Abstract
The study investigated the antifungal and phytochemical properties of three forest plants (Eucalyptus globulus, Pistacia lentiscus, and Juniperus phoenicea) against apple diseases caused by Colletotrichum gloeosporioides and Alternaria alternata. The determination of the total polyphenol and flavonoid contents in the three aqueous extracts of studied plants showed that E. globulus exhibited the highest contents than those of P. lentiscus and J. phoenicea. Furthermore, the three studied extracts showed very appreciable antioxidant activity with decreasing order: E. globulus, P. lentiscus, and J. phoenicea. The phytochemical analysis showed different common phenolic acids in the three studied plants namely: quinic acid, gallic acid, chlorogenic acid, and caffeoylquinic acid as well as other flavonoids mainly quercetin and catechin. The results of the current study demonstrated that the fungistatic activity of E. globulus EO (4 and 2 µl/ml) seemed to be the most effective under laboratory conditions with an inhibition zone diameter above 16 mm. However, the poisoned food technique indicated that the aqueous extract (80%) and the essential oil (4 µl/ml) of E. globulus exhibited the highest mycelial growth (> 67%) and spore germination (> 99%) inhibition. Preventive treatments with essential oils (4 µl/ml) and aqueous extracts (80%) applied to apple fruits inoculated with A. alternata and C. gloeosporioides resulted in the lowest lesion diameter (< 6.80 mm) and disease severity index (< 15%) and the most favorable inhibitory growth (> 85.45%) and protective potentials (> 84.92%). The results suggest that E. globulus has a brilliant future in the management of anthracnose and Alternaria rot of apple and provide a basis for further studies on its effects under field conditions.
Similar content being viewed by others
Introduction
Fungal infections are regarded as the most damaging postharvest diseases for fresh fruits, producing significant economic losses due to their negative impact on market value and fruit shelf life1. However, the establishment of fungal infections in fruit has therefore several consequences, ranging from yield lowering and quality depressing in the fields to retarding their nutritive value and rapid perishing after harvesting2,3. Furthermore, infected fruits with fungal pathogens pose an impending health risk through mycotoxins production, such as aflatoxins, ochratoxins, and fumonisin are produced by Aspergillus spp., Alternaria spp., and Fusarium spp. in contaminated fruits and vegetables4. Alternaria spp. produce numerous toxic metabolites such as tenuazonic acid, alternariol, alternariol methyl ether, and altenuene, which have been detected in a wide range of foods and feedstuffs infected by the pathogen. These secondary metabolites are toxic to animals, plants, and human cell cultures5. Tenuazonic acid is thought to be a contributing factor to onyalai, a hematological disorder in humans, and it inhibits protein synthesis6.
To date, fungicides are the principal methods of controlling fungal diseases7,8. Nevertheless, during the last few decades, their use was hampered by stricter regulatory policies limiting their doses, and their action spectrum and even restricting their use. This is due to the public rising concern about possible human health risks, undesirable environmental effects, and the development of pathogens’ resistance9,10.
In the absence of effective plant protection management, Colletotrichum gloeosporioides and Alternaria alternata can result in significant yield losses of up to 100% of total apple production2,7,11. Therefore, the development of alternative safe and natural methods for controlling fungal diseases has become an urgent need11,12. Substantial research, using microbial antagonists and extracts have been conducted to prove their efficacy as fungal diseases’ biological control agents and to reduce synthetic fungicides’ usage13. Plant extracts have also been actively explored in recent years as a potential biological agent against many postharvest diseases9. In this context, forest plants are also considered potent sources of phytochemicals such as phenols, flavonoids, and essential oils that could be exploited as a biological control against fungal diseases14.
Recently, Hajji-Hedfi et al.15 reported the chemical composition of aqueous extracts of Pistacia lentiscus and their efficacy in controlling root-knot nematodes Meloidogyne javanica and Fusarium oxysporum sp. lycopersici. Nevertheless, P. lentiscus, J. phoenicea, and E. globulus are considered important medicinal plants largely used in traditional medicine16. Indeed, numerous biological activities have been associated with Eucalyptus extracts, such as antibacterial, antifungal, antioxidant, herbicidal, and acaricidal activity17. Furthermore, the use of natural botanical ingredients is thought to be a good viable alternative to control postharvest diseases. However, few researchers have looked into the ability of plant extracts to suppress the growth of many phytopathogens18.
Examining numerous physicochemical properties of plant extracts reveals the usefulness of these products in daily life. Organoleptic characteristics, color, odor, density, refractive index, acid value, pH, and yield percentage are characteristics that indirectly affect the quality of essential oils19. These physicochemical characteristics as well as the composition, which give benchmark information to gauge an oil's appropriateness for consumption or other use, largely define the commercial importance of these products.
However, limited data are available on the biocontrol efficacy of selected plant extracts (Eucalyptus globulus, Pistacia lentiscus, and Juniperus phoenicea) against the apple rot postharvest fungal diseases and their organoleptic and phytochemical characterization.
