Abstract
Breast cancer related lymphedema (BCRL) is a chronic condition with a detrimental impact on psychosocial and physical well-being. Lymphaticovenous anastomosis has shown promising results in alleviating physical symptoms and increasing quality of life in patients with BCRL. The aim of the study is to evaluate the effect on health related quality of life (HrQol) after LVA surgery versus conservative treatment in patients with BCRL. The study is a prospective, multicenter randomized controlled trial. Adult women with unilateral BCRL, with early stage lymphedema and viable lymphatic vessels were included. The primary outcome measure was HrQol measured by the lymphedema functioning disability and health (Lymph-ICF) questionnaire. The secondary outcomes were volume difference measured by the water displacement method; the Upper Extremity Lymphedema (UEL) index; and daily use of the compression garments after 3 and 6 months. For this interim analysis 46 patients per group were included. There was a significant improvement in the domains in physical and mental function in the Lymph-ICF questionnaire in the LVA group after 6 months, (− 16.46 ± 18.5, p < 0.05, − 10.12 ± 29.5, p < 0.05 respectively). However, there was no statistical difference in the total score of the Lymph-ICF after 6 months in both groups (LVA-group; − 8.57 ± 22.6, p > 0.05, CDT-group; − 2.65 ± 18.2, p < 0.05). Furthermore, there was no significant volume reduction in both groups (LVA-group: 20.04 ± 196.40, p = 0.497, CDT: 33.98 ± 189.87, p = 0.236). In the LVA group, 41% partially of completely stopped wearing the compression garments after six months whereas in the CDT group 0% discontinued to use of compression garments. LVA resulted in improvement of the domains physical and mental function of the Lymph-ICF. Limb volume did not significantly improve after 6 months. However, around 42% could completely or partially stopped with the use of compression garments in the LVA group. The current results are promising, however longer follow up is required to assess long term effect of LVA for secondary lymphedema.
Clinical Trial Registration: NCT02790021 registered on 03/06/2016
Similar content being viewed by others
Introduction
Breast cancer related lymphedema (BCRL) is a chronic condition, characterized by an aberrant accumulation of lymph fluid due to dysfunction of the lymphatic system1,2,3. It is a well-known consequence of breast cancer treatment and it represents an important survivorship topic in patients after breast cancer4,5,6,7.
BCRL affects approximately 29.4% of breast cancer survivors within 2 years after surgery1,7. Axillary lymph node dissection, radiotherapy, mastectomy, number of positive lymph nodes and body mass are all independent risk factors for the development of BCRL6,8,9.
Nowadays, Health related quality of life (HrQol) is one of the most relevant outcomes after cancer treatment10,11. BCRL is known to have a significant negative impact on physical, psychological, and social well-being5. Physical morbidities include skin infections, altered sensation, pain, decreased range of motion, strength, and function. In addition, a larger arm sizes requires women to alter daily activities, clothing, sleeping, employment, and sport. Symptoms of anxiety, depression, sexual dysfunction, disturbance of body image, and social avoidance are often associated with BCRL12,13,14.
Currently, complex decongestive therapy (CDT) is the gold standard for the treatment of lymphedema, consisting of the use of compression garments and manual lymphatic drainage5,15. These treatment modalities however are not curative and require lifelong costly maintenance16.
The first lymphaticovenous anastomosis (LVA) was described in the 1960 as a novel approach to divert lymphatic fluid through formation of an anastomosis from the lymphatic vessels to adjacent venules17,18. The concept was revolutionized by Koshima et al. in the 90 s who introduced the concept of supermicrosurgery in lymphatic surgery19. Since then the technique has been refined with the introduction of the microscope and designated supermicrosurgical instruments. LVA surgery has been widely implemented as a surgical treatment of lymphedema and it has shown promising results20,21,22. The aim of this 6-month interim analysis is to show the first results of the first randomized controlled trial (RCT) evaluating HrQol in patients with BCRL undergoing LVA surgery in comparison to conservative treatment.
