Abstract
In this study, electrochemical and theoretical studies were performed to explain the interaction mechanism between ponatinib (PNT), a third generation tyrosine kinase inhibitor, and dsDNA. The electrochemical part was conducted in phosphate-buffered saline (PBS) at physiological pH of 7.4 and in acetate buffer with a pH of 4.7, using square wave voltammetry. A boron-doped diamond electrode was used in a bulk-incubated solution. The theoretical part was investigated using computational methods, such as the semiempirical method PM7 and density functional theory (DFT). Significant differences in the electrochemical behavior of PNT in the presence of DNA confirmed the occurrence of interactions. The results obtained in the acetate buffer strongly suggested the preferential interaction of PNT with guanine residues. However, at physiological pH, it can be concluded that PNT interacts with dGua and dAdo in the dsDNA molecule. These results are consistent with outcomes from the theoretical studies, where quantum-chemical calculations showed that both electrochemically detectable nucleobases form hydrogen bonds with the drug. These bonds appeared to be stronger with guanine than with adenine. According to the computational studies, the dsDNA major groove is the energetically preferred site for the complexation of PNT.
Similar content being viewed by others
Introduction
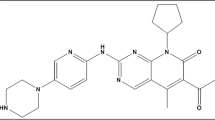
Tyrosine kinases (TK) are enzymes that act as ‘on’ and ‘off’ switches in many cellular processes, such as cell cycle regulation, proliferation, and cell death. They catalyze the transfer of the γ-phosphate of ATP to the tyrosine hydroxyl groups on target proteins1. Recent advances have implicated the role of TK in the pathophysiology of cancer. Although their activity is strictly controlled in normal, healthy cells, mutations or overexpression can lead them to acquire transforming functions that result in malignancy2. Constitutive oncogenic activation in cancer cells can be effectively blocked by selective tyrosine kinase inhibitors (TKI)2. Over the years, more than 20 TKIs effective against various cancers have been developed. Ponatinib (PNT, Fig. 1) is a multi-targeted third-generation tyrosine kinase inhibitor, approved in 2012 for the treatment of chronic myeloid leukemia (CML—a malignancy characterized by an elevated number of white blood cells3) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL). In contrast to first and second generation drugs, such as imatinib, dasatinib, or nilotinib, PNT is also active against BCR-ABL1 T315l and other mutations4,5. Nonetheless, considering the serious cardiovascular risks, heart failure, and hepatotoxicity associated with its use, PNT is generally limited to cases of second-generation drug-resistant leukemia and/or as third-line therapy in CML4,5,6. Recently, it was shown that PNT treatment resulted in a marked and dose-dependent increase in double-strand DNA break damage7.
Based on its effectiveness against chronic myeloid leukemia, ponatinib is undergoing intensive evaluation for its potential activity against other tumor types, such as liver cancer and malignant pleural mesothelioma7,8. From a chemical perspective several methods have been developed to date for the analytical determination of PNT and its main metabolite in plasma or urine1,9,10,11,12,13. Generally, chromatography has been the most commonly used technique for the determination of PNT and other TKIs. However, considering the negative ecological impact of chromatography, electrochemical techniques are becoming increasingly popular for such analyses. To our knowledge, only one study has so far demonstrated the voltammetric approach to PNT determination13. The use of electrochemical techniques is of great importance, not only in analytical studies but also in increasing our understanding of the mechanism of drug action and its pharmacokinetics. The most commonly used technique in redox mechanism evaluation is cyclic voltammetry (CV). The power of cyclic voltammetry lies in its ability to provide substantial information on the kinetics of heterogeneous electron transfer reactions and coupled chemical reactions, as well as the thermodynamics of redox processes14,15. Square wave voltammetry (SWV) is one of the most sensitive methods for the direct evaluation of chemical compound concentrations. It can be widely used for the trace analysis of biologically active compounds. An important advantage of SWV is the possibility to detect, in a single scan, the reversibility of the electron transfer. In the past decade, there has been great interest in the electrochemical approach toward drug-DNA interaction studies16,17,18. Such studies, and the proper interpretation of the results, have contributed to the elucidation of the mechanism by which DNA may be damaged by hazardous compounds.
Bearing in mind that ponatinib may cause DNA damage, and the mechanism of this process is still not clear, the goal of this work was the extensive examination of the interactions between PNT and double-stranded DNA (dsDNA). The interaction studies were performed using voltammetric techniques, mainly SWV. In general, electrochemical studies were based on the analysis of differences in the electrochemical behavior of PNT in the presence and absence of dsDNA. The investigation and clarification of the electrochemical behavior of PNT were also performed using CV and SWV. In addition, the structural properties of the PNT molecule and its complexes with dsDNA in aqueous solution, as well as the strength of interaction between them, were investigated using computational methods, such as the semiempirical method PM7 and density functional theory (DFT). The information obtained from these studies will surely contribute to a better understanding of the mechanism of interaction of PNT with nucleic acids.