The originality of natural plant extracts lies in their fungicidal properties, multifaceted approach, eco-friendly nature, and potential for innovative applications. By addressing existing challenges and continuing research, naturally occurring biologically active compounds from plants can pave the way for a more sustainable and effective future in plant disease management. Therefore, this study aims to determine the phytochemical composition and to evaluate the fungistatic and antioxidant activities of aqueous extracts and essential oils extracted from E. globulus, P. lentiscus, and J. phoenicea. These extracts will be tested to investigate their efficacy as new biological control sources against postharvest disease apple fruit rot caused by C. gloeosporioides and A. alternata. In addition, to the best of our knowledge, it is the first time to assess the aqueous and essential oils extracts from E. globulus, P. lentiscus, and J. phoenicea against C. gloeosporioides and A. alternata.
Material and methods
Plant material and extract preparations
Three species of forest trees, Pistacia lentiscus L. (Anacardiaceae), Juniperus phoenicea L. (Cupressaceae), and Eucalyptus globulus Labill. (Myrtaceae), were utilized as sources of plant material in this study. Fresh leaves were collected from the mountains of the Kairouan region in the Center East of Tunisia. Dr Aliat Taoufik from the Higher National School of Forests, Khenchela, Algeria, identified Forest plant species. No voucher specimen of these materials have been deposited in a publicly available herbarium. The plant collection complied with relevant local, institutional, national, and international guidelines, permissions, or legislation and we obtained the necessary permissions from the governorate of Kairouan. Leaves samples were carefully cleaned with tap water and then rinsed with distilled water, and shade-dried at room temperature for fifteen days. Parts of the dried leaves were ground to uniform powder using a mechanical mixer (MRC, SM 450, China). The obtained powder as well as dry leaves were stored away from moisture and light at 4 °C until further use.
Aqueous extracts
To prepare the aqueous extract, 10 g of the leaf powder of each plant was added to 100 ml of distilled water and vigorously mixed in an Erlenmeyer flask. The maceration process lasts 48 h at room temperature with periodic shaking every 2 h. The mixture was filtered using a Whatman N°1 filter paper to separate the aqueous extract. Different concentrations (C1: 80, C2: 60, C3: 30, C4: 20, and C5: 10% v/v) were prepared by diluting the stock aqueous solution to finalize the in vitro experiments9.
Essential oils extract
Hydro-distillation method was employed using a Clevenger-type apparatus in a 1 L flask. The extraction was performed for 2 h as follows: dry leaves of each plant (100 g) were first cut into small pieces to facilitate their placement in the round Pyrex glass flask that contains 1 L of water. Then the Pyrex glass was placed on an adjustable-temperature balloon heater. The ebullient vapor is carried via the vertical tube (rectification column) and then to the cooling column (also known as the refrigerator or serpentine) where the vapor is condensed. At the end of the hydrodistillation process, a collecting tube (settling column) collects the two liquids (essential oil and distillate). The volatile oil separates as an upper layer because it is immiscible and lighter than water. Following the separation of the oil from the water, it was gathered in small bottles, dried with anhydrous sodium sulfate, sealed, and kept in light-resistant vials at 4–6 °C for later use1,17,20.
Total polyphenols contents of aqueous extracts
The total polyphenols for the different samples were quantified using the folin-ciocalteu reagent according to the method described by Apolonio-Rodríguez et al.20. Briefly, 100 μl of each aqueous extract was added to 100 μl of folin-ciocalteu (1:1) and mixed. After 5 mn, 2 mL of sodium carbonate Na2CO3 (2%) was added. The mixture is vortexed and incubated for 30 min at room temperature in the dark. The volume was then adjusted to 10 ml with distilled water. Absorbance was determined, after one hour at one wavelength (750 nm) using a spectrophotometer (SP-UV5000, China). The measurement is compared to a blank and a calibration curve established by 5 increasing concentrations of gallic acid (0–1000 ppm) under the same conditions. The results were expressed as equivalent to gallic acid in milligram (EAG) mg/g of dry matter20.
Total flavonoid contents of aqueous extracts
A standard range was created based on the varying concentrations of catechin at 40, 80, 120, 160, and 200 μg/ml. For the control, 400 μL of distilled water is used. In test tubes, 400 μL of each concentration is inserted. After 5 min, 120 μL of NaNO2 and 120 μL of AlCl3 were added to each sample of aqueous extract as well as to the control. The mixture was vigorously stirred. 5 min after, a volume of 800 μL of NaOH was added. A spectrophotometer (SP-UV5000, China) was then used for an absorbance reading at 510 nm wavelength. The result was expressed in mg of catechin equivalents (mg CAT/g DW), through the calibration curve of the catechin (range 0–200 μg/mL)21.
