Abstract
This paper aimed to analyse the potato cultivar’s response to physiological, biochemical performance, yield parameters and soil physiochemical properties when subjected to quicklime (un)treated acid mine drainage (AMD) irrigation. A randomized design experiment was conducted with five water treatment levels: TW1; TW2; TW3; TW4 to TW5 replicated four times. The results showed that the quicklime treatment increased the pH of the AMD water, reduced the concentration of EC, NO3−, SO42− and ameliorated heavy metals. However, unsafe levels of heavy metals above the maximum permissible (WHO/FAO) levels were found in Pb, Mg and Mo for water (TW4 and TW5), while As, Cd and Cr for soils (ST4 and ST5) respectively. For potato tubers (TT4 and TT5) concentrations of As, Cd, Cr, and Pb were above the maximum levels. Stomatal conductance, chlorophyll content and yield parameters responded positively by increasing significantly on TW4 and TW5 treatments, but negatively (reduced) towards TW2 and TW3 treatments. A higher bioaccumulation factor was obtained for Zn ˃ Cu ˃ Mg ˃ Pb ˃ Mn, which was an indication of the contamination status of soil, with Zn being more concentrated than other metals. The findings indicate that quicklime-treated AMD is usable for potato irrigation with regular monitoring of heavy metal levels and strict observation of water reuse protocols. The use of this large source of ameliorated (AMD) water will go a long way in improving food security in South Africa and/or in countries where agriculture production is around mining areas.
Similar content being viewed by others
Introduction
With increased levels of water scarcity and its supply variability, the ability of the world to meet the growing demand for food for more people with fewer available resources per capita has become a major policy concern1. The scarcity has led to the need to consider the utilisation of alternative water sources including those discharged from industrial, commercial, and domestic activities. Interestingly, the practice has been reported to increase in recent years, particularly in countries where access to or availability of freshwater is limited. The utilization of wastewater not only conserves freshwater resources for domestic purposes such as drinking water and irrigation, but it also reduces pollution in adjacent bodies of water and the environment2,3,4,5. South Africa is ranked among 30 of the driest countries in the world and is expected to experience severe water scarcity in the future. To increase the sources of water, there are several proposals for alternatives including the re-use of treated wastewater. The country hosts plenty of abandoned and operational mines that drain acid mine drainage (AMD) water mostly into proximal waterbodies7,8,9. Although mining is a major contributor to the country's GDP, its activities can result in the release of by-products that have negative impacts on the fauna and flora of environments that surround mines10. As a result, there is a critical need to reduce toxins linked to AMD by implementing appropriate technology, eliminating waste, and implementing reuse and recycling strategies. As a result, there is a critical need to reduce toxins linked to AMD by implementing appropriate technology, eliminating waste, and implementing reuse and recycling strategies. When treated, AMD water can potentially be used for multiple purposes including the irrigation of crops and serve as an innovative solution for the current and future water crisis11.
In South Africa, agriculture accounts for more than 60% of water utilisation for its irrigation practices12. However, several published studies with varying successes have reported the use of (un)treated AMD for agricultural purposes9,13,14,15,16,17,18,19,20,21. Although some of the results have shown that it can have positive effects, in the main, the majority of the literature revealed negative effects largely caused by the activity of heavy metals. In South Africa20, reported that potato tubers (Solanum tuberosum L.) of Fianna and Lady Rosetta cultivars accumulated unsafe levels of Ni, Zn, and Sr when irrigated with Fly ash-treated AMD water. A published study by22 recorded higher concentrations of Cr, Ni, Cu, As, Cd, and Pb in potato (Solanum tuberosum), red onion (Allium cepa), and wild carrot (Daucus carota) established in multi-metal-contaminated soils relative to that recommended by the FAO/WHO, an indication that consumption of such crops could pose a risk. The findings from 23 showed significantly higher concentrations of Cd and Pb in rice grain, vegetables, and soybeans compared to the maximum permissible level in the vicinity of the Dabaoshan mine, located in southern China. When irrigating with mine wastewater24, revealed that the grain of winter wheat had significantly higher Cr, Pb, Cu and Zn relative to their counterparts irrigated with tapwater, thus implying that the irrigation with mine wastewater could result in the accumulation of heavy metals in wheat grain. When plants are exposed to stressful environmental conditions, their physiological and biochemical performances are altered25. For instance, a study by 26 examined the effects of irrigating wheat with mine wastewater (leachate of coal gangue, coal-washing wastewater, and precipitated coal-washing wastewater) on soil enzymes, physiological properties, and potential risks of heavy metal contamination. The results showed that mine wastewater irrigation caused adverse effects on rhizospheric enzymes, physiological properties, and grain yield of the winter wheat. Similarly, when wheat was supplied with mine wastewater, its growth, grain yield, leaf area, dry mass per stem, root activity, and net photosynthetic rate were markedly decreased relative to that irrigated with tap water24. However, in another study27, reported a significant increase in the height, spike length, grains spike and grain yield of wheat grown with the application of quicklime.
Potatoes (Solanum tuberosum L.) along with rice (Oryza saliva L.) and wheat (Triticum aestivum L.), are a significant staple food in various parts of the world and require an adequate supply of water to achieve a high-quality yield28. One of the most critical factors affecting potato yield and quality is the supply or availability of good unpolluted soil water29. In their results30, found that the potato crop is highly vulnerable to water stress, particularly during the tuber formation and tuber bulking growth stages and these may decrease the yield. Overall, the foremost factor that negatively influences the production of potatoes is the type of irrigation31. As one of the major crops, the cultivated potato is consumed each day by millions of people and the quality of the potato, thus, affects human health greatly. Therefore, the transfer of toxic elements from soils to plants is of great concern. Therefore, heavy metal contamination in agricultural soils, their transfer in a soil-potato system and physiological response have been of increasing concern.
In the present study, two potato cultivars Marykies and Royal were used for experimental investigation and a 3:1:1 Culterra topsoil mixture was irrigated with quicklime-treated AMD water. There are limited scientific experimental reports on the evaluation of potential heavy metals on soil properties, physiological parameters, and biochemical performance on Marykies and Royal potato cultivars when subjected to quicklime-treated acid mine drainage irrigation under greenhouse conditions.
Research objectives
This study analysed the potato cultivar's physiological, biochemical and yield parameters response and soil physicochemical properties when subjected to quicklime treatments of AMD irrigation.
The relevance of the study
This study’s findings are timely because South Africa has a water shortage and needs to utilize AMD to irrigate food crops. According to studies 32, AMD treated with lime can be used as an alternate source of irrigation water for food crops. In essence, irrigation of food crops with AMD treated with quicklime can elicit varying physiological and metabolic responses as well as rhizospheric microbial richness and diversity. It is therefore the uniqueness of this combination that merits reporting. Research on the irrigation of potatoes with quicklime-treated AMD produces results that serve as a blueprint to guide the effects such an alternative technology has on the quality of potatoes. This study seeks to address the potential of quicklime-treated AMD water for the irrigation of commercial potato cultivars. It will enable making informed decisions related to the use of treated AMD water in crop growing practices under changing climatic conditions and water deficit seasons of the current times.