Material and methods
Chemicals and reagents
Low molecular weight double-stranded DNA (dsDNA) was purchased from Sigma (Germany). A stock solution of dsDNA was prepared daily by dissolving the required mass of DNA powder in phosphate-buffered saline (PBS; Sigma, Germany)) with a pH of 7.4. The prepared solution was stored at 4 °C and was used no longer than 24 h after preparation. Ponatinib was purchased from LGC Standards (UK). A stock solution of PNT was prepared by dissolving an appropriate mass of the compound in ethanol and was then stored at 4 °C. Solutions with lower PNT concentrations were prepared by proper dilution of the stock solution. Various supporting electrolytes, namely acetate, phosphate, Britton–Robinson (BR) buffers, and phosphate buffered saline, were also prepared and used for the studies. All buffer components were purchased from Avantor (Poland) and were of analytical grade. Deionized and distilled water was used for the preparation of the solutions.
Apparatus and instrumentation
Electrochemical experiments were carried out using a μAutolab type III (Metrohm-EcoChemie, The Netherlands) coupled with an M164 electrode stand (mtm‐anko, Poland), operated with the GPES software (version no. 4.9). A conventional three-electrode system was used, comprising a boron-doped diamond working electrode (BDDE; Windsor Scientific Ltd, United Kingdom, diameter: 3 mm), a platinum wire as an auxiliary electrode, and an Ag/AgCl as a reference electrode. All studies were performed using a 0.01 L voltammetric cell.
The pH measurements of prepared buffer solutions were performed using a digital pH/mV/ion meter (Elmetron, Poland) with a combined glass electrode (Hydromet, Poland). Water was demineralized using a Polwater DL3 system (Labopol-Polwater, Poland).
Electrochemical measurements
Before each voltammetric measurement, the BDD electrode surface was polished with alumina slurry on a polishing cloth. After the polishing procedure, the electrode surface was carefully rinsed with distilled and deionized water. All measurements were performed in triplicate, at the ambient temperature of the laboratory (21–23 °C).
Cyclic voltammetry and square-wave voltammetry techniques were applied for the general characterization of the electrochemical behavior of PNT. However, interaction studies were performed using only square-wave voltammetry. The SWV conditions used were as follows: amplitude of 30 mV, frequency of 25 Hz, and step potential of 4 mV.
Interaction studies: measurements of PNT (at its fixed concentration) were conducted in the presence and absence of DNA in acetate buffer at pH 4.7 and PBS at pH 7.4. After recording the voltammogram for the blank solution, a proper amount of PNT and dsDNA were added to a voltammetric cell. In the incubation procedure, PNT was mixed with dsDNA and then incubated at room temperature for different periods. After the predetermined incubation time, voltammograms were recorded. As a control experiment, both dsDNA and PNT were also analyzed separately, without mixing each other, in the used supporting electrolyte.
Computational methods
The theoretical research included finding the most stable conformer of the PNT molecule, as well as examining the stability of its complexes with dsDNA. In both cases, a three-stage “hierarchical” approach was used, in which the accuracy of the theoretical method was increased at each subsequent stage. In the first step, the calculations were performed using the Amber99 force field of molecular mechanics. In the second step, the semiempirical method PM7 was used, and in the third step—the density functional theory (DFT) method M062X-GD319,20 with the basis set 6-31G(d,p) and the Polarizable Continuum Model (PCM)21 of water was applied.
The procedure used in the conformational search conducted for the PNT molecule alone is shown in Fig. S1 and described in Electronic Supporting Information (ESI) (see the section Procedure S1 and Fig. S2 in the ESI). The final structures were obtained from the DFT optimizations in water (PCM). Among them, two different models of PNT were chosen to test their binding to dsDNA: the lowest energy, compact structure (denoted below as PNT_LE) and the entirely different, stretched geometry (PNT_ST).
To create the initial models of the dsDNA:PNT complexes, two B-DNA dodecamers (double helix) with different sequences were taken from the RCSB Protein Data Bank: 1BNA (5′-D(*CP*GP*CP*GP*AP*AP*TP*TP*CP*GP*CP*G)-3 3′))22 and 119D ((5′-D(*CP*GP*TP*AP*GP*AP*TP*CP*TP*AP*CP*G)-3 3′))23. Using the HyperChem program24, both structures were terminated with hydrogen atoms. To mimic the presence of counterions in a real environment, one hydrogen atom was added to each phosphate group. Thus, the total charge of each molecule was equal to 0. The two dsDNA models were then fully optimized in the program MOPAC25 with the PM7 method and the MOZYME procedure. In these calculations, the effect of the solvent (water) was included by using the Conductor-like Screening Model (COSMO)26. For these structures, single point energy calculations were performed in water using the DFT method M062X-GD3/6-31G(d,p)/PCM.
