Crop sci. 11(6), 929–931 (1971).
Basal O. The effects of drought stress on soybean physiology, yield and quality. PhD dissertation, University of Debrecen http://hdl.handle.net/2437/300871 (2021).
Michel, B. E. & Kaufmann, M. R. The osmotic potential of polyethylene glycol 6000. Plant physiol. 51(5), 914–916 (1973).
Cakmak, I. & Marschner, H. Decrease in nitrate uptake and increase in proton release in zinc deficient cotton, sunflower and buckwheat plants. Plant Soil. 129, 261–268 (1990).
Schreiber, U. S. C. H. L. I. W. A., Schliwa, U. & Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62 (1986).
Wellburn, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144(3), 307–313 (1994).
Wilson, P. J., Thompson, K. E. N. & Hodgson, J. G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 143(1), 155–162 (1999).
Bates, L. S., Waldren, R. A. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant soil. 39, 205–207 (1973).
Trevelyan, W. E. & Harrison, J. S. Studies on yeast metabolism: 1 Fractionation and microdetermination of cell carbohydrates. Biochem. J. 50(3), 298 (1952).
Duncan, D. B. Multiple range and multiple F tests. Biometrics. 11(1), 1–42 (1955).
Wang, Y. et al. Polyamines are involved in chilling tolerance in tobacco (Nicotiana tabacum) seedlings. Plant Growth Regul. 89, 153–166 (2019).
Tan, D. X. et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 63(2), 577–597 (2012).
Cui, G. et al. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 118, 138–149 (2017).
Rahman, M. M. et al. Ethanol positively modulates photosynthetic traits, antioxidant defense and osmoprotectant levels to enhance drought acclimatization in soybean. Antioxidants 11(3), 516 (2022).
Jiang, Y. P. et al. Hydrogen peroxide functions as a secondary messenger for brassinosteroids-induced CO2 assimilation and carbohydrate metabolism in Cucumis sativus. J. Zhejiang Univ. Sci. B. 13(10), 811–823 (2012).
Abass, S. M. & Mohamed, H. I. Alleviation of adverse effects of drought stress on common bean (Phaseolus vulgaris L) by exogenous application of hydrogen peroxide. Bangladesh J. Bot. 40(1), 75–83 (2011).
Cao, L., Jin, X. J. & Zhang, Y. X. Melatonin confers drought stress tolerance in soybean (Glycine max L) by modulating photosynthesis, osmolytes, and reactive oxygen metabolism. Photosynthetica 57(3), 812–819 (2019).
Das, A. K. et al. Ethanol treatment enhances physiological and biochemical responses to mitigate saline toxicity in soybean. Plants 11(3), 272 (2022).
Mohammadkhani, N. & Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J 3(3), 448–453 (2008).
Du, J., Chen, X., Li, W. & Gao, Q. Osmoregulation mechanism of drought stress and genetic engineering strategies for improving drought resistance in plants. For. Stud. China 6, 56–62 (2004).
Ozturk, M. et al. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 172(2), 1321–1335 (2021).
Zulfiqar, F., Akram, N. A. & Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 251, 1–17 (2020).
Darmanti, S., Hastuti, E. D., & Suedy, S. W. A. Exogenous hydrogen peroxide induces an antioxidative defense system against drought stress in soybean [Glycine max (L) merr] crops. JAPS J. Anim. Plant Sci., 31(1) (2021).
Khan, M. N. et al. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 140, 111597 (2019).
Meng, J. F. et al. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 57(2), 200–212 (2014).
Hayat, S. et al. Role of proline under changing environments: A review. Plant signaling behav. 7(11), 1456–1466 (2012).
Kaczmarek, M., Fedorowicz-Strońska, O., Głowacka, K., Waśkiewicz, A. & Sadowski, J. CaCl 2 treatment improves drought stress tolerance in barley (Hordeum vulgare L). Acta Physiol. Plant. 39, 1–11 (2017).
Iqbal, N. et al. Imbalance water deficit improves the seed yield and quality of soybean. Agronomy 8(9), 168 (2018).
Du, Y. et al. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 146, 1–12 (2020).
Moloi, M. J. & Van der Merwe, R. Drought tolerance responses in vegetable-type soybean involve a network of biochemical mechanisms at flowering and pod-filling stages. Plants 10(8), 1502 (2021).
Anjum, S. A., Farooq, M., Xie, X. Y., Liu, X. J. & Ijaz, M. F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 140, 66–73 (2012).
Bandurska, H. et al. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 118, 427–437 (2017).
Zahoor, R., Zhao, W., Abid, M., Dong, H. & Zhou, Z. Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol. 215, 30–38 (2017).
Lum, M. S., Hanafi, M. M., Rafii, Y. M., & Akmar, A. S. N. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. JAPS: J. Anim. Plant Sci., 24(5) (2014).
