Abstract
The study aimed to assess the efficacy of transcranial direct current stimulation (tDCS) in the treatment of neuropsychiatric (NP) symptoms of the post-acute sequelae of SARS-CoV-2 infection (PASC), known as the long COVID. A double-blind, randomized, sham-controlled study compared the efficacy and safety of prefrontal cortex active tDCS to sham-tDCS in treating NP-PASC. Patients diagnosed with NP-PASC, with a Fatigue Impact Scale (FIS) score ≥ 40, were eligible for the study. Twenty tDCS sessions were administered within four weeks, with continuous, end-of-treatment, and follow-up measurements. The primary outcome was a change in the FIS at the end-of-treatment, analyzed in the intention-to-treat population. Data from 33 patients assigned to active (n = 16) or sham-tDCS (n = 17) were analyzed. After the treatment, a decrease in the FIS score was more pronounced in the sham than in the active group, yet the intergroup difference was insignificant (11.7 [95% CI −11.1 to 34.5], p = 0.6). Furthermore, no significant intergroup differences were observed regarding anxiety, depression, quality of life, and cognitive performance. The small cohort sample, differences in baseline FIS scores between groups (non-stratified randomization), or chosen stimulation parameters may have influenced our findings. However, it might also be possible that the expected mechanism of action of tDCS is insufficient to treat these conditions.
Similar content being viewed by others
Introduction
The post-acute sequelae of SARS-CoV-2 infection (PASC), known as the post-COVID syndrome or the long COVID, is a set of distinct symptoms persisting more than one month after COVID-191. Neuropsychiatric (NP) and neurocognitive symptoms are among the most common manifestations of PASC1,2. These include functional (such as fatigue (37%), sleep disturbances (31%), muscle pain (18%) or loss of sense of smell (12%)] and cognitive (such as brain fog (32%), memory (27%) or attention deficit (22%)) impairment, and emotional dysregulation (such as anxiety (23%) or depression (12%))3. They can vary from mild to severe symptoms, affecting the patient's daily life for several months4. Thus, PASC can represent a severe limitation that leads to reduced work capacity and quality of life and creates a critical need for therapeutic intervention.
Current therapeutic strategies for managing SARS-CoV-2-induced neuroinfection mainly involve pharmacological approaches with unclear results5. Given their limited efficacy in many patients, it is relevant to seek alternative therapeutic approaches. These could be based on influencing the pathophysiological mechanism of PASC, probably associated with persistent microvascular endotheliopathy, autoantibodies, localized inflammation, or reactivation of latent pathogens6. Transcranial direct current stimulation (tDCS), together with some other treatment strategies outlined in the consensus guidelines of the multidisciplinary collaboration7, might represent one such targeted treatment option for PASC treatment.
TDCS represents a Non-Invasive Brain Stimulation (NIBS) method, safe and user-friendly (portable and home-use)8 that has been proposed as an effective treatment tool for functional (e.g., fatigue)9, cognitive (e.g., attention or working memory impairment) impairment10,11, and emotional dysregulation (e.g., depression)12,13,14. In addition, tDCS has also been introduced as a treatment for fatigue15 in neuroimmune-based diseases (such as multiple sclerosis or post-polio syndrome) for its potential effect on restoring autonomic balance16,17. Current evidence suggests that tDCS can indirectly ameliorate inflammation manifestations by intervening in neuroplasticity processes18 or directly affecting NP symptoms persisting after infection19, raising the possibility of influencing the systemic immune response by targeting the frontal region20,21. Thus, tDCS might represent a potential therapeutic modality for managing the sequelae of COVID-19 infection not only as an adjunctive treatment to improve cognitive and physical rehabilitation22 but also to manage persistent symptoms after illness, such as fatigue or pain19.
Recently, the evidence for the effect of tDCS in the treatment of COVID-19 was mainly based on case reports8,23, but the results of several RCTs24,25,26,27,28,29,30,31 are now available. The first clinical RCTs in tDCS management of NP symptoms of acute and chronic stages of COVID-19 have confirmed its effect on influencing functional impairment, specifically alleviating fatigue24,25,26,27, cognitive impairment28, olfactory impairment29, and anxiety25. In addition, tDCS impacts other PASC symptoms, such as dyspnea30, and electrophysiological improvements31, such as heart rate variability or oxygen saturation. Those RCTs and case reports aimed at treating NP symptoms after COVID-19 targeted mainly the prefrontal (PFC) areas, such as the left dorsolateral24, the medial PFC8,31,32,33, or the primary motor cortex25,26,27, and found several positive results on fatigue24,25,26,27, anxiety8,23,25,32,33, and depression8,23,33,34 alleviation, and cognitive performance8,25,28,29,33,34,35,36 and quality of life25 improvement. However, research in this area is limited, and the evidence needs to be supported by the results of further studies.