To study the dsDNA:PNT complexes, for each of the two dsDNA models, four typical sites for binding PNT were considered: external binding (ExB), intercalation (InC), major groove (MaG) and minor groove (MiG). The final structures were obtained from partial optimizations (during which the dsDNA structure was kept frozen, while the PNT molecule was fully relaxed) performed with the M062X-GD3/6-31G(d,p) method in water (PCM) for the complexes selected after the semiempirical PM7 calculations. The calculations were performed using the Gaussian 16 package27. The details of the procedure used to explore the configurational space of the dsDNA:PNT complexes are presented in Fig. 2 and the section Procedure S2 in the ESI.
From the semiempirical calculations the complexation enthalpies Hcompl were calculated as:
where Hcompl is the complexation enthalpy, while \({H}_{{\text{dsDNA}}:{\text{PNT}}}^{{\text{OPT}}}\), \({H}_{{\text{dsDNA}}}^{{\text{OPT}}}\) and \({H}_{{\text{PNT}}\_{\text{LE}}}^{{\text{OPT}}}\) are, respectively, the heats of formation of the dsDNA:PNT complex and of the isolated dsDNA and PNT_LE molecules, fully optimized with the PM7 method in water (COSMO).
From the DFT calculations, the complexation energies Ecompl were computed according to a similar formula:
where Ecompl is the complexation energy, \({E}_{{\text{DNA}}:{\text{PNT}}}^{{\text{POPT}}}\) is the energy of partially optimized complex dsDNA:PNT, while \({E}_{{\text{dsDNA}}}^{{\text{SP}}}\)—the energy obtained from the single point DFT calculations for the dsDNA structures obtained after the PM7 optimization, \({E}_{{\text{PNT}}\_{\text{LE}}}^{{\text{OPT}}}\)—the DFT energy of the most stable conformer of ponatinib (PNT_LE) obtained in water (PCM).
Results and discussion
Voltammetric behavior of PNT on the BDD electrode
It is well known that the composition of the supporting electrolyte and its pH affect the observed electrode reaction and kinetics of the charge transfer process during voltammetric measurements. Thus, the pH-dependent oxidation process of PNT was initially studied in the wide pH range of 1.7–9.0 using BR buffers. As can be seen in Fig. 3, PNT signals were observed through the whole pH range. It was found that PNT provided one oxidation peak at a potential of approximately 1.3 V in a strong acidic medium. For higher pH values, a second signal appeared. As the acidity of the supporting electrolyte decreased, the peaks shifted toward less positive potential values, and the second peak became better separated. For pH 5.0 and higher, the separation of the two signals was sufficient for reliable measurements of the peak currents. For the first peak (the one of the right), the slope of the Ep–pH dependence was 13 mV pH−1, showing that both protons and electrons are involved in the oxidation process of PNT, but it is difficult to determine the ratio between them. Such results indicated a complex electrode process presumably involving a multi-step reaction28. For the second peak (the one of the left), the slope of the same dependence was 51 mV pH−1, which is close to the theoretical value of 59 mV pH−1. This result suggests the participation of an equal number of protons and electrons in this part of the reaction.
Bearing in mind the sufficient separation of PNT signals for pH approximately 5.0 and higher, the influence of other buffers (such as acetate, phosphate, phosphate buffered saline; pH range of 4.5–8.0) on the analyte signals was also tested (data not shown). In all the tested buffers, the obtained peak currents and the separation of the signals were satisfactory and analogous to those obtained in the BR buffer. No significant improvement or deterioration of recorded signals was observed. Therefore, considering the fact that the main aim of the present work was DNA interaction studies, further measurements were performed in phosphate buffered saline at physiological pH and acetate buffer at pH 4.7. These buffers are commonly used for interaction studies3,15,29.
In the next experimental step, cyclic voltammetry was applied to gain insight into the electrochemical oxidation process of PNT using both chosen supporting electrolytes, i.e. PBS and acetate buffer. As can be seen in Fig. 4a PNT exhibited either two or one anodic peaks, depending on the pH of the supporting electrolyte. In PBS two well-separated signals were observed, which are in excellent agreement to the SWV voltammograms. In acetate buffer, only one signal was observed ca. 1.2 V, suggesting that during the measurement, two signals visible in square wave experiments overlapped. Based on the cyclic voltammograms it may be also observed that in the reverse scan, no corresponding cathodic signals were visible. Thus, it may be concluded that the oxidation of ponatinib on BDD electrode is an irreversible process. This was also confirmed by using the SW voltammetry, in which the observed forward and backward components of PNT signals tipped in the same direction for both buffers (Fig. 4b and c), thus proving the irreversible character of the electrochemical oxidation of PNT.
(a) Cyclic voltammograms of PNT (1.0 × 10–4 mol L−1), obtained at scan rate of 50 mV s−1, black line represents the voltammograms recorded in acetate buffer of pH 4.7, gray line in PBS of pH 7.4. Inset: Represents the same voltammograms but in the wider potential range; (b) SW voltammograms of PNT (1.0 × 10−5 mol L−1, net current—solid line) and the forward (dashed line) and backward (dotted line) components of PNT signals recorded in acetate buffer of pH 4.7; (c) SW voltammograms of PNT (1.0 × 10−5 mol L−1, net current—solid line) and the forward (dashed line) and backward (dotted line) components of PNT signals recorded in PBS of pH 7.4.