Methods
From 2018 to 2022, 100 women with BCRL, with stage 1 or 2a, according to the international society of lymphology (ISL) with viable lymphatic vessels and ICG stage II–III according to Narushima measured by near infrared fluoroscopy (NIRF) were included23,24. The patients were allocated into two groups, an LVA group and CDT group. The full protocol for the trial has been previously published25. The protocol and related documents were approved by the Dutch Medical ethical assessment committee (NL67059.068.18/ METC18-024) registered on 19/12/2018. Clinical Trial Registration: NCT02790021 registered on 03/06/2016. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Design
The trial was an multi-center randomized controlled trial (RCT). Patients were included in the Maastricht University Medical Center + , Radboud University Medical Center, Zuyderland Medical Centre and the Canisius-Wilhelmina Hospital in the Netherlands. All eligible patients were invited to participate in the RCT. Written informed consent was obtained from all participants.
Randomization
Surgeon investigators (S.S.Q, H.T, D.U) determined whether the patients were eligible for surgery. The collection of outcomes was performed at the outpatient clinic (J.W, S.H and Y.J). After inclusion and informed consent, participants were randomly assigned to either the LVA or conservative (CDT) group with a 1:1 allocation as per a computer-generated randomization schedule stratified by site using block randomization. This computer-generated randomization was done within the electronic Case Report Form in CASTOR EDC.
Interventions
CDT
All patients in both groups underwent at least 3 months of CDT prior to inclusion. The patients allocated in the CDT group continued to be treated according to standard protocol known as the ‘Verdonkmethod’ which is implemented in all patients receiving CDT in the Netherlands25. The CDT consisted of two stages, the first phase entailed skincare, MLD, exercises aimed at improvement of mobility/range of motion, and compression therapy. The second phase was focused on maintenance of the achieved limb volume reduction through compression therapy with therapeutic elastic compression garment for the arm. Skincare, mobility exercises and MLD is continued in this phase if needed.
Using this standardized protocol we were able to compare the outcomes within the CDT group. The conservative treatment and the frequency is controlled by the skin therapist. During follow up all changes in conservative therapy were noted by each patient in the patient diary.
LVA
The eligibility for participation in the study was determined with near infrared fluorescence imaging (NIRF) during the outpatient clinic. 0.01 to 0.04 ml of indocyanine green (ICG) (5 mg/ml) was injected into the second and fourth finger webspace intradermally of the lymphedematous limb. Using NIRF the viable lymph vessels were identified, marked, and photographed23.
The procedure was performed under general or local anesthesia. This was left at the surgeon’s discretion. During surgery viable lymphatic collecting vessel and similarly sized adjacent recipient venules were identified in the subdermal plane. Subsequently an anastomosis was performed in an end-to-end fashion with 11–0 suture between the lymphatic collecting vessel and the venule, with the use of a surgical microscope. After the anastomosis was made, the patency was confirmed under the microscope. Between 1 and 5 anastomosis were performed in a lymphedematous arm. The superficial wound was closed using 4–0 Ethilon covered by adhesive plasters.
Two weeks after the surgery the patients were able to continue in the maintenance phase of the CDT protocol.
Outcomes variables
All data was collected according to a standardized protocol. The primary outcome was HrQoL, which was measured by the lymphedema international classification of functioning (Lymph-ICF) questionnaire (Dutch version)26. The Lymph-ICF is a validated quality of life questionnaire for patients with upper extremity lymphedema. The questionnaire is divided into five domains: physical function, mental function, household, mobility, life and social activities. A low score on the questionnaire indicates a better quality of life and a higher score indicates of a bad quality of life. A decrease in the sum of scores of more than 10 points, and an increase of more than 9, was considered statistically significant (p < 0.05). A decrease in the sum of scores of more than 15 points was considered clinically significant27.
The secondary outcomes were volume reduction, measured by the water displacement method, circumference reduction measured by the upper extremity lymphedema (UEL) index, the complete or partial discontinuation rate of the use of compression garments, reported through a patient diary. Lastly adverse-, and serious adverse events were reported during follow up28,29. The data was collected after 3, 6, 12, 18 and 24 month follow up. The data used for the current article are based on the first six months follow up.
Statistical analysis
We made the following assumptions for the calculation of the sample size to show a statistically significant and clinically relevant difference in quality of life between treatment groups follow-up as measured with the Lymph-ICF questionnaire:
Comparing LVA group to CDT group, the minimal difference in HRQoL that is considered as clinically relevant is 15 points on the Lymph-ICF questionnaire.