Here, we investigated the effect of PFC-tDCS on fatigue alleviation in PASC. To this end, in a double-blind, parallel-group, sham-controlled study in patients with NP symptoms of PASC presenting chronic fatigue, we administered a 4-week tDCS procedure. We hypothesized that active tDCS would induce significantly higher fatigue relief than sham tDCS. As a secondary objective, we examined the effects of PFC-tDCS on changes in affective and anxiety symptoms, cognition, and quality of life.
Results
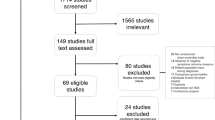
Seventy-one patients were screened for eligibility between April 2022 and May 2023, and 35 met the inclusion criteria and consented to participate. One patient from each group withdrew from the study before the initial treatment. The final sample for analyses consists of thirty-three patients (16 in the active and 17 in the sham groups) who entered the blinded phase, and all consequently completed the four-week study. Within the one-month post-study observation, one participant from the active group was lost to follow-up (Fig. 1).
Twenty-three of thirty-three participants were women (70%), and the mean age was 42.2 ± 10.5 years. The duration of PASC was 13.3 ± 7.6 months. Eleven participants (33%) had a history of mental disorders, primarily anxiety disorders. Fifteen participants (46%) were taking antidepressants, and four (12%) were using benzodiazepines (BZDs) or anticonvulsants (AC) at the time of study entry.
The baseline characteristics of groups were comparable across various demographic and clinical parameters; however, except for a significant difference in the fatigue severity, patients in the sham group reported significantly higher scores in the Fatigue Impact Scale (FIS) total and cognitive and psychosocial domains (Table 1).
At the end of the four-week randomized control trial (Fig. 2), there was a substantial decrease in fatigue severity according to the FIS scale, but no difference between groups was revealed (the mixed model for repeated measures (MMRM); effect of time: F(2,55.6) = 11.88, p < 0.001; effect of treatment: F (1,23.6) = 0.10, p = 0.8; treatment x time interaction: F (2,53.3) = 1.07, p = 0.35). The least squares (LS) mean difference between groups in the FIS total score changes from the baseline to week 4 (the primary outcome endpoint) was 11.7 points (95% CI −11.1 to 34.5, t = 1.36, pcorr = 0.6). Within-group comparisons showed significant improvement only in the sham group (sham: −22.2, 95% CI −40.3 to −4.1), t = 3.6, pcorr = 0.005; active: −10.5, 95% CI −28.0 to 7.0, t = 1.76, pcorr = 0.7). Continuous alleviation of fatigues was also observed at the end of follow-up, however still nonsignificant in the active group (active vs sham: 11.3 (95% CI −11.7 to 34.4), t = 1.31, pcorr = 0.7; sham: −27.1 (95% CI −45.2 to −9.1), t = 4.40, pcorr < 0.001; active: −15.8 (95%CI −33.7 to 2.1), t = 2.59, pcorr = 0.13) (Fig. 3a). Improvement of fatigue from baseline to end of the study and to follow-up were also found for cognitive, physical, and psychosocial domains; again, neither achieved significant between-group differences (Table 2).
Scheme of the study. Post-COVID-19 Symptoms Assessment Questionnaire (A-PASC); Assessment of Quality of Life—six dimensions (AQoL-6D); Clinical Global Impression (CGI); Fatigue Impact Scale (FIS); Generalised Anxiety Disorder (GAD-7); Patient Health Questionnaire (PHQ-9); Transcranial Direct Current Stimulation (tDCS).
Clinical outcomes. (a) Change in Fatigue Impact Scale (FIS); (b) change in Post-COVID-19 Symptoms Assessment Questionnaire (A-PASC); (c) change in self-assessment of depression using Patient Health Questionnaire (PHQ-9); (d) change in self-assessment of anxiety using Generalised Anxiety Disorder (GAD-7). Symbols represent least-squares means and error bars their 95% confidence intervals.
In the more specific assessment for PASC, i.e., Post-COVID-19 Symptoms Assessment Questionnaire (A-PASC), the score decrease was revealed at week four compared to baseline (time: F(2,56.8) = 12.62, p < 0.001) with similar change within either group (active: −14.4 (95% CI −26.1 to −2.8), t = 3.62, pcorr = 0.004; sham: −16.6 (95% CI −26.7 to −4.6), t = 4.12, pcorr = 0.001), thus no between-group differences (treatment: F(1, 24.3) = 1.80, p = 0.19; treatment × time: F(2, 56.8) = 0.17, p = 0.8; active vs sham: 2.2 (95%CI −13.1 to 17.4), t = 0.30, pcorr > 0.9) (Fig. 3b). For subscales (cognitive, emotional, physical, and functional), analogous changes were found (Table 2). A-PASC was not administered at the follow-up visit.