Materials and methods
First, we confirm that all methods including experiments and analyses were performed in accordance with relevant protocols, guidelines, and regulations. These methods were approved by the University of South Africa, and we also confirm that informed and ethical consent was obtained from all relevant institutions.
Experimental study area, water sampling, pre-treatment, and physicochemical analysis
The greenhouse experimental study was conducted at the University of South Africa (UNISA), Florida Science Campus, Johannesburg, Gauteng Province (S 26° 10′ 30″ S, 27° 55′ 22.8″ E) over two growing seasons, the first season from August to November 2018 and second season from February to May 2019. The greenhouse's temperature ranged from 20 to 25 degrees, which was aligned with the requirements for potato growth. Potato seeds were donated from McCain Delmas Mpumalanga, South Africa. Before planting, water samples were collected from a gold mine, Mogale City in Gauteng and accurately measured into 2 L (L) containers. A total of five experimental treatments were used, however, only two different solution ratios (amount (g) of quicklime (QL) and 100 percentage of AMD) as shown below:
-
1.
Treatment 1 (TW1) = 0:0, Tapwater.
-
2.
Treatment 2 (TW2) = 0:100, AMD water.
-
3.
Treatment 3 (TW3) = 1:100, 1 g Quicklime and AMD water.
-
4.
Treatment 4 (TW4) = 2:100, 2 g Quicklime and AMD water
-
5.
Treatment 5 (TW5) = 2:75:100, 2 g Quicklime, 75% FA and AMD water.
Before irrigating with (un)treated AMD, as alluded to above, the water was treated with quicklime powder following33 protocol. Quicklime (QL) was obtained from All Lime Services Pty located at Elandsfontein in Johannesburg. For the Lab segment of the experiment, 2.5 g weight of QL was added into a 1 L beaker that contained 100% AMD water (1 g of QL equivalent to 1 L of AMD water). The AMD water that contained QL was stirred using a mechanical stirrer. The method to carry out the experiments is known as the Jar test, a well-known active treatment technique (Fig. 1). After the QL was added, the AMD water colour changed to orange and precipitation was simply observed. Triplicate water samples from each experimental treatment level were denoted as TW1 to TW5. The physicochemical characteristics which included pH, electric conductivity (EC), DO, NO3–, SO42– were recorded using a pH meter (A329, Thermo Scientific, Indonesia) and ICP-EOS (Agilent Technologies 700 series ICP-OES). The concentration of micronutrients (Cu, Fe, Mn, and Zn) and heavy metals (As, Cd, Co, Cr, Ni, Mg, Mn, and Mo) were analysed using an inductively coupled optical emission spectrometer (Agilent Technologies 700 series ICP-OES, USA).
Jar test showing AMD water treatment used for irrigation (a) before and (b) after reaction between AMD and quicklime (Source: adapted from33).
Experimental soil sampling and analyses
A mixture of 3:1:1 of Culterra topsoil, vermiculite and river sand was used for planting potato seeds. The soil samples were collected immediately before planting and after harvesting from soil layers of 0–20 cm. They were collected in triplicates from each treatment, air-dried through a 2 mm mesh sieve and preserved in plastic bags before analysis. A similar method as above was used after harvesting before the measurements of the physicochemical parameters.
Pot experimental design, potato planting schedule and irrigation water treatments
The Marykies and Royal potato cultivars were grown in the pots using a statistical approach suggested by20 in the greenhouses. The factorial experiment was randomized and designed into blocks which comprised six pots (2 × 5), in which potato tubers of almost equal diameters between 30 and 60 mm were planted in each 25 × 25 cm pot (Fig. 2a–c). After planting, from emergence until crop maturity, irrigation with the various AMD treatments (TW1–TW5) was applied every two days (until senescence). An Irrometer Soil Moisture Meter (SN: 946,776) (Model 30–KTCD–NL, Riverside, California) was used to accurately schedule irrigation. When the Irrometer reading was between 60 and 100 centibars, 500 mm irrigation water was applied to all experimental pots at every cycle.
Physiological, biochemical and yield parameters measurements
To determine how irrigation with quicklime-treated AMD water affects the physiological and biochemical traits of the two potato varieties (Marykies and Royal), plant growth parameters, including yield, chlorophyll content, and stomatal conductance (DAP) were measured. The 4th matured completely expanded leaf (abaxial and adaxial) apex of the two potato cultivars was used for both chlorophyll content and stomatal conductance measurements at weekly intervals from 40 to 72 days after planting using a hand-held Minolta SPAD (Soil Plant Analysis Development: 502 m, 2900 PDL, Spectrum Technologies, Inc.) and Porometer (Model SC–1 Leaf Porometer, Pullman, USA) respectively. All tubers were rinsed with tap water after harvesting, dried with paper towels, and weighed. The UW4200H top loading Balance scale was used to determine the tuber fresh weights per plant. Fresh tubers per individual plant were freeze-dried and weighed to determine tuber dry matter using a freeze dryer (Free Zone Plus 2.5 L Cascade Benchtop Freeze Dry System Vacutec, USA).
Determination of levels of heavy metals on potato tubers
To evaluate the effect of heavy metal content on the potato tuber, hydrochloric-nitric acids HNO3–HCl method adopted from34 was used. The method was employed because hydrochloric-nitric acids, HNO3–HCl, have shown to be the best acid combination suitable for potato tuber samples due to their capacity to liberate metal ions from such complex matrices of tuber materials and, as a result, to limit noise levels during the detection technique. A UW4200H top loading balance scale was used to weigh a total of 1 g potato tubers powder from each of the five treatments. The samples were then placed in microwave-safe jars and mixed with 9 mL of nitric acid (65%) and 3 mL of hydrochloric acid (37%). The digesting process, as previously mentioned by35, was carried out at 175 °C for 60 min at 6 W energy. The materials were digested, allowed to cool, and then centrifuged for 10 min at 10,000×g. The supernatant was then collected, brought up to a volume of 50 mL in each tube, and diluted with deionized water (1:3) before being filtered through the Whatman No. 1 filter paper. Overnight, the suspension settles at room temperature before the measurement of the concentration of micronutrients (Cu, Fe, Mn, and Zn) and heavy metals (As, Cd, Co, Cr, Ni, Mg, Mn, and Mo) using an inductively coupled optical emission spectrometer (Agilent Technologies 700 series ICP-OES, USA).