To be able to achieve a power of 80%, a total of 45 patients are needed per treatment group, when the SD is 25%, using an alpha of 0.05. If a drop-out rate (loss-to-follow-up and patients with missing data) of 25% is taken into account, a sample size of 60 patients per study group is required and a total of 120 patients will be randomized. Due to the COVID pandemic outpatient clinic at hospitals in the Netherlands were cancelled and there was a lower inclusion rate. In concordance with the ethical committee an amendment was approved to reduce the sample size to 100 patients considering the low dropout rate (n = 1).
Continuous variables were reported as mean with standard deviation. Categorical data were reported as frequencies. To examine the effect of LVA, the paired-samples t-test was used to evaluate the changes between baseline, 3 and 6 months within the groups for the Lymph ICF questionnaire, the relative volume difference measured by water displacement and the UEL index. To measure the effect of the LVA between groups the independent-samples t-test was performed for the above-mentioned variables.
Furthermore, a linear regression was used to determine the relationship between the number of LVAs, follow-up months, lymphedema onset, BMI and ICG stage and for the HrQoL and volume reduction (measured by volume displacement). Results were expressed as regression coefficient with a 95% confidence interval (CI). The use of compression garments, adverse and serious adverse events were reported as frequency. All analysis were performed with IBM SPSS version 25 (IBM Corp., Armonk, N.Y).
Ethical approval
The study was carried out in accordance to the declaration of Helsinki. The study was approved by the Ethics Committee of Maastricht University Medical Center on 19 December 2018 (NL67059.068.18/ METC18-024). Written consent has been obtained from all participants.
Results
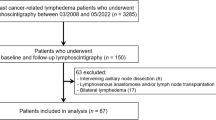
One hundred consecutive patients with unilateral BCRL were eligible for inclusion. Ninety-two patients finished at least 6 months follow up and were included in the interim analysis. The other eight patients had not finished the 6 month follow up. The patient characteristics are listed in Table 1. Ninety patients had stage 2a lymphedema according to the ISL classification. Eighty-five patients had an ICG stage III lymphedema. After 6 months one patient in the CDT group had discontinued follow up.
Primary outcome
HrQoL
In the LVA group the mean difference in the total score of the Lymph ICF between baseline and follow up after three and six months was 8.93 ± 22.71 (p > 0.05) and 8.57 ± 22.56 (p > 0.05), respectively. For the domains physical function and mental function a decrease of more than 10 points was observed after 3 and 6 months, representing a statistically significant improvement (p < 0.05). In the CDT group there was no difference observed in the total score of the Lymph-ICF between baseline and follow up. The mean difference in the total score of the Lymph-ICF was 4.57 ± 14.46 (p > 0.05) after 3 months and 2.65 ± 18.21 (p > 0.05) after 6 months. All data respecting HrQol is presented in Table 2.
When comparing the total score of the Lymph-ICF between the two groups a statistical difference was observed in physical function after 3 (p = 0.006) and 6 months (p = 0.001), presented in Table 3. No relationship between the total score of the Lymph-ICF and preoperative ICG stage, the amount of anastomosis, lymphedema onset, and BMI was found (see Table 4).
Secondary outcomes
Volume reduction
The excess volume was measured by the difference in affected and non-affected arm. The absolute volume difference was 24.80 ± 179.93 mL (p = 0.398) after 3 months and 20.04 ± 196.40 mL (p = 0.497) after 6 months for the LVA group. For the CDT group, 13.88 ± 193.36 mL (p = 0.640) and 33.98 ± 189.87 mL (p = 0.236) after three months and six months, respectively. All data respecting volume measurements is presented in Table 5. There was no significant difference observed between the two groups, − 2.82 (p = 0.737) after 6 months.
Furthermore, there was no correlation between volume difference and preoperative ICG stage, the amount of anastomosis, lymphedema onset, and BMI (see Table 6).
Limb circumference
The mean absolute difference in UEL index for the LVA group 3.65 ± 7.24 (p = 0.002) after 3 months and 1.84 ± 14.6, (p = 0.497) after 6 months, for the CDT group the mean absolute difference was respectively 3.30 ± 31.57 (p = 0.521) and − 0.84 ± 14.6 (p = 0.189) after three and six months. The data is presented in Table 7. Furthermore, there is no significant difference in UEL index between the LVA and CDT group, 2.43 (p = 0.458).
Discontinuation of compression garments
After 3 months 8 patients (17.0%) in the LVA group completely discontinued the use of compression garments. After 6 months the discontinuation rate increased to 10 patients (21.3%). Furthermore after 3 and 6 months, 10 patients (21.7%) partially discontinued use of compression garments. None of the patients in the CDT group discontinued the use of compression garments in the first 6 months.