Anxious and depressive symptoms were of mild to moderate severity at baseline and continued to decrease throughout the study and follow-up. Again, the decline in scores on the respective self-assessment questionnaires (Generalised Anxiety Disorder (GAD-7) and Patient Health Questionnaire (PHQ-9) was comparable between groups at all post-baseline visits (Fig. 3c,d, Table 2).
There was no difference in the quality of life as assessed by the Assessment of Quality of life—six dimensions (AQoL-6D) between groups during the study and follow-up (Fig. 4a, Table 2), except in the dimension of senses in follow-up measurement (Table 2), which also significantly differed between groups at baseline in favor of the sham group (Table 1). Subjects' performance on cognitive tests assessed by Digit Span—Forward (DSF)—attention, Digit Span—Backward (DSB)—working memory, and Digit Symbol Substitution Test (DSST)—psychomotor tempo were comparable between groups at all visits after the baseline examination. (Fig. 4b–f, Table 2).
Quality of life and cognition outcomes. (a) Assessment of Quality of life—six dimensions (AQoL-6D); (b) correct symbols in Digit Symbol Substitution Test (correctcount); (c) two-error maximum length, the traditional measure of a participant's forward digit span. It is the last digit span a participant gets correct before making two consecutive errors (fTE_ML); (d) the maximal forward digit span that a participant recalled correctly during all 14 trials (fML); (e) two-error maximum length, the traditional measure of a participant's backward digit span. It is the last digit span a participant gets correct before making two consecutive errors (bTE_ML); (f) maximal backward digit span that a participant recalled correctly during all 14 trials. It is set to 0 before the start of the Digit Span (bML); Symbols represent least-squares means and error bars their 95% confidence intervals.
During the initial 2 weeks of the RCT, eight of the sixteen patients (50%) receiving active tDCS and nine of the seventeen (53%) patients assigned to the sham tDCS group experienced mild to moderate side effects such as burning or tingling. The incidence of side effects subsided as the study continued, with five patients in each group (31 and 29%) reporting side effects by the end of the trial. After the one-month post-study follow-up, none of the patients reported ongoing side effects, indicating their transient nature.
Discussion
So far, only a few studies have been published on the effect of tDCS in NP-PASC involving fatigue. Our study evaluated the efficacy and safety of tDCS in treating NP-PASC. The primary outcome was the FIS (fatigue) change at the endpoint, analyzed in the intention-to-treat population. Secondary outcome measures were a change in the FIS at follow-up and changes in A-PASC (post-covid symptoms), GAD-7 (anxiety symptoms), PHQ-9 (depressive symptoms), AQoL-6D (quality of life), and the cognitive tests: DSF (attention)/DSB (working memory) and DSST (psychomotor tempo) at the endpoint and the follow-up measurement. No intergroup difference was found in the FIS change and changes of secondary outcomes (A-PASC, GAD-7, PHQ-9, AQoL-6D, cognitive tests) at the endpoint or follow-up between the active and sham groups, except the change in the dimension of senses in the AQoL-6D in follow-up measurement, which we consider to be an incidental finding. There was no intergroup difference in the frequency, type, and severity of side effects37 monitored during the tDCS application. Predisposing factors for PASC (hypertension, hypothyroidism, type 2 diabetes mellitus, dyslipidemia) did not affect treatment outcomes.
Sample size
At the time of study design, we could not rely on any RCT with this clinical population to estimate the sample size needed for the expected outcome (difference between active and placebo tDCS). It would also be difficult to rely on existing clinical RCTs focusing on other causes of fatigue, such as multiple sclerosis38, which have also included small samples.
It is questionable whether the results of this study may have been influenced by the small sample size, which was, however, adequately chosen with respect to recruitment opportunities between March 2022 and March 2023 in Czechia. The justification for expanding the sample size would have been warranted if this study had shown at least a trend favoring the active intervention. However, the results indicated the opposite—a non-significant difference and even a trend toward improvement in the sham group. The Bayesian factor (BF10 = 0.163) for the primary outcome (FIS total score) suggests that the observed data are about 6.12 times more likely under the null hypothesis (no difference between groups or sham is better) compared to the alternative hypothesis (active is better than sham), providing moderate to strong evidence against the benefit of active tDCS. Therefore, we believe our data reflect true negative results rather than false negatives.
PASC cohort
The heterogeneous nature of PASC, based on differences in the clinical symptomatology of the PASC cohort, may also cause our findings. However, our analyses did not confirm the impact of the intergroup difference in the degree of clinical manifestation in baseline PASC characteristics (except for differences in FIS score—total and cognitive) or PASC predisposing factors such as severity of COVID-19, duration of PASC, predisposing physical illnesses, and history of psychiatric illness.