In order to check whether the oxidation of PNT is a diffusion- or adsorption-controlled process the scan rate studies were performed. The measurements were carried out within the scan rate (v) range from 50 to 500 mV s−1. A linear dependence between the peak currents (Ip) and scan rates were observed in the case of both supporting electrolytes, suggesting adsorption-controlled nature of the PNT electrode processes. This evidence was further verified with construction of the logarithmic plot Ip vs. log v. The log Ip–log v slopes were found to be 0.71 and 0.97 for PBS and acetate buffer, respectively, where values close to 1.0 are specific for processes controlled by adsorption14,30. Based on these results, it can be stated that electrochemical process in the acetate buffer is controlled by adsorption, whereas in phosphate buffered saline, a mixed diffusion-adsorption controlled process was observed.
Electrochemical studies of PNT-dsDNA interaction
The studies of the interaction between PNT and DNA were initially performed by incubating a solution of 5.0 µmol L−1 PNT with 80 mg L−1 of dsDNA. Both supporting electrolytes, i.e., acetate buffer of pH 4.7 and PBS of pH 7.4 were used for that purpose; however, the results obtained in PBS were insufficiently repeatable to draw reliable conclusions. Thus, the following results are the outcome of interaction studies performed via the incubation procedure in acetate buffer. The SW voltammograms were recorded after different times of incubation (10, 30, 60, and 90 min). The surface of the boron doped diamond electrode was renewed between every measurement to avoid the blockage of the electrochemically active electrode surface via adsorption of the oxidation products of PNT and/or dsDNA. After the addition of dsDNA to the voltammetric cell with PNT, significant changes in recorded voltammograms were observed, and the extension of incubation time deepened the changes (Fig. 5). As can be seen in Fig. 5, PNT exhibited two signals, the left one at approximately 1.15 V and the right one at approximately. 1.25 V (dashed line). After 10 min upon the addition of dsDNA, the left signal significantly increased, the right one decreased, and one additional signal at approximately 1.4 V appeared. The left signal, after the initial increase, gradually decreased with the incubation time, while the right signal (approximately 1.25 V) current progressively decreased in a time-dependent manner. It is worth noting here that dsDNA is an electrochemically active compound, and in the applied potential range two signals are expected31, one corresponding to the oxidation of deoxyguanosine (dGua) at a lower potential value, and the second one to the oxidation of deoxyadenosine (dAdo) at a higher potential. In the present work, the electrochemical behavior of dsDNA was recorded as a control experiment, and two signals were observed at approximately 1.05 V and 1.4 V (Fig. 6a, dotted line). Thus, based on the results shown in the Fig. 5, it may be stated that the peak current at approximately 1.15 V initially increased after the short incubation with dsDNA due to some conformational changes in PNT molecules, which initially facilitated the oxidation of the drug by exposing the group responsible for oxidation. The further gradual decrease of the oxidation peak indicated a preferential interaction between PNT and dGua residues in the dsDNA. It can also presumed that the signal at 1.4 V corresponds to the oxidation of dAdo. As no significant changes occurred with the signal at approximately 1.4 V during the incubation, it may be presumed that the presence of PNT did not affect the oxidation of deoxyadenosine, and condensation or aggregation of DNA strands was not observed32. During the evaluation of PNT-dsDNA interaction in the incubated solution, no significant shifts of the peak potentials were observed. As a shift of the peak potential is commonly observed in intercalative types of interaction, we can rather exclude this type of interaction between PNT and DNA. What is more, DNA oxidative damage was not detected, as oxidation signals of 8-oxoguanine or 2,8-dihydroxyadenine32 were not observed, which means that PNT did not induce oxidative damage to double-stranded DNA.
(a) SW voltammograms of PNT (dashed, black line), PNT in the presence of dsDNA (solid, gray line), and dsDNA (dotted, black line) recorded in acetate buffer of pH 4.7; cPNT = 5.0 µmol L−1, cdsDNA = 80 mg L−1; (b) SW voltammograms of PNT (dashed, black line) recorded in PBS of pH 7.4, in the presence of an increasing amount of dsDNA (solid, gray lines), cPNT = 5.0 µmol L−1, cdsDNA = 10—80 mg L−1; (c) SW voltammograms of PNT (dashed, black line) recorded in acetate buffer of pH 4.7, in the presence of increasing amount of dsDNA (solid, gray lines), cPNT = 5.0 µmol L−1, cdsDNA = 10–80 mg L−1; and (d) SW voltammograms of dsDNA and its increasing concentration recorded in acetate buffer of pH 4.7, cdsDNA = 10–80 mg L−1.