Adverse events (AE)
Within the first 3 months 7 AE’s were observed.
In the CDT group one subject had a mild episode erysipelas, treated with oral antibiotics and in one patient moderate erysipelas occurred, where treatment with intravenous antibiotics were required. One patient had COVID, one patient was diagnosed with muscular rheumatism.
In the LVA group one patient had moderate erysipelas, treated with intravenous antibiotics, one patient had a skin infection and 1 patient had pneumonia.
After 6 months, 5 AEs occurred in the CDT group and 3 AE in the LVA group occurred. In the CDT group 4 patients had erysipelas, in the LVA group 3 patients. All patients received oral antibiotics for the treatment of erysipelas. One patient in the LVA group had an allergic reaction of unknown origin.
Severe adverse events (SAE)
After 3 months no SAE were reported. After six months one patient in the LVA group reported recurrence of her breast cancer but she remained in the study.
Discussion
Previous published studies on the efficacy of the LVA surgery have shown promising results30,31,32,33. A decrease on subjective complaints and a volume reduction between 0 and 61% have been reported34,35,36. However, most of the studies included a small heterogeneous population and have a retrospective design28,37,38,39,40. The reported studies did not compare LVA surgery with other conservative treatment modalities and use different assessment tools resulting in disparate results35,41,42.
To our knowledge, this the first large scale prospective randomized multicenter study assessing the effectiveness of LVA surgery compared with conservative treatment in patients with BCRL with HrQoL as primary outcome. Currently, there is a wide variety of quality of life questionnaires in lymphedema. The Lymph-ICF for the upper extremity has been well rated in regards to content validity, reliability, and construct validity based on good-quality evidence43.
Intra group analyses demonstrated that physical function and mental function were significantly improved in the LVA group after 3 and 6 months. Moreover, the physical function was significantly improved in the LVA group compared to the CDT group. Indicating that the patients in the LVA group experience a significant improvement in physical symptoms such as heaviness, swelling, weakness, tingling and tightness of the arm as early as 3 months after LVA surgery. Previous studies reported an overall improvement of the subjective symptoms after LVA surgery21,37,44,45. However so far, the total score of the Lymph-ICF in the LVA and CDT group showed no statistically significant improvement.
Notwithstanding, the total score of the Lymph-ICF in the LVA group are promising in comparison to the CDT group where smaller differences in total score of the Lymph-ICF were seen. Furthermore, there was a high variability in the overall cohort. Moreover, patients with lymphedema present a wide array of complaints and symptoms. While some patients experience a detrimental effect in the arm volume, other patients experience more loss of mobility, pain and heaviness5.
After 3 and 6 months no significant changes in arm volume were observed for both groups. Interestingly, after 3 months UEL index significantly improved in the LVA group, however this trend did not persist after 6 months. The volume reductive effect of the LVA treatment could not be evidenced during the first 6 months in the current study. This is concordant with previous studies21,25,45.
However, the absence of volume reduction does not equate treatment failure. Even though the population had early stage lymphedema a discrepancy was seen between the ISL stage and ICG stage. Most of the cohort had ISL-stage 2a lymphedema, however using NIRF we mostly saw patients with ICG stage 3. Moreover, the patients in our cohort have had lymphedema for an average of ~ 7 years. Altogether this could indicate that even in early stage (ISL stage 2a) lymphedema determined by the ISL classification, the lymph vessels in the subdermal plane could already be nonfunctioning. Furthermore, in ISL stage 2a lymphedema changes in the tissue have been established using Dual Energy X-Ray Absorptiometry, such as lymph vessel dysfunction, fat deposition and, fibrosis. However, little is known about the exact physiological progression of lymphedema over time, which makes it difficult to establish which patients will benefit from LVA surgery46,47,48,49.
Other systematic reviews have demonstrated that limb circumference significantly decreased, however these studies have a smaller patient population, reported heterogeneous assessment modalities and a had longer follow up39,50. There are many factors that might influence the outcome of the LVA, however there is a lack of consensus on what these factors are42,51,52,53. A positive correlation between Lymph-ICF and the amount of anastomosis has been reported21. As of yet, in our study no correlation was found between Lymph-ICF the amount of anastomosis, ICG stage, BMI and onset of lymphedema. However, in the study by Qiu et al. patients received multiple LVA surgeries and a longer follow up period then our current cohort21.