According to current knowledge, PASC can be caused by different pathophysiological mechanisms such as direct cytopathic response39, contribution to coagulation and vasculature vasculature-related issues6,40,41, dysregulation of the immune response facilitating reactivation of latent infections40, or by various psychological mechanisms41. Even the different variants of SARS-CoV-2 may directly affect the central nervous system to varying degrees42. Our study did not investigate which SARS-CoV-2 variant in the enrolled patients was responsible for NP-PASC. Considering that the enrolled patients underwent COVID-19 between April 2020 and November 2022 and that different SARS-CoV-2 virus variants, specifically 20/A; 20/B; 20/C; 20/E; Alpha: 20/I; Beta: 20/H; Gamma: 20/J; Kappa: 21/B; Delta: 21/A, 21/E, 21/I, 21/J; Omicron: 21/K, 21/L, 22/A, 22/B, 22/C, 22/D, 22/E, 22/F (from https://covariants.org/) occurred in Czechia during this time, we can infer that the heterogeneity of the cause of NP-PASC in the sense of SARS-CoV-2 variant may also have influenced the outcome of our study.
Area of neuromodulation
The electrode placement (anode/cathode corresponding to the F3/F4 regions) for tDCS application was chosen based on the positive findings of one of the first published case reports in this area of research8,23. According to the visualization of the electric field simulation using SimNIBS 4.0 software, it corresponds to neuromodulation of the mPFC area (Fig. 5). In addition, this placement has been recently proven ineffective, for example, in treating depression43. It can be assumed that our choice of electrode placement may have accounted for the different outcomes of recent studies in PASC that have applied tDCS to other cortical regions, either to the left dorsolateral prefrontal cortex (DLPFC) or to the primary motor (M1) cortex. Specifically, left DLPFC tDCS showed relief from fatigue24 (change in the Modified FIS (MFIS) physical fatigue subdimension) and affected severity of depression24 (change in the Beck Depression Inventory (BDI)). Similarly, the tDCS of the M1 area alleviated fatigue (change in the MFIS or Fatigue Assessment/Severity Scale (FAS, and FSS)25,26,27, improved the subjective health (VAS-Health; EQ-5D-5L VAS]27, anxiety (Hamilton Anxiety Rating Scale)25, and quality of life (WHOQOL-BREF)25. Moreover, the M1 region seems suitable for tDCS application in other causes of fatigue, such as multiple sclerosis15 or stroke44.
Stimulator parameter
Furthermore, the chosen stimulation parameters may have also influenced the outcome of this study.
Session duration
The results of our study may have been influenced by the duration of each tDCS session, which lasted 30 min. Thus, treatment protocols with different session duration (e.g., 20 min) may have yielded different results. It appears that the efficacy of tDCS does not necessarily increase with a longer duration of tDCS session45,46 and may even have the opposite effect46. Since some RCTs in PASC24,26 or other diseases causing fatigue47,48,49 have benefited from tDCS using a session duration of 20 min, it can be inferred that the chosen duration of the session and maybe even smaller number of sessions15,47,50,51,52 may have partially influenced our study results.
Intensity: We used a DC of 2 mA for the tDCS application. However, some existing studies on non-clinical populations suggest that excitatory after-effects are nonlinear with increasing intensity of tDCS45,53. Nevertheless, most NP clinical studies13, especially those focused on fatigue relief after tDCS, including PASC24,25,26,27 or multiple sclerosis49, have confirmed the beneficial effects of tDCS using a DC of 2 mA or higher intensity.
tDCS as an augmentation of rehabilitation
PASC treatment typically involves a multidisciplinary approach, including intervention from different medical disciplines, with treatment plans for NP-PASC often focusing on psychopharmacological treatment of symptoms, such as antidepressants or physical or cognitive rehabilitation tailored to the individual patient's needs. In our case, tDCS was not used to augment an established rehabilitation procedure as another NP-PASC tDCS study did25,28. TDCS was applied during patient activities (reading, housework, etc.) that were individually difficult—no specific rehabilitation program was performed during tDCS. However, the choice of tDCS as an adjunct to targeted rehabilitation, as shown in some other studies25,28,54, may have led to different outcomes.
Systemic factors and tDCS
Taking the possible reasons for our study findings more generally, it should be noted that tDCS has been investigated primarily for its potential to modulate neuronal activity13. Although recent studies have demonstrated an effect of this method on restoring autonomic balance16,17 or its indirect effect on manifestations of inflammation through neuroplasticity processes18, these mechanisms may be insufficient, especially when fatigue or other NP-PASC symptoms may be caused by systemic factors such as inflammation, immune dysregulation, or their combination. They may not directly address the complex underlying mechanisms that contribute to PASC.
The main study limits
The main limitation of our study was the differences in baseline FIS scores between the active and sham groups. Patients assigned to the placebo group had a significantly higher FIS score at the baseline visit than those in the active group, which may have influenced the outcome. This could have been addressed by setting a higher FIS ceiling as an inclusion criterion or by stratified randomization at the time of study design.