Bioaccumulation of metals in soil-potato system
To understand the relationship of the bioaccumulation of metals in the soil-potato system, the soil-to-plant transfer can be predicted using a transfer factor (PTF)36. Metal concentrations in the extracts of soils and potatoes were calculated based on dry weight. The soil-to-plant transfer factor was calculated as37.
where Cpotato tuber and Csoil represent the heavy metal concentration in extracts of plants and soils on a dry weight basis, respectively. The transfer coefficient may differ considerably between plant, soil, and metal types under investigation38.
Statistical analysis
Data were subjected to two-way analysis of variance (ANOVA) carried out using TIBCO Statistica version 14.0 (StaSoft Inc., Tulsa, OK, USA) package (2020). Means separation was done using Tukey’s Honest Significant Difference (HSD) at p < 0.05.
Results and discussion
Physicochemical properties of the water and soil
Physicochemical parameters of different water treatments and soils recorded in the present study are summarised in Table 1 with a significant difference at p < 0.05 observed across the 5 treatments for all the measured parameters. After the application of quicklime in the AMD water, the pH value increased from 3.85 (TW2) to 6. 23–8.63 (TW3–TW4) and 8.85 (TW5), respectively. These values are within the permissible limit of World Health Organization (WHO) standards39. According to previous research33,40,41,42 quicklime and fly ash treatments have been shown to raise water pH to values suitable for crop irrigation. This is explained by the two substances' capacity to neutralize the acid produced by the AMD43. The EC and Total Dissolved Solids (TDS) values of treated water (TW3, TW4 and TW5) decreased when compared to untreated water (TW2); and were also within the recommended limit39. The study by44 reported that high EC values might make it difficult for plants to absorb ions from the soil solution. While, the TDS has a significant impact on plant growth, yield, and quality the values ranged from 853.14 to 2431.16 mg/L, and the EC of treated mine water ranged from 421.64 (TW4), 434.61 (TW5), to 917.43 (TW3). A study by45 showed a positive correlation between EC and TDS. This could account for the concurrent decrease of both parameters in this study under the ameliorating effect of QL and FA (Table 1). Only TW3 (1 g of QL) exceeded the maximum permitted limits for irrigation water’s EC (700 S/cm) and TDS (1000 mg/L). The investigation done by9 reported high EC (3100–13,000 S/cm) values of treated mine water that were over the maximum allowable limit for irrigation water in another investigation. Similar to the current study’s findings, the EC values of the examined samples were taken from mine wastewater at Eshidiya Mines in South Jordan and ranged from 3689 to 3795 S/cm with a mean value of 3724 12 S/cm, while those from another mine wastewater site ranged from 3869 to 3960 S/cm with a mean value of 3919 11 S/cm, both of which were above the WHO standard value46. This suggests that irrigation of crops with water that has been treated with 1 g of QL (TW3) is not recommended. The salinity appropriateness of irrigation water is assessed using the EC, or specific conductance, of a water sample. Increased soil salt levels caused by excessive soil salinization can harm crops47. Growing plants in salty conditions can restrict or hinder their ability to absorb water and nutrients, which results in stunted growth and lower yields. As a result of this study’s findings, it was determined that the selected potato cultivars could be watered with a solution made by combining AMD with 2 g of QL (TW4) and 2 g of QL and spiking with 75% of FA (TW5). Quicklime and fly ash treatment for AMD precipitates the sulphates linked to it, resulting in a decrease in its concentration40,48. In agreement with our findings, quicklime, and fly ash treatments (TW4 and TW5) reduced the sulfates compared to irrigation with raw AMD (TW2). Values, however, remained over the irrigation standard that is advised39. Sulfate can hinder a plant’s ability to absorb other nutrients when present in high concentrations, as reported by49. For the studied values, the physiochemical properties of soil irrigated with treated AMD water were significant (p < 0.05) among treatments (Table 1). In comparison to untreated AMD (TW2) and tapwater (TW1), there was a significant (p < 0.05) rise in the pH of the soil irrigated with treated AMD with ST3:5.67, ST4: 6.70, and ST5:7.23 respectively. In addition50, also noted a rise in pH value for soil irrigated with treated AMD water, which is consistent with our findings. Soil pH has a significant impact on the mobility and bioavailability of heavy metals51. The pH plays a significant role in plant health and growth by changing the chemistry of the soil, especially when it comes to boosting the number of nutrients that are available in the soil52.
For all the evaluated parameters, the treated AMD water displayed a significant difference (p < 0.05) see Table 1. When compared to the untreated 100% AMD water (TW2), the treated AMD water (TW3, TW4, and TW5) had lower concentrations of As, Co, Cu, Cd, Cr, Fe, Mg, Mn, Ni, and Zn (T2). According to40 treating AMD with QL eliminated 99% of As, Cd, Co, Cu, Fe, Mn, Ni, and Zn, which is consistent with our findings. Additionally33, found that the concentration of As, Cd, and Cr decreased when AMD was treated with 2 g of QL equivalent to 1 L of AMD. Except for Pb, Mg, and Mo, most of the heavy metals in TW4 and TW5 were decreased to levels below those stated in standards when this study’s findings were compared to the stipulated standard39. TW3 did not satisfy any recommended standard with As, Cd, Cr, Fe, Ni, and Zn. According to53, the differences between the three treatments may be due to the amount of QL used and the addition of fly ash to the QL treatment. High concentrations of metals in irrigation water, according to9, contaminate agricultural soils and cause crops grown on these soils to absorb metals. Therefore, it is necessary to maintain irrigation water's heavy metal content within a predetermined threshold.
Effect of the irrigation water on the physiological parameters, biochemical and yield attributes of potato cultivars
-
(a)
Plant height. The effects of irrigation water were reported in Fig. 3a and b to show the response of the two cultivars to the different treatments of AMD water. A significant difference (p < 0.05) was observed across the treatments for both cultivars. In addition, the progress growth of the crops was recorded as shown in Fig. 3a and b. In general, the Marykies cultivar responded better than the Royal across the treatments. This could be attributed to differences in the physiological response of the two cultivars as impacted by their molecular properties under AMD environment54. The study conducted by55 also indicated that different plants respond differently in the synthesis of proteins that could play a crucial role in their adaptation and survival under AMD conditions. The differential protein abundance in the Marykies or the plant–microbe interactions could have played a crucial role in their better adaptation under the treated AMD condition. However, there is a need for further studies to validate this assumption. Among the treatment levels, TW4 and TW5 enhanced the plant height of the two cultivars better regarding the AMD sample (TW2) and control (TW1). This may be due to lime and fly ash's beneficial benefits in reducing soil acidity, as they are well-known for their strong acid-neutralizing capabilities, which can effectively eliminate existing acid, increase biological activity, and minimize heavy metal toxicity52,56. For instance, a study conducted by57, applying lime to acid soil increased barley height, fresh biomass, dry biomass, grain yields, harvest index, and P-uptake. Furthermore, the use of lime enhanced maize growth and yield, owing to the reduction in Al toxicity. For this study, TW2 and TW3 exhibited low plant height as compared to TW1, TW4 and TW5. Similar results were reported by24 who observed negative effects on the growth and grain yield of winter wheat irrigated with acid mine wastewater.