In our study there was no correlation shown between volume reduction and the number of anastomosis performed. This concurs with the study conducted by Winters et al. where there was no correlation between the amount of anastomosis and volume reductive effect of the LVA28. In the current cohort there was no relationship between the volume, the duration and severity of lymphedema. Concurrently, a meta-analysis conducted by Nacchiero et al. reported that the stage and duration of lymphedema were not dependent on the success of the operation39,54.
Lastly approximately 42% of the patient population in the LVA group completely of partially discontinued the use of compression garments. The discontinuation rate in our population is line with previously published articles21,44,45. Unfortunately, we did not include the reason for discontinuation in our questionnaire, which might be interesting to investigate in the future.
This study is a 6-month interim analysis where 92% of the population completed six-month follow up. At the time of analysis, one patient had dropped out. Because the design of the current study eliminates any form of selection bias, this study more closely resembles results of the general population in comparison to other smaller and retrospective studies. In our study, patients were only able to receive one LVA surgery whereas in other previously published studies patients were able to receive more sessions, and ultimately more LVA’s21,55. Although the results of the first 6 months may be encouraging, a longer follow up period is required the assess the true effect of the LVA in comparison to CDT for secondary lymphedema.
Conclusion
Lymphaticovenous anastomosis resulted in improvement of physical and mental function in patients with BCRL. Limb volume and limb circumference did not significantly improve after 6 months in both groups. However, in the LVA group around 40% could completely or partially stopped with the use of compression garments. The current results are promising, however longer follow up is required to assess the long term effect of LVA.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Adverse events
- BCRL:
-
Breast cancer related lymphedema
- BMI:
-
Body mass index
- CDT:
-
Complex decongestive therapy
- CI:
-
Confidence interval
- HrQoL:
-
Health related quality of life
- ICG:
-
Indocyanine green
- ISL:
-
International society of lymphology
- LVA:
-
Lymphaticovenous anastomosis
- Lymph-ICF:
-
Lymphedema functioning, disability and health questionnaire
- MLD:
-
Manual lymphatic drainage
- NIRF:
-
Near infrared fluoroscopy
- RCT:
-
Randomized controlled trial
- SAE:
-
Severe adverse events
- UEL-index:
-
Upper extremity lymphedema index
- VAS:
-
Visual analogue score
- WDM:
-
Water displacement method
References
Gillespie, T. C. et al. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 7(4), 379–403 (2018).
Fu, M. R. Breast cancer-related lymphedema: Symptoms, diagnosis, risk reduction, and management. World J. Clin. Oncol. 5(3), 241–247 (2014).
Wanchai, A. et al. Breast cancer-related lymphedema: A literature review for clinical practice. Int. J. Nurs. Sci. 3(2), 202–207 (2016).
Morrell, R. M. et al. Breast cancer-related lymphedema. Mayo Clin. Proc. 80(11), 1480–1484 (2005).
Anbari, A. B., Wanchai, A. & Armer, J. M. Breast cancer-related lymphedema and quality of life: A qualitative analysis over years of survivorship. Chronic Illn. 17(3), 257–268 (2021).
Petrek, J. A. & Heelan, M. C. Incidence of breast carcinoma-related lymphedema. Cancer 83(S12B), 2776–2781 (1998).
Hayes, S. C. et al. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J. Clin. Oncol. 26(21), 3536–3542 (2008).
Kwan, M. L. et al. Risk factors for lymphedema in a prospective breast cancer survivorship study: The Pathways Study. Arch. Surg. 145(11), 1055–1063 (2010).
Ren, Y. et al. Burden of lymphedema in long-term breast cancer survivors by race and age. Cancer 128(23), 4119–4128 (2022).
Kim, K. & Yoon, H. Health-related quality of life among cancer survivors depending on the occupational status. Int. J. Environ. Res. Public Health 18(7), 3803 (2021).
de Ligt, K. M. et al. The impact of health symptoms on health-related quality of life in early-stage breast cancer survivors. Breast Cancer Res. Treat. 178(3), 703–711 (2019).
Ahmed, R. L. et al. Lymphedema and quality of life in breast cancer survivors: The Iowa Women’s Health Study. J. Clin. Oncol. 26(35), 5689–5696 (2008).