Another shortcoming of the study is that, with the exception of cognitive tests, the reported results were obtained only from subjective questionnaires. The study lacks objective scales to assess clinical changes objectively.
Also, the study design did not include another active stimulation (e.g., with different electrode positions or with anode and cathode swapping) as a control group to verify the effect/non-effect demonstrated by tDCS.
The study was also limited by the severity of the participants' PASC—patients with severe symptoms would not be able to participate (inability to complete self-assessment questionnaires, self-operation of tDCS). In addition, most participants enrolled were searched through Facebook groups, indicating that this was a population that was able to actively seek information about PASC.
Another study flaw is the omission of a pre-treatment assessment of participants' expectations/perceptions of effects and the post-treatment recording of their guesses regarding their group allocation. Notably, higher expectations of a positive outcome may have contributed to the increased improvement in the placebo group (or the absence of a difference between groups). Indirectly, high expectancy may be inferred from the high retention rate in the study, as all participants who started the intervention completed the study.
Conclusion
We did not find the active tDCS superior to sham tDCS in fatigue, anxiety, depression, and other PASC symptoms relief or quality of life and cognitive performance improvement in PASC. The small cohort sample, differences in FIS scores between groups at baseline, or chosen neuromodulation parameters, such as tDCS session duration or selection of mPFC instead of M1 cortex as a target area, may influence our findings. Also, it might be possible that the expected mechanism of action of tDCS is insufficient to treat these conditions.
Patients and methods
Subjects
Outpatients of the National Institute of Mental Health (NIMH) with a history of moderate or severe COVID-19 infection dispensed by an outpatient physician for neuropsychiatric problems (fatigue, anxiety, depressed mood, sleep disturbance, etc.) within the PASC were eligible for study participation. They were approached through advertising via Facebook groups, advertising on the NIMH website (https://www.nudz.cz), or by referral from their physician or psychiatrist. Recruitment for the study ran from March 2022 to March 2023. Before the study, patients' health status was assessed by a physician. Written informed consent was obtained from all subjects. The ethics committee of the NIMH approved the study (No. 46/23), and the study has been registered at ISRCTN with trial ID ISRCTN10942585. All methods used on the study subjects were performed in accordance with relevant guidelines and regulations.
Inclusion criteria were females or males aged 18 to 75 years; PCR RNA SARS-CoV-2 negativity at the time of screening/pre-study entry; symptom duration > 1 month after detection of COVID-19; FIS questionnaire score ≥ 40; the presence of neuropsychiatric symptoms of PASC as determined by the A-PASC questionnaire with a minimum total score ≥ 25; psychopharmacological medication (if used) on a stable dose for ≥ 4 weeks; competent to give informed written consent.
Patients were excluded if one of the following conditions was present: contraindications to tDCS (skin disease, superficial injury, and fracture or skull fracture in the area of stimulation, epilepsy, metal plates in the head); history of any other DSM-IV Axis I diagnosis prior to COVID-19, except a) depressive disorder, anxiety disorder, and sleep disorders or substance use disorder, which may be present in the history but with at least six months of documented symptom remission; pregnancy or breastfeeding; patients with severe or unstable somatic disorders (cardiovascular disease, neoplasms, endocrinological disorders, etc.); patients suffering from a neurological disorder (e.g. epilepsy, head injury with loss of consciousness).
Procedure
The screening phase was conducted on days −2 to −7 before starting the active phase. After initial screening and study enrollment (see Clinical Assessment below), informed consent was obtained, and patients (n = 35) were randomized in a 1:1 ratio to one of two study groups: active DLPFC-tDCS and placebo-tDCS (block randomization). A baseline visit (day 0) was conducted on the day of tDCS initiation. Patients were subsequently clinically examined and assessed in the NIMH outpatient clinic at two weeks (day 14 ± 2) and at the end (day 28 ± 2) of four weeks of tDCS administration and after a four-week follow-up (FU-4W) visit (day 56 ± 2).
Assessment of clinical status and cognition
A medical history (somatic status, presence of neuropsychiatric symptoms), including a detailed history of COVID-19 (course and duration of the disease, history of COVID-19 treatment, etc.), was taken at inclusion in the study.
Clinical assessment, including FIS assessment as the primary outcome at the end of the tDCS course, was performed using self-assessment scales (FIS; A-PASC; PHQ-9; GAD-7; AQOL-6D) and objective assessment (CGI) by the physician at each visit, except A-PASC (was not used in the FU-4W visit) and FIS (was not used in the visit at week two). Examination of cognitive functions (attention, working memory, and psychomotor tempo) was performed using computerized cognitive tests (Digit Span, DSST) with the assistance and instruction of a psychologist at each visit. TDCS tolerability was assessed using the tDCS self-assessment questionnaire37 after two weeks and at the end of treatment. The patients and the objective assessors were blinded to the patient group (active versus placebo-tDCS).