-
(b)
Chlorophyll content. Plants exhibit dynamism that cuts across physiological, metabolic, and molecular responses in their struggle to survive adverse environmental conditions58. A study by59 stated that chlorophyll concentration, stomatal conductance, and biomass of roots, stems, leaves, and fruits can all be used to determine a plant's physiological growth. Several studies have suggested that factors such as water stress and soil types might affect the chlorophyll concentration of plant leaves60. For this study, the chlorophyll content (mg/m2) and stomatal conductance (mmol m2 s1) of two cultivars of potato grown under the irrigation of treated AMD water were measured to ascertain whether their physiological response could promote their survival under (un)treated AMD water stress. The measured physiological parameters showed significant differences (p < 0.05) across the two cultivars. Royal cultivar produced more chlorophyll content than the Marykies (Table 2). Like in these findings20, also observed variation in the chlorophyll response when different cultivars of potato were treated with AMD water. This could be linked to crops' physiological responses to stress, which vary depending on the type and level of crops, as well as the type of crops involved57. The chlorophyll content (chlorophyll a & b) determined on the leaf of the selected cultivars was significantly affected by long-term irrigation with the different quicklime AMD treatments. For example, the highest chlorophyll content a & b for both cultivars responding to treatments was recorded on TW2 and TW3 as compared to the controls (TW1) and TW4 and TW5 for both seasons (Table 2). The increase in chlorophyll can be due to the possible accumulation of metals (toxic effect) in comparison with the other treatments. A similar trend was observed in season 2 as well. The chlorophyll content varied on days after planting (DAP) with the highest recorded on D5 which is day 56 after planting (56 DAP). There was a trend in that the chlorophyll content decreased as the day of planting increased. Congruent to our study, other studies have also observed variation in the chlorophyll content with DAP as impacted the AMD treatment20,61. For this study, the implication is that long exposure of the plants to QL treated AMD could impair the physiological processes of the crops.
Table 2 Effects of AMD water treated with quicklime on Marykies and Royal cultivars chlorophyll content (Season 1 and 2). -
(c)
Stomatal conductance. Stomatal conductance is referred to as a measure of the degree of physical resistance to gas movement between air and leaf interior62. Such an exchange supports the exchange in CO2 intake and water loss (transpiration) through the stomatal aperture. Stomatal adjustments help to maintain plant water status under varying soil moisture and atmospheric conditions. Acid mine drainage is known to constitute an environmental stress to plants, and this culminates into diverse physiological and morphological responses by plants that include reduction in transpiration rate and stomatal conductance63,64. The two measured physiological parameters showed significant differences (p < 0.05) across the two cultivars. Royal cultivar produced greater stomatal conductance than the Marykies (Table 3). Investigation from20 also observed variation in stomatal conductance between Fiana and Lady Rosetta cultivars that were treated with AMD water. In concurrence with the findings of this study, in other research studies conducted under extreme environmental stress, plants exhibit a plethora of physiological adjustment which reduces stomatal conductance and chlorophyll content as a mechanism to aid their survival65,66,67,68. The treatment of AMD with QL was promising as the treatments were able to improve the stomatal conductance of the two cultivars. Similarly, there were significant differences (p < 0.05) in the stomatal conductance on DAP across the different days of measurement with D5 also showing the highest stomatal conductance (Table 3). Stomatal conductance continued to decrease as the plant grew older.
Table 3 Effects of acid mine drainage (AMD) water treated with quicklime on the stomatal conductance of Marykies and Royal (Season 1 & 2). -
(d)
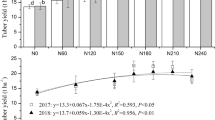
Yield parameters and their components. A two-way ANOVA analysis showed that the quicklime and fly ash treatments of AMD were significant (p < 0.05) across the treatments for the tuber yield, fresh tuber weight, and dry tuber weight for both cultivars (Table 4) with subtle variation between them. The treated AMD water samples (TW3, TW4, and TW5) improved all the yield parameters for the two potato cultivars with T2 showing higher potential in the improvement of the yield (Table 4). Maize (Zea mays) and sunflower (Helianthus annuus) grown in a heavy metal-enriched AMD environment showed enhanced growth and copper uptake, as reported in the69 findings. Additionally, using copper-resistant Pseudomonas strains improved Zn and Pb bioaccumulation as well as plant growth-promoting indole-3-acetic acid (IAA), iron chelating siderophore, and mineral phosphate and metals solubilization capacity70. The increased crop yield observed under TW2 could have been because of the presence of growth-promoting bacteria that could have promoted the growth promotion and heavy metal bioaccumulation of the potatoes but might have also enhanced the remediation function through plant microbes interactions71. Results on season 2 (Table 4) revealed a slight variation between treatments compared to season 1. Marykies had a higher number of tubers on TW4 and TW5 and this enhancement in yield can be in response to quicklime and fly ash application and other environmental factors in the greenhouse. Several research including72,73,74 reported that fly ash has the potential to improve the yield of wheat (Triticum aestivum), rice (Oryza sativa), maize (Zea mays), mung bean (Vigna unguiculata), eggplant (Solanum melongena), onion (Alium cepa) and chickpea (Cicer arietinum) cultivated on different types of soils. Irrigation with AMD water generally causes a shift in the parameters of soils, has the potential to positively alter microbial diversity and plays vital roles in the ecology of the rhizosphere of plants through the maintenance of soil health and therefore increasing the yield of crops75,76,77. The decrease in the yield of crops irrigated with treated AMD water could be a function of the important microbe reduction during the process of treatment6. Hence, there is a need to evaluate a system where AMD treatment can protect the important microbial communities while removing the harmful substance that plants can translocate from the soil.
Table 4 Effects of treated quicklime AMD water on the Marykies and Royal yield.