Pusic, A. L. et al. Quality of life among breast cancer patients with lymphedema: A systematic review of patient-reported outcome instruments and outcomes. J. Cancer Surviv. 7(1), 83–92 (2013).
Rockson, S. G. et al. Cancer-associated secondary lymphoedema. Nat. Rev. Dis. Primers 5(1), 22 (2019).
Zasadzka, E. et al. Comparison of the effectiveness of complex decongestive therapy and compression bandaging as a method of treatment of lymphedema in the elderly. Clin. Interv. Aging 13, 929–934 (2018).
Ridner, S. H. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 13(11), 904–911 (2005).
Nielubowicz, J. & Olszewski, W. Surgical lymphaticovenous shunts in patients with secondary lymphoedema. Br. J. Surg. 55(6), 440–442 (1968).
Jacobson, J. H. 2nd. & Suarez, E. L. Microvascular surgery. Dis. Chest 41, 220–4 (1962).
Koshima, I. et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J. Reconstr. Microsurg. 16(6), 437–442 (2000).
Park, K. E. et al. Surgical management of lymphedema: A review of current literature. Gland Surg. 9(2), 503–511 (2020).
Qiu, S. S. et al. Outcomes following lymphaticovenous anastomosis (LVA) for 100 cases of lymphedema: Results over 24-months follow-up. Breast Cancer Res. Treat. 184(1), 173–183 (2020).
Chang, D. W., Suami, H. & Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 132(5), 1305–1314 (2013).
Narushima, M. et al. Indocyanine green lymphography findings in limb lymphedema. J. Reconstr. Microsurg. 32(1), 72–79 (2016).
The Diagnosis and Treatment of Peripheral Lymphedema. 2016 consensus document of the international society of lymphology. Lymphology 49(4), 170–184 (2016).
Wolfs, J. et al. Improving the quality of life of patients with breast cancer-related lymphoedema by lymphaticovenous anastomosis (LVA): Study protocol of a multicentre randomised controlled trial. BMJ Open 10(1), e035337 (2020).
Devoogdt, N. et al. Lymphoedema functioning, disability and health questionnaire (Lymph-ICF): Reliability and validity. Phys. Ther. 91(6), 944–957 (2011).
De Vrieze, T. et al. Revision of the lymphedema functioning, disability and health questionnaire for upper limb lymphedema (Lymph-ICF-UL): Reliability and validity. Lymphat. Res. Biol. 17(3), 347–355 (2019).
Yamamoto, T. et al. Upper extremity lymphedema index: A simple method for severity evaluation of upper extremity lymphedema. Ann. Plast. Surg. 70(1), 47–49 (2013).
Sagen, A. et al. Validity for the simplified water displacement instrument to measure arm lymphedema as a result of breast cancer surgery. Arch. Phys. Med. Rehabil. 90(5), 803–809 (2009).
Mihara, M. et al. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br. J. Surg. 101(11), 1391–1396 (2014).
Auba, C. et al. Lymphaticovenular anastomoses for lymphedema treatment: 18 months postoperative outcomes. Microsurgery 32(4), 261–268 (2012).
Akita, S. et al. Suitable therapy options for sub-clinical and early-stage lymphoedema patients. J. Plast. Reconstr. Aesthet. Surg. 67(4), 520–525 (2014).
Chen, W. F. et al. Indocyanine green lymphographic evidence of surgical efficacy following microsurgical and supermicrosurgical lymphedema reconstructions. J. Reconstr. Microsurg. 32(09), 688–698 (2016).
Baumeister, R. G. & Siuda, S. Treatment of lymphedemas by microsurgical lymphatic grafting: What is proved?. Plast. Reconstr. Surg. 85(1), 64–74 (1990).
Carl, H. M. et al. Systematic review of the surgical treatment of extremity lymphedema. J. Reconstr. Microsurg. 33(6), 412–425 (2017).
Masià, J., Pons, G. & Rodríguez-Bauzà, E. Barcelona lymphedema algorithm for surgical treatment in breast cancer-related lymphedema. J. Reconstr. Microsurg. 32(05), 329–335 (2016).
Winters, H. et al. The efficacy of lymphaticovenular anastomosis in breast cancer-related lymphedema. Breast Cancer Res. Treat. 165(2), 321–327 (2017).
Yamamoto, T. et al. Navigation lymphatic supermicrosurgery for the treatment of cancer-related peripheral lymphedema. Vasc. Endovasc. Surg. 48(2), 139–143 (2013).