Next, the SW voltammograms of PNT (at its fixed concentration) in the absence and presence of increasing amount of dsDNA were recorded in an acetate buffer of pH 4.7 and PBS of 7.4; the obtained results were illustrated in Fig. 6. In Fig. 6d, SW voltammograms of dsDNA and its increasing amount in acetate buffer were shown for comparison purposes. In these experiments, the signals were recorded immediately after the addition of dsDNA to the PNT solution. The voltammograms recorded in the acetate buffer of pH 4.7 (Fig. 6c) significantly differed from the voltammograms obtained in PBS of pH 7.4 (Fig. 6b). In the second case, both observed signals gradually decreased upon the addition of increasing amounts of dsDNA. What is more, both signals slightly shifted toward more positive potential values when compared to PNT signals. In acetate buffer, the right signal increased whereas the left decreased upon the addition of increasing amounts of dsDNA. Moreover, one additional signal appeared at approximately 1.4 V, and its peak current gradually increased upon the addition of dsDNA. In the acetate buffer, the signal shift was so slight as to be insignificant. In conclusion, both supporting electrolytes allowed us to observe the occurring interaction; however, based on the obtained results, it can be concluded that the pH of the supporting electrolyte strongly affected the direction of the interaction. In general, PNT induced conformational modifications in dsDNA molecules33. The decrease of the signals and the shift observed in PBS may be correlated with DNA double helix condensation and PNT intercalation33. However, the observed shift in the peak potential is much smaller than shifts described by other authors for intercalation34, making it rather difficult to definitively identify the observed interaction type. As signal decrease was observed in the case of both signals in PBS, and no additional signal appeared, it can be concluded that PNT interacts with both dGua and dAdo at physiological pH. Whereas the dependencies observed in the acetate buffer strongly suggested the interaction between PNT and guanine residues in the dsDNA17, which is consistent with the results obtained in the incubation procedure. In the acidic environment, as no significant shift in the peak potential was observed, it can be stated that intercalation likely did not occur.
Results of the DFT calculations
More detailed information on the structural properties of the tested compounds, as well as on the stability of the dsDNA:PNT complexes and the intermolecular interactions occurring within them, can be obtained using theoretical chemistry methods. Since the electrochemical measurements were performed in an aqueous solution, the solvent effect was also incorporated into the quantum chemical calculations using implicit models.
First, the properties of PNT in water were explored through an extensive conformational search. The final M062X-GD3/6-31G(d,p)/PCM results allowed the identification of the most stable structure of PNT, but at the same time revealed that the energy of other conformers is not much higher, so they may also be present in the solution. Therefore, for further studies on complexes of dsDNA:PNT, two different PNT structures were selected, both of which are shown in Fig. 7. The first one, PNT_LE, is the lowest energy conformer in water as indicated by the results of DFT calculations. It has a twisted structure, with the methylpiperazine group located above the two aromatic rings. The second, PNT_ST, is the first fully stretched structure found among all tested conformers ordered by increasing energy. It is worth mentioning that, in this order, PNT_ST is numbered 157, and its total energy is higher than that of PNT_LE by only 8.4 kcal mol−1. Thus, within this relatively narrow energy window, there are many other intermediate structures between PNT_LE and PNT_ST.
The two dodecamers (double-helix), based on the crystal structures (1BNA and 119D) and optimized with the semiempirical method PM7 in water, which were used to create initial models of the dsDNA:PNT complexes, are shown in Fig. 8. As can be seen, due to the sequence differences, the two strands of B-DNA in the model 1BNA appear to be closer than in 119D, which may have some effect on stability of the dsDNA:PNT complexes.
The four sites in dsDNA considered for PNT binding are depicted in Fig. 9. The final PM7 complexation enthalpies (Hcompl) and DFT complexation energies (Ecompl) obtained at these sites with the two dsDNA models and two PNT conformers are compared in Fig. 10. The most stable structures, as indicated by the DFT results for the four models of the dsDNA:PNT complex, are presented in Fig. 11a, while all structures corresponding to the results shown in Fig. 10, are displayed in Figs. S3–S6 in the ESI.
Structures of the most stable configurations obtained from the DFT calculations for the four different models of the dsDNA:PNT complexes (a) and the corresponding schematic images of the dsDNA sequence, on which the nucleobases closest to PNT are outlined with a blue line (b). In (a), the dsDNA atom colors are the same as in Fig. 8, while the PNT molecule is colored pink. Nucleobases symbols in (b): A—adenine, G—guanine, C—cytosine, T—thymine.
As can be seen, the results obtained with both PM7 and DFT methods indicate that in all four sites the PNT molecule forms stable complexes with dsDNA, regardless of the DNA model used. For a given B-DNA sequence and a specific PNT conformer, the trends in the PM7 enthalpies Hcompl and the DFT complexation energies Ecompl for the different interaction sites are similar, but the Hcompl values are significantly more negative than the corresponding Ecompl values.