Effect of water quality on potato tubers and irrigated soil
Due to the potential for crops to absorb heavy metals, irrigation of crops with AMD water, whether it is sourced from industrial, municipal, or sewage and whether it has been treated or not, has been documented to be harmful to crops and agricultural soil9,78. In this study, quicklime and fly ash were used to water different potato cultivars, and the number of heavy metals in the tubers was measured. The findings revealed that heavy metals were present in the tubers and that their quantities varied significantly (p ≤ 0.05) depending on the treatments Table 5. When compared to the 100% AMD, there was a decrease in the concentration of several heavy metals in the tubers, especially after quicklime and fly ash treatments (TT3, TT4, and TT5) (TT2). This is probably due to the treatment's success in lowering the level of heavy metal in the 100% AMD. The creation of several metabolites that are essential for the signalling, sequestration, and transportation of heavy metals like Fe, Cu, Zn, and Cd is observed to rise because of heavy metal stress, according to several studies79,80,81,82,83. The concentration of some of the heavy metals decreased, however not all the lowered heavy metal concentrations fell under the39 recommended tolerable limits. The following heavy metals such as Al, Co, Cu, Fe, Mg, Mn, Ni, and Zn met the requirements. In contrast, the levels of As, Cd, Cr, Pb, and Mo were higher than what is considered safe by39 criteria. Hence, further studies that could unveil the underlying shift in a metabolite that could be responsible for the crop not sequestrating many of the heavy metals are recommended. Such research may also shed light on the mechanisms underlying the heavy metal hyperaccumulation capacity of certain potato cultivars. Additionally, increased concentrations of heavy metals such As, Cd, Pb, and Zn were found in the soils and vegetables grown in Portugal under irrigation using water from locations near mines84. While 85 revealed that fresh vegetable samples that had As and Pb concentrations that were higher than allowed by international food standards had contaminated irrigation in areas close to mining zones. For example, in Guangdong province, South China, around the Lechang Pb/Zn mine86, high levels of metals such as Cd, Pb, Cu, and Zn were measured in 11 edible vegetables, including Solanum tuberosum (potato). The results revealed that local mining activity caused heavy metal contamination, with Cd concentration exceeding the required standards for all vegetables. In another study, paddy fields in a karst region of Guangxi Province, South China, were found to be heavily contaminated with Cd, Zn, Pb, and Cu87. In the same Lechang mining area, Guangdong Province, South China, irrigation with mining effluent contaminated a paddy field and rice grain with Cd88. According to a study by89, Pb and Cd concentrations in rice grain are above China's maximum allowable limits. The study looked at the extent to which heavy metals (Cu, Zn, Pb, and Cd) contaminate soils, vegetables, and rice growing near the Dabaoshan mine in South China. In Potosi (Bolivia), it was shown that potato tubers irrigated with streams affected by mining had higher Cd concentrations than those irrigated with spring water18. Similarly, in this study, higher than required levels of As, Cd, Pb, and Zn were found in the heavy metal content of potato tubers cultivated in acid mine water released from mining enterprises in Potosi19.
The study also evaluated the concentration of selected heavy metals in the soils irrigated with quicklime and fly ash-treated AMD. An analysis was done to delineate the impact of heavy metal contamination of the soil on the crops (Table 6). Studies have shown that when plants are raised in soils that are contaminated with heavy metals, they absorb and accumulate these in their edible parts of plants and these could be beyond the permissible limits, which could be harmful to humans it consumed90. The present results showed significant differences (p < 0.05) across the treatments for both cultivars. The soil irrigated with treated AMD (ST3, ST4, and ST5) showed variation in the concentrations of heavy metals and were within the permissible limit of the WHO except for As, Cd and Cr. As expected, the soil that was irrigated with untreated AMD water (ST2) showed a higher concentration of heavy metals in most of the measured metals that were not within the stipulated standard. This is due to the transfer of heavy metals from the untreated AMD and the inability of the crops to sequester such high concentration91. In agreement with these findings, some studies have also reported an increase in the concentration of heavy metals in soil polluted with heavy metal-laden waste92. A study by93 investigated the degree of contamination of heavy metals in paddy soil irrigated with acid mine drainage and showed that Cu, Zn, and Cd in topsoil exceeded the maximum permissible concentrations for Chinese agricultural soil. Another study91 observed similar findings on paddy fields that had been extensively polluted by Cu, Zn, and Cd due to long-term irrigation with nearby stream water contaminated by acid mine wastes. In their findings94, reported higher concentrations of heavy metals and soil salinity during the experimental period for plots irrigated with mine wastewater, when compared to plots irrigated with fresh water. Overall, a significant number of studies on heavy metals in plants have been conducted in Chinese paddy fields, which is likely due to a large amount of mining in that region of the world, which has resulted in AMD accumulation,23,95.
Soil-to-plant transfer factor
PTF provides a useful indication of the metal availability from soil to plants. The PTF values for Cd, Cu, Fe, Ni, Mn, Pb and Zn ranged from 0.01 to 5.17, 0.25 to 1.80, 0.00 to 0.01, 0.00 to 2.90, 0.04 to 0.10, 0.16 to 2.90 and 0.14 to 0.52, respectively. The mean value of PTF for each heavy metal is shown in Fig. 4. The higher the value of the transfer factor, the more elements would be accumulated by plants. The trend of transfer factor and thus the availability of heavy metals for potatoes was in the order of Sr > Zn > Cu > Mg > Mn > Pb > Cd > As > Mo > Cr > Ni > Fe and Co. The availability of heavy metals in potato tubers was significantly (P < 0.05) different among heavy metals and the accumulation of Cd in potatoes was highest. These results agree with previous investigations. The findings96 reported that the transfer factor for Cd and Cu was higher than other metals in vegetables. The other findings97,98 reported that the accumulation of Cd and Zn was higher than that of Ni in rice. There was a significant correlation between total Cd concentrations in soil and two potato cultivars (Fig. 4). There was no significant correlation between other metal concentrations in soils.
Conclusion
This research aimed to evaluate the possible use of quicklime to treat acid mine water for crops and assess the effects on soil properties and potato cultivars Marykies and Royal physiological, biochemical, and yield parameters when subjected to quicklime treated acid mine drainage irrigation under greenhouse conditions. It was found that the quicklime-treated mine water had increased pH levels indicating normal alkalinity which is equivalent to the permissible limits. The observed pH levels and metal removal capacity during the experimental period indicate the potential long-term effectiveness of quicklime in treating AMD. The results showed that soil irrigated with treated AMD water exhibited a noticeable decrease in pH and EC, as well as an increase in sulphate content when compared to the treated AMD water. This might be explained by how plants and bacteria interact, which has the potential to change the soil's ecology and make it more conducive to crop growth. The presence of sulphate-oxidizing bacteria in the AMD is linked to the rise in sulphates in the environment that is polluted by AMD. Since water is a scarce resource in South Africa, these findings make it possible to consider the possibility of using treated AMD in agriculture, without negative consequences to plants and by extension, to human life. Since AMD is available abundantly and quicklime is also cheap to obtain, this study presents a great opportunity to ameliorate AMD water for food security. Soil-to-plant transfer factor revealed that there were high concentrations of total heavy metals in soil and potatoes and that the PTFs were higher for Zn, Cu, Mg, Pb and Mn than other metals. Thus, the transfer of Pb from soils to potatoes may exhibit potential health risks for people who regularly consume heavy metals contaminated potatoes. However, to avoid the eventual risks, the use of AMD must be regularly monitored, and the reuse standards should be developed and strictly observed.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to university policy but are available from the corresponding author on reasonable request.