If the patient was taking psychopharmaceuticals (antidepressants, anxiolytics, hypnotics) for any reason (e.g., neurological indication, pain management, depression, or insomnia) at study entry, the patient was eligible to enter the study only if he/she had been taking the medication for at least four weeks before study entry and agree to continue taking it at an unchanged dose for the duration of study participation. The only additional psychopharmacological treatment for the patient's participation in the study was clonazepam (up to 1 mg daily) for anxiety and zolpidem (up to 10 mg daily) for insomnia for all groups.
tDCS application
The HDCStim programmable stimulator (Newronica, Italy) was used for tDCS application in a double-blind design. The stimulating electrodes were placed as follows: the anode on the F3 area (the area for EEG electrode placement according to the international 10/20 system) and the cathode contralaterally on the F4 area. The electrodes with sponges filled with saline were fixed with the help of Mind-cap to the stimulating areas (F3—above the left DLPFC; F4—above the right DLPFC), which allows for possible home use (ensuring identical electrode placement during repeated application).
A total of 20 tDCS sessions (Monday to Friday) were administered to patients over a four-week period. During active tDCS, a DC of 2 mA intensity (current density 0.08 mA/m2) was applied for 30 min with an initial ramp-up and final ramp-down of current intensity, each lasting 30 s. Placebo-tDCS with an identical electrode assembly involved only an initial 30 s ramp-up phase, immediately afterward 30 s ramp-down phase to simulate sensations similar to active stimulation, then was stopped and followed by 29 min rest.
The tDCS applicator was blinded to the study participant's affiliation with the active or placebo group. For home use, the device was unlabelled, pre-programmed (active or placebo), and secured against off-study use (only one application per day possible). Home administration under the visual control of a physician (involving tDCS and its entire course) was allowed as an option for participants after thorough training (with online physician assistance and visual monitoring of tDCS engagement and progress) to reduce the burden of daily commuting to the NIMH. During each tDCS administration, patients performed activities (reading, housework, etc.) that they found difficult after the COVID-19 infection (not applied at rest to activate a dysfunctional cortical circuit).
Data analysis
The baseline demographic and clinical characteristics between groups were compared using unpaired t-tests, Mann–Whitney tests, or Fisher's exact tests. Efficacy and safety data were obtained from the modified intention-to-treat (mITT) dataset, consisting of patients randomized to treatment and receiving at least one tDCS session. Data were treated as observed without imputation of missing data. To estimate changes in the primary outcome measurement, the FIS total score, from baseline to week four and follow-up, a mixed model for repeated measures (MMRM) with a restricted maximum likelihood (REML) approach and Kenward-Roger adjustment of degrees of freedom was employed. The model included fixed effects for the time, treatment, and their interaction, subjects treated as random effects, and baseline score, age, BMI, PASC duration, depression (PHQ-9), and anxiety (GAD 7) as covariates. The first-order autoregressive (AR1) correlation structure was used, but alternative structures were also assessed using the Akaike Information Criterion (AIC). Least-squares (LS) means, within and between-group differences in LS means, and corresponding 95% confidence intervals were calculated, and Sidak correction was applied. Secondary outcomes (FIS subscales, A-PASC total score and subscales, PHQ-9, GAD-7, cognitive tests, and AQOL-6D) were subjected to the same MMRM analysis, with covariate selection adapted to each outcome. The occurrence of side effects was compared by Fisher´s exact test. All statistical analyses were conducted using Stata Statistical Software 15 (StataCorp. 2017).
Data availability
After the publication of this article, de-anonymized data will be made available for non-commercial academic projects. Data can be obtained by request to the corresponding author. The de-anonymized data files with a dictionary will be provided via a secure data transfer service.
References
Carfì, A., Bernabei, R., Landi, F., & for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–605 (2020).
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 (2021).
Premraj, L. et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 434, 120162 (2022).
Rolin, S., Chakales, A. & Verduzco-Gutierrez, M. Rehabilitation strategies for cognitive and neuropsychiatric manifestations of COVID-19. Curr. Phys. Med. Rehabil. Rep. 10, 182–187 (2022).
Mudd, P. A. et al. Targeted immunosuppression distinguishes COVID-19 from influenza in moderate and severe disease. Preprint https://doi.org/10.1101/2020.05.28.20115667 (2020).
Ahamed, J. & Laurence, J. Long COVID endotheliopathy: Hypothesized mechanisms and potential therapeutic approaches. J. Clin. Invest. 132, 33 (2022).
Herrera, J. E. et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients. PM R 13, 1027–1043 (2021).