According to the PM7 results, for both dsDNA models, the conformer PNT_ST is more strongly bound in all four sites than PNT_LE. A similar tendency is observed in the DFT complexation energies for 119D:PNT, while for 1BNA:PNT the trend is the opposite, except for ExB_ST, for which Ecompl is lower than that for ExB_LE. Thus, the DFT results suggest that the binding of PNT by dsDNA depends on the order of nucleotides in the DNA. In the case of 1BNA:PNT, the major and minor grooves are preferred sites for the complexation of both PNT_LE and PNT_ST conformers. The most negative Ecompl of − 56.6 kcal mol−1 is obtained for the MaG_LE complex, but for the other sites it differs by only a few kilocalories per mole. An exception is the InC_ST configuration, for which the complexation energy is only − 6.5 kcal mol−1. In contrast, this particular configuration appears to be very stable in the case of 119D:PNT, where it has the complexation energy of − 59.7 kcal mol−1. As already mentioned, the stretched structure of PNT in general fits much better to the 119D double helix; the most stable configuration for 119D:PNT is MaG_ST with the complexation energy of -65.6 kcal mol−1. The significant difference between the InC_ST complexes formed by PNT_ST with the two dsDNA models may be due to the fact that in 1BNA the two strands are more tightly coiled than in 119D, so intercalation of PNT into 1BNA causes stronger deformation of the latter. Among the complexes of 119D, a surprisingly small Ecompl (-0.2 kcal mol−1) was obtained for the InC_LE configuration. In this case, steric hindrance may be responsible for so small stabilization of this complex.
A closer inspection of the most stable structures indicated for each dsDNA:PNT model by the DFT results (Fig. 11a) allows for the identification of the nucleobases that directly interact with PNT (Fig. 11b). The specific arrangement of the two electrochemically detectable nucleobases, guanine and adenine, is illustrated in Fig. 12. PNT is a relatively large molecule, and as a result, it can interact with several nucleobases. As expected, for the stretched conformer PNT_ST, there are more such interactions. In the case of guanine, the interaction most often occurs via its oxygen atom, which is usually involved in hydrogen bonding with the H-N or H-C bonds of PNT. The O…H distances in the O…H–N hydrogen bonds range from 1.96 (upper guanine in 119D MaG_ST in Fig. 12) to 2.37 Å (upper guanine in 1BNA MaG_LE), while in the O…H–C hydrogen bonds: from 2.32 (lower guanine in 119D MaG_ST) to 2.42 Å (upper guanine in 1BNA MaG_ST). According to the Jeffrey’s categorization35,36, these are moderate or weak hydrogen bonds but, considering their number, they can be quite important and may have some effect on the electrochemical activity of dsDNA. Adenine forms various hydrogen bonds, but their geometrical parameters (e.g. longer H…X distances) suggest that they are weaker than those formed by guanine. The most prominent hydrogen bond observed for adenine is the one in the complex 1BNA MaG_LE, between its NH2 hydrogen atom and the oxygen atom in PNT (the H…O distance is 2.20 Å). In other configurations, they are formed between one of the nitrogen atoms in adenine and H-C bonds in PNT. Of course, apart from hydrogen bonds, other interactions between dsDNA and PNT (such as electrostatic and dispersion forces) also contribute to the stability of the complex.
Magnification of fragments of the most stable complexes dsDNA:PNT shown in Fig. 11; the PNT molecule (always on the right) and the nearest adenine (A) and guanine (G) are rendered with spheres and cylinders. Atom colors are the same as in Figs. 7 and 8. The black dashed lines connect the closest atoms in each base and PNT.
Conclusion
The interaction of PNT, a third-generation tyrosine kinase inhibitor, with double-stranded DNA was investigated for the first time using electrochemical and computational techniques. The voltammetric studies were conducted using two approaches. The first approach involved experiments performed in an incubated solution, where both the concentration of the analyte and DNA were fixed, and different incubation times were tested. The second approach relied on measurements performed in a solution containing PNT and dsDNA in different concentration ratios. Based on the voltammetric results, obtained at physiological pH, it was observed that PNT interacted with both dGua and dAdo residues in dsDNA molecules. In an acidic environment, voltammetric data suggest that PNT interacts mainly with dGua residues. It is known that evaluating the type of interaction with electrochemical techniques should be confirmed with other methods, as they only allow for the formulation of a hypothesis based on the peak potential shift. In this study, conclusions drawn from the peak potential shift may be summarized as follows: in the acidic environment, since no shift in the peak potential was observed, it can be concluded that intercalation likely did not occur. Hence, groove binding is suggested to be more prevalent. At physiological pH, a shift in the peak potential value was observed; however, it was so insignificant that it was not possible to draw a definitive conclusion about the ongoing interaction type.