References
Dinar, A., Tieu, A. & Huynh, H. Water scarcity impacts on global food production. Glob. Food Secur. 23, 212–222 (2019).
Khalid, S. et al. Influence of groundwater and wastewater irrigation on lead accumulation in soil and vegetables: Implications for health risk assessment and phytoremediation. Int. J. Phytoremed. 19(11), 1037–1046 (2017).
Chamorro, S. et al. Chemical characterization of organic micro contaminant sources and biological effects in riverine sediments impacted by urban sewage and pulp mill discharges. Chemosphere 90(2), 611–619 (2013).
Jaramillo, M. F. & Restrepo, I. Wastewater reuse in agriculture: A review about its benefits. Int. J. Sustain. 9, 1734 (2017).
Libutti, A. et al. Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric. Water Manag. 196, 1–14 (2018).
Grewar, T. South Africa’s options for mine-impacted water re-use: A review. J. S. Afr. Inst. Min. Metall. 119(3), 321–331 (2019).
Pulles, W., Banister, S. & van Biljon, M. The development of appropriate procedures towards and after closure of underground gold mines from a water management perspective. In Water Research Commission, Pretoria Report No. 1215/1/05 (2005).
Ochieng, G. M. M., Seanego, E. S. & Nkwonta, O. I. Impacts of mining on water resources in South Africa: A review. Sci. Res. Essays 5(22), 3351–3357 (2010).
Shabalala, A. N. & Ekolu, S. O. Assessment of the suitability of mine water treated with pervious concrete for irrigation use. Mine Water Environ. 38, 798–807 (2019).
McCathy, T. S. The impact of acid mine drainage in South Africa. S. Afr. J. Sci. 107(5), 7–12 (2011).
El Chami, D. & El Moujabber, M. Drought, climate change and sustainability of water in agriculture: A roadmap towards the NWRS2. S. Afr. J. Sci. 112(9/10), 1–4 (2016).
Nkondo, M.N., van Zyl, F.C., Keuris, H. & Schreiner, B. proposed national water resource strategy 2 (NWRS2): Summary. In Cape Town: Department of Water Affairs, South Africa (2012).
van Zyl, H. C., Maree, J. P., van Niekerk, A. M., van Tonder, G. J. & Naidoo, C. Collection, treatment, and re-use of mine water in the Olifants River Catchment. J. S. Afr. Inst. Min. Metall. 101, 41–46 (2001).
Lin, C. et al. Environmental impacts of surface mining on mined lands, affected streams and agricultural lands in the Dabaoshan Mine region, southern China. Land Degrad. Dev. 16(5), 463–474 (2005).
Annandale, J. G. et al. Prediction of the environmental impact and sustainability of large-scale irrigation with gypsiferous mine-water on groundwater resources. Water SA 32(1), 21–28 (2006).
Annandale, J. G., Jovanoic, N. Z., Tanner, P. D., Benadé, N. & Du Plessis, H. M. The sustainability of irrigation with gypsiferous mine water and implications for the mining industry in South Africa. Mine Water Environ. 21, 81–90 (2002).
Annandale, J. G. et al. The influence of irrigation with gypsiferous mine water on soil properties and drainage water. In Water Research Commission Report No. K5/858, Pretoria, South Africa (2001).
Oporto, C., Vandecasteele, C. & Smolders, E. Elevated cadmium concentrations in potato tubers due to irrigation with river water contaminated by mining in Potosí, Bolivia. J. Environ. Qual. 36, 1181–1186 (2007).
Garrido, A. E., Strosnider, W. H. J., Wilson, R. T., Condori, J. & Nairn, R. W. Metal-contaminated potato crops and potential human health risk in Bolivian mining highlands. Environ. Geochem. Health 39(3), 681–700 (2017).
Nemutanzhela, M. V., Modise, D. M., Siyoko, K. J. & Kanu, S. A. Assessment of growth, tuber elemental composition, stomatal conductance and chlorophyll content of two potato cultivars under irrigation with fly ash-treated acid mine drainage. Am. J. Potato Res. 94(4), 367–378 (2017).
Nevhulaudzi, T., Kanu, S. A. & Ntushelo, K. The interaction effect of Bacillus subtilis co-inoculation and mine water irrigation on cowpea’s growth, physiology, and nutritional quality. Sci. Afr. 9, 1–32 (2020).
Islam, M. S. et al. Health risk assessment due to heavy metal exposure from commonly consumed fish and vegetables. Environ. Syst. Dec. 36, 253–265 (2016).
Zhuang, P., Lu, H., Li, Z., Zou, B. & McBride, M. B. Multiple exposure and effects assessment of heavy metals in the population near mining area in South China. PLoS ONE 9(4), e94484 (2014).
Ma, S. C. et al. Effects of mine wastewater irrigation on activities of soil enzymes and physiological properties, heavy metal uptake and grain yield in winter wheat. Ecotoxicology 113, 483–490 (2015).
Hasanuzzaman, M., Nahar, K., Alam, M. M., Roychowdhury, R. & Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14(5), 9643–9684 (2013).
Ma, S. C., Ma, S. T., Shao, Y., Jiang, L. N. & Li, C. X. Effects of irrigation with mine wastewater on physiological characters and heavy metals accumulation of winter wheat. J. Appl. Ecol. 24(11), 3243–3248 (2013).
Kamaruzzaman, M., Mohammad, S. M. R., Rasel, M. & Nurul-Md, I. Effect of lime on yield contributing characters of wheat in Barind tract of Bangladesh. IOSR-JAVS. 4(6), 39–46 (2013).
Levy, D. & Tai, G. C. C. Differential response of potatoes (Solanum tuberosum L.) to salinity in an arid environment and field performance of the seed tubers grown with fresh water in the following season. Agric. Water Manag. 116, 122–127 (2013).
Yuan, B. Z., Nishiyama, S. & Kang, Y. Effects of different irrigation regimes on the growth and yield of drip-irrigated potato. Agric. Water Manag. 63, 153–167 (2003).
Lerna, A. Water management in potato. In Potato Production Worldwide 87–100 (2023)
Elzner, P., Jůzl, M. & Kasal, P. Effect of different drip irrigation regimes on tuber and starch yield of potatoes. Plant Soil Environ. 64(11), 546–550 (2018).
Skousen, J. Overview of acid mine drainage treatment with chemicals. In Acid Mine Drainage, Rock Drainage, and Acid Sulfate Soils (eds. Jacobs, J. A. et al.) (Springer, 2014).
Othman, A., Sulaiman, A. & Sulaiman, S. K. The use of quicklime in acid mine drainage treatment. Chem. Eng. Trans. 56, 1585–1590 (2017).