Eilam-Stock, T. et al. Telehealth transcranial direct current stimulation for recovery from post-acute sequelae of SARS-CoV-2 (PASC). Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 14, 1520–1522 (2021).
Lefaucheur, J.-P. et al. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol. Clin./Clin. Neurophysiol. 47, 173–184 (2017).
Leffa, D. T. et al. Transcranial direct current stimulation improves long-term memory deficits in an animal model of attention-deficit/hyperactivity disorder and modulates oxidative and inflammatory parameters. Brain Stimul. 11, 743–751 (2018).
Flöel, A. tDCS-enhanced motor and cognitive function in neurological diseases. NeuroImage 85, 934–947 (2014).
Hyde, J. et al. Efficacy of neurostimulation across mental disorders: Systematic review and meta-analysis of 208 randomized controlled trials. Mol. Psychiatry 27, 2709–2719 (2022).
Lefaucheur, J.-P. et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92 (2017).
Brunoni, A. R. et al. Transcranial direct current stimulation for acute major depressive episodes: Meta-analysis of individual patient data. Br. J. Psychiatry 208, 522–531 (2016).
Ferrucci, R. et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation 34, 121–127 (2014).
Chaudhuri, A. & Behan, P. O. Fatigue in neurological disorders. Lancet 363, 978–988 (2004).
Okano, A. H. et al. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br. J. Sports Med. 49, 1213–1218 (2015).
Suchting, R., Colpo, G. D., Rocha, N. P. & Ahn, H. The effect of transcranial direct current stimulation on inflammation in older adults with knee osteoarthritis: A Bayesian residual change analysis. Biol. Res. Nurs. 22, 57–63 (2020).
Baptista, A. F. et al. Neuromodulation and inflammatory reflex: Perspectives on the use of non-invasive neuromodulation in the management of disorders related to COVID-19. Preprint https://doi.org/10.2139/ssrn.3601048 (2020).
Iseger, T. A., van Bueren, N. E. R., Kenemans, J. L., Gevirtz, R. & Arns, M. A frontal-vagal network theory for major depressive disorder: Implications for optimizing neuromodulation techniques. Brain Stimul. 13, 1–9 (2020).
Dantzer, R. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol. Rev. 98, 477–504 (2018).
Simpson, R. & Robinson, L. Rehabilitation after critical illness in people with COVID-19 infection. Am. J. Phys. Med. Rehabil. 99, 470 (2020).
Gómez, L., Vidal, B., Cabrera, Y., Hernández, L. & Rondón, Y. Successful treatment of post-COVID symptoms with transcranial direct current stimulation. Prim. Care Companion CNS Disord. 23, 38522 (2021).
Oliver-Mas, S. et al. Transcranial direct current stimulation for post-COVID fatigue: A randomized, double-blind, controlled pilot study. Brain Commun. 5, 117 (2023).
Santana, K. et al. Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC). Brain Stimul. 16, 100–107 (2023).
Workman, C., Boles-Ponto, L., Kamholz, J., Bryant, A. & Rudroff, T. Transcranial direct current stimulation and post-COVID-19-fatigue. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 14, 1672–1673 (2021).
Rudroff, T., Fietsam, A., Deters, J., Bryant, A. & Kamholz, J. tDCS improves perceptions of fatigue in patients with post-COVID-19-symptoms that are less than 6 months post-infection. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 16, 249 (2023).
Brunoni, A. R. et al. Efficacy of transcranial direct current stimulation and cognitive training for the neurocognitive symptoms of long covid-19. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 16, 192 (2023).
Vestito, L. et al. Impact of tDCS on persistent COVID-19 olfactory dysfunction: A double-blind sham-controlled study. J. Neurol. Neurosurg. Psychiatry 94, 87–88 (2023).
Andrade, S. M. et al. Efficacy and safety of HD-tDCS and respiratory rehabilitation for critically ill patients with COVID-19. The HD-RECOVERY randomized clinical trial. Brain Stimul. 15, 780–788 (2022).
Pinto, T. P. et al. Prefrontal tDCS modulates autonomic responses in COVID-19 inpatients. Brain Stimul. 16, 657–666 (2023).
Shinjo, S. K., Brunoni, A. R., Okano, A. H., Tanaka, C. & Baptista, A. F. Transcranial direct current stimulation relieves the severe anxiety of a patient with COVID-19. Brain Stimul. 13, 1352–1353 (2020).
Cavendish, B. A. et al. Combination of transcranial direct current stimulation with online cognitive training improves symptoms of post-acute sequelae of COVID-19: A case series. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 15, 1375–1377 (2022).
Esmaeili, L. & Ramezanpoor, A. The effectiveness of transcranial direct current stimulation of the brain (tDCS) on depressive syndrome in improved individuals from Covid-19. Neuropsychology 8, 49–58 (2022).