The results of the theoretical research confirm that groove binding of PNT is energetically more favorable. According to the semiempirical PM7 results, the stretched structure PNT_ST is, in general, more strongly bound than its most stable structure PNT_LE and in both forms of dsDNA the major groove is the preferred site for the complexation of PNT. The complexation enthalpies corresponding to the MaG_ST configurations with the 1BNA and 119D models of dsDNA are − 77 and − 80 kcal mol−1, respectively. The absolute values of the DFT complexation energies are smaller than those obtained from the PM7 calculations and suggest that the stability of complexes may depend on both the structure of PNT and the order of nucleotides in DNA. For both dsDNA models, the MaG site is favored, but in the case of 1BNA the complex with PNT_LE is the most stable (Ecompl = − 57 kcal mol−1), while for 119D the binding of PNT_ST is energetically more profitable (Ecompl = − 66 kcal mol−1). Nevertheless, given the relatively minor differences in Ecompl for some of the other alternative configurations, it is likely that these configurations will also form in parallel under certain conditions. Analysis of the structures of the complexes obtained from quantum-chemical calculations showed that both electrochemically detectable nucleobases, adenine and guanine, form hydrogen bonds with the drug. The geometric characteristics of these bonds suggest that those formed with guanine are stronger than those formed with adenine.
In general, based on both voltammetric and computational results it can be concluded that an intercalative type of interaction can likely be excluded, whereas major groove binding is more probable to occur. Both approaches also suggest that PNT interacts with guanine and adenine residues in dsDNA molecules, with a preference toward guanine.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Kadi, A. A., Darwish, H. W., Attwa, M. W. & Amer, S. M. Detection and characterization of ponatinib reactive metabolites by liquid chromatography tandem mass spectrometry and elucidation of bioactivation pathways. RSC Adv. 6, 72575–72585. https://doi.org/10.1039/C6RA09985H (2016).
Paul, M. K. & Mukhopadhyay, A. K. Tyrosine kinase—role and significance in cancer. Int. J. Med. Sci. 1, 101–115 (2004).
Diculescu, V. C., Vivan, M. & Brett, A. M. O. Voltammetric behavior of antileukemia drug glivec. Part III: In situ DNA oxidative damage by the glivec electrochemical metabolite. Electroanalysis 18, 1963–1970. https://doi.org/10.1002/elan.200603602 (2006).
Zeng, P. & Schmaier, A. Ponatinib and other CML tyrosine kinase inhibitors in thrombosis. Int. J. Mol. Sci. 21, 1–16. https://doi.org/10.3390/ijms21186556 (2020).
Sparidans, R. W., Kort, A., Schinkel, A. H., Schellens, J. H. M. & Beijnen, J. H. Liquid chromatography–tandem mass spectrometric assay for ponatinib and N-desmethyl ponatinib in mouse plasma. J. Chromatogr. B 1023–1024, 24–29. https://doi.org/10.1016/j.jchromb.2016.04.049 (2016).
Hoy, S. M. Ponatinib: A review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs 74, 793–806 (2014).
Yang, Y.-W. et al. Ponatinib is a potential therapeutic approach for malignant pleural mesothelioma. Exp. Lung Res. 47, 9–25. https://doi.org/10.1080/01902148.2020.1836691 (2021).
Liu, C. et al. Ponatinib inhibits proliferation and induces apoptosis of liver cancer cells, but its efficacy is compromised by its activation on PDK1/Akt/mTOR signaling. Molecules 24, 1363 (2019).
Merienne, C. et al. High throughput routine determination of 17 tyrosine kinase inhibitors by LC–MS/MS. J. Pharm. Biomed. Anal. 150, 112–120. https://doi.org/10.1016/j.jpba.2017.11.060 (2018).
Huynh, H. H. et al. Development and validation of a simultaneous quantification method of 14 tyrosine kinase inhibitors in human plasma using LC-MS/MS. Ther. Drug Monit. 39, 43–54. https://doi.org/10.1097/FTD.0000000000000357 (2017).
Kadi, A. A., Darwish, H. W., Attwa, M. W. & Amer, S. M. Validated LC-MS/MS method for the quantification of ponatinib in plasma: Application to metabolic stability. PLoS ONE 11, e0164967. https://doi.org/10.1371/journal.pone.0164967 (2016).
Attwa, M. W., Kadi, A. A., Darwish, H. W., Amer, S. M. & AlRabiah, H. LC-ESI-MS/MS identification and characterization of ponatinib in vivo phase I and phase II metabolites. Clin. Chim. Acta 485, 144–151. https://doi.org/10.1016/j.cca.2018.06.035 (2018).
Koszelska, K., Ciesielski, W. & Smarzewska, S. First electrochemical approach to voltammetric behavior and sensing of anticancer drug ponatinib. J. Electrochem. Soc. 169, 046523 (2022).
Gosser, D. K. Cyclic Voltammetry (VCH, 1994).
Dogan-Topal, B., Bozal-Palabiyik, B., Ozkan, S. A. & Uslu, B. Investigation of anticancer drug lapatinib and its interaction with dsDNA by electrochemical and spectroscopic techniques. Sens. Actuators B Chem. 194, 185–194. https://doi.org/10.1016/j.snb.2013.12.088 (2014).