Uddin, A. B. M. H., Khalid, R. S. & Abba, S. Determination of heavy metal concentration of different traditional medicine formulations available at the East Coast Region of Malaysia. Afr. J. Pharm. Pharacol. 6, 1487–1491 (2016).
Iloms, E., Ololade, O. O., Ogola, H. J. O. & Selvarajan, R. Investigating industrial effluent impact on municipal wastewater treatment plant in Vaal, South Africa. Int. J. Environ. Res. Public Health 17, 1096 (2020).
Kachenko, A. G. & Singh, B. Heavy metal contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 169, 101–123 (2006).
Cui, Y. J. et al. Transfer of metals from soil to vegetables in an area near a smelter in Nanning China. Environ. Int. 30(6), 785–791 (2004).
Alexander, P. D., Alloway, B. J. & Dourado, A. M. Genotypic variation in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 144, 736–745 (2006).
WHO. Health guidelines for the use of wastewater in agriculture and aquaculture. In Report of a WHO Scientific Group, Geneva (2006).
Tolonen, E. T., Sarpola, A., Hu, T., Ramo, J. & Lassi, U. Acid mine drainage treatment using by-products from quicklime manufacturing as neutralization chemicals. Chemosphere 117, 419–424 (2014).
Chen, H. et al. In-situ remediation of acid mine drainage from abandoned coal mine by filed pilot-scale passive treatment system: Performance and response of microbial communities to low pH and elevated Fe. Bioresour. Technol. 317(123985), 1–11 (2020).
Liang, J. et al. Effect of quicklime on microbial community in strong acidic soil. J. Soil Sci. Plant Nutr. 2021, 1–11 (2021).
Geldenhuys, A. J., Maree, J. P., De Beer, M. & Hlabela, P. An integrated limestone/lime process for partial sulphate removal. J. S. Afr. Inst. Min. Metall. 103, 345–354 (2003).
Oladeji, O. S., Adewoye, A. O. & Adegbola, A. A. Suitability assessment of groundwater resources for irrigation. J. Appl. Eng. Sci. Technol. 1(3), 437–445 (2012).
Oyem, H. H., Oyem, I. M. & Ezeweali, D. Temperature, pH, electrical conductivity, total dissolved solids and chemical oxygen demand of groundwater in Boji-BojiAgbor/Owa area and immediate suburbs. Environ. Res. 8, 444–450 (2014).
Al-Hwaiti, M. S., Brumsack, H. J. & Schnetger, B. Suitability assessment of phosphate mine wastewater for agricultural irrigation: An example from Eshidiya Mines South Jordan. Environ. Earth Sci. 75(276), 1–17 (2016).
Mansilha, C. et al. Irrigation with coal mining effluents: Sustainability and water quality considerations (São Pedro da Cova, North Portugal). Water 13(16), 2157 (2021).
Qureshi, A., Jia, Y., Maurice, C. & Öhlander, B. Potential of fly ash for neutralisation of acid mine drainage. Environ. Pollut. 23, 17083–17094 (2016).
Natsheh, B. Impact of short-term irrigation with different water types on some chemical and physical soil properties. Open J. Soil Sci. 11, 389–401 (2021).
Nigam, R., Srivastava-Prakash, S. & Srivastava, M. M. Cadmium mobilisation and plant availability—the impact of organic acids commonly exuded from roots. Plant Soil 230, 107–113 (2001).
Roohi, M. et al. Low C/N ratio raw textile wastewater reduced labile C and enhanced organic-inorganic N and enzymatic activities in a semiarid alkaline soil. Environ. Pollut. 24, 3456–3469 (2017).
Shirin, S., Jamal, A., Emmanouil, C. & Yadav, A. K. Assessment of characteristics of acid mine drainage treated with fly ash. Appl. Sci. 11(3910), 1–10 (2021).
Farhad, M. S., Babak, A. M., Reza, Z. M., Hassan, R. S. M. & Afshin, T. Response of proline, soluble sugars, photosynthetic pigments, and antioxidant enzymes in potato (Solanum tuberosum L.) to different irrigation regimes in greenhouse condition. Aust. J. Crop Sci. 5(1), 55–60 (2011).
Kiiskila, J. D., Sarkar, D. & Datta, R. Differential protein abundance of vetiver grass in response to acid mine drainage. Plant Physiol. 173(3), 829–842 (2021).
Ameyu, T. A review on the potential effect of lime on soil properties and crop productivity improvements. J. Environ. Sci. 9(2), 17–23 (2019).
Achalu, C. H., Gebrekidan, H., Kibret, K. & Tadesse, A. Response of barley to liming of acid soils collected from different land use systems of Western Oromia. J. Biodivers. Environ. Sci. 2(7), 1–13 (2012).
Kalu, C. M., Rauwane, M. E. & Ntushelo, K. Microbial spectra, physiological response and bioremediation potential of phragmites australis for agricultural production. Front. Sustain. Food Systs. 5, 696196 (2021).
Ebrahim, M. K. Comparison, determination and optimizing the conditions required for rhizome and shoot formation, and flowering of in vitro cultured calla explants. Sci. Hortic. 101(3), 305–313 (2004).
Shu, W. S., Ye, Z. H., Lan, C. Y., Zhang, Z. Q. & Wong, M. H. Acidification of lead/zinc mine tailings and its effect on heavy metal mobility. Environ. Int. 26, 389–394 (2001).
Ali, B. et al. 5-aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L.. J. Plant Growth Regul. 32(3), 604–614 (2013).
Pietragalla, J. & Pask, A. Stomatal conductance. In Physiological Breeding II: A Field Guide to Wheat Phenotyping (eds. Pask, A. et al.) 15–17 (International Maize and Wheat Improvement Center CIMMYT, 2012).
Zhang, Z., Tao, Z., Zhou, B., Zhang, X. & Zhao, Z. Plant stomatal conductance determined transpiration and photosynthesis both contribute to the enhanced negative air ion (NAI). Ecol. Indic. 130, 108114 (2021).
Zhang, Z. et al. Validation and in situ application of a modified thermal dissipation probe for evaluating standing water use of a clumped bamboo: Bambusa chungii. Agric. Forest Meteorol. 239, 15–23 (2017).
Khaleel, R. I., Ismail, I. & Ibrahim, M. H. The impact of wastewater treatments on seed germination and biochemical parameters of Abelmoschus Esculentus L.. Afr. J. Soc. Behav. sci. 91, 453–460 (2013).
Manisha, P. & Angoorbala, B. Effect of sewage on growth parameters and chlorophyll content of Trigonella foenum-graecum (Methi). Int. Res. J. Environ. Sci. 2(9), 5–9 (2013).
Sahay, S., Iqbal, S. & Inam, A. Wastewater irrigation in the regulation of soil properties, growth determinants, and heavy metal accumulation in different Brassica species. Environ. Monit. Assess. 191(107), 1–21 (2019).