Khan, N. et al. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot 52(2), 355–363 (2020).
Mwenye, O. J., Van Rensburg, L., Van Biljon, A. & Van der Merwe, R. The role of proline and root traits on selection for drought-stress tolerance in soybeans: A review. S. Afr. J. Plant Soil. 33(4), 245–256 (2016).
Shaw, B., Thomas, T. H. & Cooke, D. T. Responses of sugar beet (Beta vulgaris L.) to drought and nutrient deficiency stress. Plant Growth Regul. 37, 77–83 (2002).
Zhang, J., Liu, J., Yang, C., Du, S. & Yang, W. Photosynthetic performance of soybean plants to water deficit under high and low light intensity. S. Afr. J. Bot. 105, 279–287 (2016).
Guler, N. S. & Pehlivan, N. Exogenous low-dose hydrogen peroxide enhances drought tolerance of soybean (Glycine max L.) through inducing antioxidant system. Acta Biologica Hungarica 67, 169–183 (2016).
Muhammad, A. A. et al. Interactive effect of shade and PEG-induced osmotic stress on physiological responses of soybean seedlings. J. Integr. Agric. 20(9), 2382–2394 (2021).
Rahman, M. et al. Acetic acid improves drought acclimation in soybean: An integrative response of photosynthesis, osmoregulation, mineral uptake and antioxidant defense. Physiol. Plant. 172(2), 334–350 (2021).
Dai, Y. et al. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 65(2–3), 177–182 (2009).
Rahbarian, R., Khavari-Nejad, R., Ganjeali, A., Bagheri, A., & Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietinum L.) genotypes. Acta biologica Cracoviensia. Series botânica, 53(1) (2011).
Weraduwage, S. M., Chen, J., Anozie, F. C., Morales, A., Weise, S. E., & Sharkey, T. D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 167 (2015).
Verma, K. K. et al. Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 5(37), 24145–24153 (2020).
Gavili, E., Moosavi, A. A. & Haghighi, A. A. K. Does biochar mitigate the adverse effects of drought on the agronomic traits and yield components of soybean?. Ind. Crops Prod. 128, 445–454 (2019).
Wei, Y., Jin, J., Jiang, S., Ning, S. & Liu, L. Quantitative response of soybean development and yield to drought stress during different growth stages in the Huaibei Plain. China. Agronomy 8(7), 97 (2018).
Basal, O. & Szabó, A. Ameliorating drought stress effects on soybean physiology and yield by hydrogen peroxide. Agriculturae Conspectus Scientificus 85(3), 211–218 (2020).
Kuchlan, M. K., Kuchlan, P. & Husain, S. M. Effect of foliar application of growth activator, promoter and antioxidant on seed quality of soybean. Legume Res. 40(2), 313–318 (2017).
Razmi, N., Ebadi, A., Daneshian, J. & Jahanbakhsh, S. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Interact. 12(1), 457–464 (2017).
Shi, G. et al. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ. Exp. Bot. 111, 127–134 (2015).
Xiong, L., Wang, R. G., Mao, G. & Koczan, J. M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 142(3), 1065–1074 (2006).
Zegada-Lizarazu, W. & Monti, A. Deep root growth, ABA adjustments and root water uptake response to soil water deficit in giant reed. Ann. Bot. 124(4), 605–615 (2019).
Bashir, W., Anwar, S., Zhao, Q., Hussain, I. & Xie, F. Interactive effect of drought and cadmium stress on soybean root morphology and gene expression. Ecotoxicol. Environ. Saf. 175, 90–101 (2019).
Li, C., Bian, B., Gong, T. & Liao, W. Comparative proteomic analysis of key proteins during abscisic acid-hydrogen peroxide-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J. Plant Physiol. 229, 185–194 (2018).
Deng, X. P. et al. Exogenous hydrogen peroxide positively influences root growth and metabolism in leaves of sweet potato seedlings. Aust. J. Crop Sci. 6(11), 1572–1578 (2012).
Acknowledgements
Project No. TKP2021-NKTA-32 has been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the TKP2021-NKTA funding scheme and. This study was also supported by the National Research, Development and Innovation Office of Hungary under Grant: RRF-2.3.1-21-2022-00008.
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Contributions
O.B.: methodology, measurements, statistical analyses, writing the manuscript. T.B.Z.: measurements, preparing the manuscript for submission. V.S.: methodology, manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basal, O., Zargar, T.B. & Veres, S. Elevated tolerance of both short-term and continuous drought stress during reproductive stages by exogenous application of hydrogen peroxide on soybean. Sci Rep 14, 2200 (2024). https://doi.org/10.1038/s41598-024-52838-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52838-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.