Rosen, A. C. et al. TDCS in a patient with dreadlocks: Improvements in COVID-19 related verbal fluency dysfunction. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 15, 254–256 (2022).
Wysokiński, A., Szczepocka, E. & Szczakowska, A. Improved cognitive performance, increased theta, alpha, beta and decreased delta powers after cognitive rehabilitation augmented with tDCS in a patient with post-COVID-19 cognitive impairment (brain-fog). Psychiatry Res. Case Rep. 2, 100164 (2023).
Antal, A. et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 128, 1774–1809 (2017).
Kan, R. L. D. et al. Effects of non-invasive brain stimulation in multiple sclerosis: Systematic review and meta-analysis. Ther. Adv. Chronic Dis. 13, 20406223211069200 (2022).
Gonzalez-Garcia, P. et al. From cell to symptoms: The role of SARS-CoV-2 cytopathic effects in the pathogenesis of COVID-19 and long COVID. Int. J. Mol. Sci. 24, 8290 (2023).
Proal, A. D. et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. https://doi.org/10.1038/s41590-023-01601-2 (2023).
Mukaetova-Ladinska, E. B. & Kronenberg, G. Psychological and neuropsychiatric implications of COVID-19. Eur. Arch. Psychiatry Clin. Neurosci. 271, 235–248. https://doi.org/10.1007/s00406-020-01210-2 (2021).
Turner, S. et al. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 34, 321–344 (2023).
Burkhardt, G. et al. 572. Transcranial direct current stimulation as add-on to selective serotonin reuptake inhibitors in adults with major depressive disorder: Results: from the depression DC trial. Biol. Psychiatry 93, S325–S326 (2023).
De Doncker, W., Ondobaka, S. & Kuppuswamy, A. Effect of transcranial direct current stimulation on post-stroke fatigue. J. Neurol. 268, 2831–2842 (2021).
Batsikadze, G., Moliadze, V., Paulus, W., Kuo, M.-F. & Nitsche, M. A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 591, 1987–2000 (2013).
Hassanzahraee, M., Nitsche, M. A., Zoghi, M. & Jaberzadeh, S. Determination of anodal tDCS intensity threshold for reversal of corticospinal excitability: An investigation for induction of counter-regulatory mechanisms. Sci. Rep. 10, 16108 (2020).
Forogh, B. et al. Repeated sessions of transcranial direct current stimulation evaluation on fatigue and daytime sleepiness in Parkinson’s disease. Neurol. Sci. 38, 249–254 (2017).
Dong, X.-L. et al. A randomized controlled trial to explore the efficacy and safety of transcranial direct current stimulation on patients with post-stroke fatigue. Medicine 100, 45 (2021).
Chalah, M. A. et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J. Neurol. Sci. 372, 131–137 (2017).
Tecchio, F. et al. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J. Neurol. 261, 1552–1558 (2014).
Tecchio, F. et al. Brain plasticity effects of neuromodulation against multiple sclerosis fatigue. Front. Neurol. 6, 42 (2015).
Cancelli, A. et al. Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult. Scler. 24, 1366–1374 (2018).
Jamil, A. et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiol. 595, 1273–1288 (2017).
Dobbs, B. et al. Generalizing remotely supervised transcranial direct current stimulation (tDCS): Feasibility and benefit in Parkinson’s disease. J. NeuroEng. Rehabil. 15, 114 (2018).
Acknowledgements
We are grateful to all the patients who agreed to participate in the study and the clinicians who helped us with the patient recruitment. This study was funded by the grants Nos. NU22-D-133 and NU22-04-00192 of the MH CR and the Charles University research program Cooperatio-Neurosciences.
Author information
Authors and Affiliations
Contributions
M.K.: Conceptualization, Methodology, Investigation, Project Administration, Data Collection, Visualization, Writing Original Draft Preparation, Supervision. A.A.: Conceptualization, Project Administration, Data Collection, Writing Original Draft Preparation. N.B.: Data Collection, Writing Reviewing, and Editing. O.L.: Data Collection, Writing Reviewing, and Editing. V.R.: Conceptualization, Writing Reviewing, and Editing. Z.S.: Data Curation, Writing Reviewing, and Editing. K.O.: Visualization, Writing Reviewing, and Editing. T.N.: Conceptualization, Formal analysis, Writing Original Draft Preparation, Writing Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
ZS: Hospital České Budějovice, a.s., České Budějovice, Czechia; KO: Second Faculty of Medicine, Charles University, Prague, the Czech Republic. Other authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klírová, M., Adamová, A., Biačková, N. et al. Transcranial direct current stimulation (tDCS) in the treatment of neuropsychiatric symptoms of long COVID. Sci Rep 14, 2193 (2024). https://doi.org/10.1038/s41598-024-52763-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52763-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.