Naz, A. et al. Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment. Int. J. Environ. Res. Public Health 12, 7400–7416 (2015).
Li, K. & Ramakrishna, W. Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazar Mater. 189, 531–539 (2011).
Rathi, M. & Yogalakshmi, K. N. Brevundimonas diminuta MYS6 associated Helianthus annuus L. for enhanced copper phytoremediation. Chemosphere 263, 128–195 (2021).
Tripti, A. K. et al. Bioaugmentation with copper tolerant endophyte Pseudomonas lurida strain EOO26 for improved plant growth and copper phytoremediation by Helianthus annuus. Chemosphere 266(128983), 1–12 (2021).
Pandey, V. C., Singh, J. S., Kumar, A. & Tewary, D. D. Accumulation of heavy metals by chickpea grown in fly ash treated soil: Effect on antioxidants. Clean. Soil Air Water 38, 1116–1123 (2010).
Singh, A., Sarkar, A. & Agrawal, S. B. Assessing the potential impact of fly ash amendments on Indian paddy field with special emphasis on growth, yield, and grain quality of three rice cultivars. Environ. Monit. Assess. 184(8), 4799–4814 (2012).
Parab, N., Mishra, S. & Bhonde, S. R. Mycorrhizal dependency, yield, and biochemical parameters of onion in response to fly ash amendment with different biofertilizers. Int. J. Environ. Sci. 2(1), 34–41 (2013).
Narendrula-Kotha, R. & Nkongolo, K. K. Microbial response to soil liming of damaged ecosystems revealed by pyrosequencing and phospholipid fatty acid analyses. PLoS ONE 12(1), 1–22 (2017).
Li, Y., Yuan, L., Xue, S., Liu, B. & Jin, G. The recruitment of bacterial communities by the plant root system changed by acid mine drainage pollution in soils. FEMS Microbiol. Lett. 367(15), 458 (2020).
Xin, R. et al. Contrasting seasonal variations of geochemistry and microbial community in two adjacent acid mine drainage lakes in Anhui Province, China. Environ. Pollut. 268, 115826 (2021).
Singh, A. & Agrawal, S. B. Response of mung bean cultivars to fly ash: Growth and yield. Ecotoxicol. Environ. Saf. 73, 1950–1958 (2010).
Johnson, A. A. et al. Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron-and zinc-biofortification of rice endosperm. PLoS One 6(9), 1–11 (2011).
Zhou, X. et al. Genome-wide identification, classification, and expression profiling of nicotianamine synthase (NAS) gene family in maize. BMC Genom. 14, 238 (2013).
Yang, G. et al. Molecular cloning and characterization of MxNAS2, a gene encoding nicotianamine synthase in Malus xiaojinensis, with functions in tolerance to iron stress and misshapen flower in transgenic tobacco. Sci. Hortic. 183, 77–86 (2015).
Jalmi, S. K. et al. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 9(12), 1–21 (2018).
Safdarian, M., Askari, H., Shariati, V. & Nematzadeh, G. Transcriptional responses of wheat roots inoculated with Arthrobacter nitroguajacolicus to salt stress. Sci. Rep. 9, 1–12 (2019).
Ávila, P. F., Ferreira da Silva, E. & Candeias, C. Health risk assessment through consumption of vegetables rich in heavy metals: The case study of the surrounding villages from Panasqueira mine Central Portugal. Environ. Geochem. Health. 39(3), 565–589 (2017).
Bui, A. T. K. et al. Accumulation and potential health risks of cadmium, lead and arsenic in vegetables grown near mining sites in Northern Vietnam. Environ. Monit. Assess. 188, 525 (2016).
Dong, J., Yang, Q. W. & Sun, L. Assessing the concentration and potential dietary risk of heavy metals in vegetables at a Pb/Zn mine site, China. Environ. Earth Sci. 64, 1317–1321 (2011).
Li, Y. T. et al. Microbial biomass, enzyme, and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biol. Biochem. 41(5), 969–977 (2009).
Yang, Q. W., Lan, C. Y., Wang, H. B., Zhang, P. & Shu, W. S. Cadmium in soil—rice system and health risk associated with the use of untreated mining wastewater for irrigation in Lechang, China. Agric. Water Manag. 84, 147–152 (2006).
Zhuang, P., Zou, B. & Li, N. Y. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: Implication for human health. Environ. Geochem. Health 31, 707–715 (2009).
Ahmad, P. et al. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLos One 10(1), e0114571 (2015).
Rambabu, K. et al. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2, 100024 (2020).
Roy, M. & McDonald, L. M. Metal uptake in plants and health risk assessments in metal-contaminated smelter soils. Land Degrad. Dev. 26, 785–792 (2015).
Qu, L., Xie, Y. & Lu, G. Distribution, fractionation, and contamination assessment of heavy metals in paddy soil related to acid mine drainage. Paddy Water Environ. 15, 553–562 (2017).
Hu, X. F. et al. Effects of mining wastewater discharges on heavy metal pollution and soil enzyme activity of the paddy fields. J. Geochem. Explor. 147, 139–150 (2014).
Rimawi, O., Jiries, A., Zubi, Y. & El-Naqa, A. Reuse of mining wastewater in agricultural activities in Jordan. Environ. Dev. Sustain. 11, 695–703 (2009).
Sun, H., Zhao, F., Zhang, M. & Li, J. Behaviour of rare earth elements in acid coal mine drainage in Shanxi Province, China. Environ. Earth Sci. 67, 205–213 (2012).
Khan, S., Rehman, S., Khan, A. Z., Khan, A. M. & Shah, M. T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 73, 1820–1827 (2010).
Kashem, M. A. & Singh, B. R. Solid phase speciation of Cd, Ni and Zn in some contaminated and non-contaminated tropical soils. In Trace Elements in Soil, Bioavailability, flux, and Transfer (eds. Iskandar, I. K. & Krikham, M. B.) 213–227 (Lewis, 2001).
Kashem, M. A., Singh, B. R. & Kawai, S. Mobility and distribution of cadmium, nickel, and zinc in contaminated soil profiles from Bangladesh. Nutr. Cycl. Agroecosyst. 77, 187–198 (2007).
Acknowledgements
We acknowledge the support from the University of South Africa and Horticulture Research Centre for providing research facilities at the UNISA Science campus.
Funding
The study was funded by the University of South Africa.
Author information
Authors and Affiliations
Contributions
R.M. was involved in study design, crop planting and data collection, data analysis and write-up, D.M.M. was involved in manuscript arrangement, review, comments, and corrections.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Munyai, R., Modise, D.M. Potato (Solanum tuberosum L.) cultivars physiological, biochemical performance and yield parameters response to acid mine water irrigation and soil physiochemical properties. Sci Rep 14, 1958 (2024). https://doi.org/10.1038/s41598-024-52507-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52507-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.