Oliveira Brett, A. M. et al. Comprehensive Analytical Chemistry Vol. 49, 413–437 (Elsevier, 2007).
Diculescu, V. C. & Oliveira-Brett, A. M. In situ electrochemical evaluation of dsDNA interaction with the anticancer drug danusertib nitrenium radical product using the DNA-electrochemical biosensor. Bioelectrochemistry 107, 50–57. https://doi.org/10.1016/j.bioelechem.2015.10.004 (2016).
Shahzad, S. et al. Electrochemical, spectroscopic and molecular docking studies on the interaction of calcium channel blockers with dsDNA. Bioelectrochemistry 127, 12–20. https://doi.org/10.1016/j.bioelechem.2018.12.007 (2019).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts 120, 215–241. https://doi.org/10.1007/s00214-007-0310-x (2008).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799. https://doi.org/10.1002/jcc.20495 (2006).
Tomasi, J., Mennucci, B. & Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 105, 2999–3094. https://doi.org/10.1021/cr9904009 (2005).
Drew, H. R. et al. Structure of a B-DNA dodecamer. Conformation and dynamics. Proc. Natl. Acad. Sci. USA 78, 2179–2183 (1981).
Leonard, G. A. & Hunter, W. N. Crystal and molecular structure of d(CGTAGATCTACG) at 2.25 A resolution. J. Mol. Biol. 234, 198–208 (1993).
HyperChem(TM) Professional, version 8.0.10. (Hypercube, Inc, 2011).
Stewart, J. J. P. MOPAC2016, Stewart Computational Chemistry, ver. 21.186L (2016) http://OpenMOPAC.net.
Klamt, A. & Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2, 799–805. https://doi.org/10.1039/P29930000799 (1993).
Gaussian 16, Revision C.01 (Gaussian, Inc., 2016).
Stanković, D., Mehmeti, E., Svorc, L. & Kalcher, K. New electrochemical method for the determination of β-carboline alkaloids, harmalol and harmine, in human urine samples and in Banisteriopsis caapi. Microchem. J. 118, 95–100. https://doi.org/10.1016/j.microc.2014.08.007 (2015).
Morawska, K., Popławski, T., Ciesielski, W. & Smarzewska, S. Interactions of lamotrigine with single- and double-stranded DNA under physiological conditions. Bioelectrochemistry 136, 107630. https://doi.org/10.1016/j.bioelechem.2020.107630 (2020).
Guziejewski, D. et al. Lactofen—electrochemical sensing and interaction with dsDNA. Electroanalysis 30, 94–100. https://doi.org/10.1002/elan.201700472 (2018).
Oliveira-Brett, A. M., Piedade, J. A. P., Silva, L. A. & Diculescu, V. C. Voltammetric determination of all DNA nucleotides. Anal. Biochem. 332, 321–329. https://doi.org/10.1016/j.ab.2004.06.021 (2004).
Buoro, R. M. et al. In situ evaluation of gemcitabine–DNA interaction using a DNA-electrochemical biosensor. Bioelectrochemistry 99, 40–45. https://doi.org/10.1016/j.bioelechem.2014.05.005 (2014).
Chiorcea-Paquim, A.-M. & Oliveira-Brett, A. M. DNA electrochemical biosensors for in situ probing of pharmaceutical drug oxidative DNA damage. Sensors https://doi.org/10.3390/s21041125 (2021).
Temerk, Y. & Ibrahim, H. Binding mode and thermodynamic studies on the interaction of the anticancer drug dacarbazine and dacarbazine–Cu(II) complex with single and double stranded DNA. J. Pharm. Biomed. Anal. 95, 26–33. https://doi.org/10.1016/j.jpba.2014.02.010 (2014).
Jeffrey, G. A. An Introduction to Hydrogen Bonding (Oxford University Press, 1997).
Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 41, 48–76. https://doi.org/10.1002/1521-3773(20020104)41:1%3c48::AID-ANIE48%3e3.0.CO;2-U (2002).
Acknowledgements
This work was supported by the University of Lodz, Poland (Grant No. B2311113000177.07). DFT calculations have been carried out using resources provided by Wroclaw Centre for Networking and Supercomputing (https://wcss.pl), grant No. 443. Sylwia Smarzewska and Kamila Koszelska want to thank Dariusz Guziejewski for the scientific discussion.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.K., A.I., S.S.; Formal analysis: K.K., A.I.; Investigation: K.K., A.I., S.S.; Methodology: K.K., A.I.; Software: K.K., A.I.; Validation: K.K., A.I.; Visualization: K.K., A.I.; Funding acquisition: S.S.; Project administration: S.S.; Writing—original draft: K.K., A.I., S.S.; Writing—review and editing: K.K, A.I.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smarzewska, S., Ignaczak, A. & Koszelska, K. Electrochemical and theoretical studies of the interaction between anticancer drug ponatinib and dsDNA. Sci Rep 14, 2278 (2024). https://doi.org/10.1038/s41598-024-52609-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52609-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.