Nacchiero, E. et al. Lymphovenous anastomosis for the treatment of lymphedema: A systematic review of the literature and meta-analysis. Lymphology 53, 172–194 (2020).
Will, P. A. et al. Supermicrosurgical treatment for lymphedema: A systematic review and network meta-analysis protocol. Syst. Rev. 11(1), 18 (2022).
Wang, S. et al. Supermicrosurgical lymphoevenous anastomosis for the treatment of peripheral lymphedema: A systematic review of the literature. Chin. J. Plast. Reconstr. Surg. 3(3), 155–160 (2021).
Rosian, K. & Stanak, M. Efficacy and safety assessment of lymphovenous anastomosis in patients with primary and secondary lymphoedema: A systematic review of prospective evidence. Microsurgery 39(8), 763–772 (2019).
Paramanandam, V. S. et al. Self-reported questionnaires for lymphoedema: A systematic review of measurement properties using COSMIN framework. Acta Oncol. 60(3), 379–391 (2021).
Wolfs, J. et al. Correlation between patency and clinical improvement after lymphaticovenous anastomosis (LVA) in breast cancer-related lymphedema: 12-month follow-up. Breast Cancer Res. Treat. 179(1), 131–138 (2020).
Cornelissen, A. J. M. et al. Lymphatico-venous anastomosis as treatment for breast cancer-related lymphedema: A prospective study on quality of life. Breast Cancer Res. Treat. 163(2), 281–286 (2017).
Mihara, M. et al. Lymphatic venous anastomosis can release the lymphedema-associated pain of upper limb after breast cancer treatment. J. Reconstr. Microsurg. Open 03(01), e1–e7 (2018).
Garza, R. M. et al. The relationship between clinical and indocyanine green staging in lymphedema. Lymphat. Res. Biol. 17(3), 329–333 (2019).
Zhang, J., Hoffner, M. & Brorson, H. Adipocytes are larger in lymphedematous extremities than in controls. J. Plast. Surg. Hand Surg. 56(3), 172–179 (2022).
Jørgensen, M. G. et al. Indocyanine green lymphangiography is superior to clinical staging in breast cancer-related lymphedema. Sci. Rep. 11(1), 21103 (2021).
Basta, M. N., Gao, L. L. & Wu, L. C. Operative treatment of peripheral lymphedema: A systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast. Reconstr. Surg. 133(4), 905–913 (2014).
Tsai, P.-L. et al. Determining factors in relation to lymphovascular characteristics and anastomotic configuration in supermicrosurgical lymphaticovenous anastomosis—A retrospective cohort study. Int. J. Surg. 81, 39–46 (2020).
Klingelhöfer, E. et al. Factors affecting outcomes after supermicrosurgical lymphovenous anastomosis in a defined patient population1. Clin. Hemorheol. Microcirc. 73, 1–11 (2019).
Chen, W. F. How to get started performing supermicrosurgical lymphaticovenular anastomosis to treat lymphedema. Ann. Plast. Surg. 81(6S), S15–S20 (2018).
Chang, D. W. Lymphaticovenular bypass for lymphedema management in breast cancer patients: A prospective study. Plast. Reconstr. Surg. 126(3), 752–758 (2010).
Pandey, S. K. et al. Supermicrosurgical lymphaticovenular anastomosis <i>vs. </i> vascularized lymph vessel transplant—Technical optimization and when to perform which. Plast. Aesthet. Res. 8, 47 (2021).
Funding
This work was supported by ZonMw, grant number 80-85200-98-91044.
Author information
Authors and Affiliations
Contributions
The study conception and design were contributed by S.S.Q., J.W., R.vdH., D.U., H.T., S.H., X.K. Data collection was contributed by J.W., S.H., Y.J. Surgeries were performed by S.S.Q., H.T., D.U. Analysis and interpretation of results were contributed by Y.J. and S.vK. Draft manuscript preparation was contributed by Y.J. All authors refined and contributed of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jonis, Y.M.J., Wolfs, J.A.G.N., Hummelink, S. et al. The 6 month interim analysis of a randomized controlled trial assessing the quality of life in patients with breast cancer related lymphedema undergoing lymphaticovenous anastomosis vs. conservative therapy. Sci Rep 14, 2238 (2024). https://doi.org/10.1038/s41598-024-52489-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52489-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.