Antioxidant activity of aqueous extracts
Antioxidant activity is based on the reduction of DPPH (1,1-diphény l-2-picryl-hydrazyl), a stable free radical violet in solution with a distinctive absorbance between 512 and 517 nm. DPPH was reduced to diphenylpicryl-hydrazine by a compound with anti-free radical properties. DPPH (0.0078 g) was dissolved in methanol to have a volume of 100 ml of DDPH solution. The tests were performed three times with 1 ml of DPPH solution added to 1 ml of extracts. The absorbance was measured against a blank at 517 nm (SP-UV5000 spectrophotometer, China). The inhibition activity of DPPH radical of the studied extracts is then expressed in % and calculated according to the following formula (1) 20:
where Ac is the absorbance of the control of DPPH, and Ae is the absorbance of the sample in the presence of DPPH.
Phenolic profile of aqueous extracts by HPLC–DAD
The phenolic compounds were quantified using a Shimadzu UFLC XR system (Kyoto, Japan), equipped with a SIL-20 AXR auto-sampler, a CTO-20 AC column oven, an LC binary pump-20ADXR and a 2020 quadruple detector system.
In this part of the study, we used the aqueous extracts (previously prepared), and separation was carried out by HPLC (high-performance liquid chromatography). The combinations of these different products (H2O (95%), methanol (5%), acetic acid (0.15%), and (acetonitrile (50%), H2O (50%), folic acid (0.1%) have been used as mobile phases A and B, respectively. Phenolic compounds were identified by comparison with the retention time of phenolic standards and results were reported as mean ± standard error22.
Organoleptic and physicochemical properties of essential oils
A benchmark for the quality of oils is provided by their physicochemical properties. General aspects of essential oils’ physicochemical properties were determined. Color and odor were scored using a scale from 1 to 8; 1 = extremely weak intensity, 2 = very weak intensity, 3 = weak intensity, 4 = moderate intensity, 5 = slight intensity 6 = strong intensity, 7 = very strong intensity, 8 = extremely strong intensity23,24. pH using a pH meter (HI 9125-Hanna-USA), density according to AFNOR. NF ISO 279, (Standard NFT 75–111)25, refractive index using an Atago (Atago pal-α, Japan)26, acid index by referring to ISO 1242:1999/NF ISO 1242:1999 (T 75–103)27, and percentage of yield, which was determined about the dry mass (100 g) of the original sample.
Essential oils composition: gas chromatography analyses
GC–MS analyses were performed with a gas chromatograph (Agilent 7890A) equipped with an HP-5MS capillary column (30 m × 0.25 mm) and associated with a mass selective detector (Agilent 5975C inter MSD). The flow of the carrier gas (helium) was 0.8 ml/min. The oven temperature was programmed from 60 to 240 °C at 4 °C/min. The injector temperature was maintained at 250 °C. The temperatures of the quadrupole and the source were 150 and 230 °C, respectively. The mass scan ranged from 50 to 550 m/z at 70 eV. Essential oil components were identified by comparison of their retention times with those of authentic standards available in the laboratory of plant biotechnology, National Institute of Applied Science and Technology (Tunis, Tunisia), and also by comparison of their retention indices according to the literature. The retention indices were calculated according to a series of n-alkanes (C9–C24). The identification was also completed by comparison of their mass spectra with those stored in NIST08 and W8N08 libraries28.
Fungal strains
Alternaria alternata (Fr. keissl.) (Pleosporaceae) and Colletotrichum gloeosporioides (Penz.) (Glomerellaceae), causal agents of apple rot, used in the present study were obtained from the laboratory of Plant Protection and Biological Sciences, Higher Agronomic Institute of Chott-Meriem (Sousse, Tunisia). These phytopathogens were isolated from infested apple fruits (cv. Zina) collected from fields cultivated with apple trees (Sbiba, Kasserine, Tunisia).
Fungistatic activity of essential oils
The relative efficacy of essential oils on inhibition zone diameter (IZD) of A. alternata and C. gloeosporioides was studied in vitro using the disc diffusion method, as described by Perczak et al.29.
Fungistatic activity is revealed by the absence or presence of mycelia proliferation. It results in a translucent halo around the disc identical to sterile agar30,31.
Effect of essential oils and aqueous extract on mycelium growth and spore germination inhibition
The effect of E. globulus, J. phoenicea, and P. lentiscus essential oils on the spore germination of the pathogenic fungi was tested in potato dextrose broth (PDB) as described by Moreira et al.32 and Droby et al.33. The percentage of inhibition of spore germination was estimated using the formula (2):
where C, Number of germinated spores in the control treatment, and D, Number of germinated spores in the experimental treatment.
Different concentrations of aqueous extracts (10%, 20%, 30%, 60% and 80%) and essential oils (4, 2, 1, 0.5, 0.25 and 0.125 µl/ml PDA) containing 0.05% dimethylsulfoxide (DMSO) each were prepared to evaluate their fungistatic activities against A. alternata and C. gloeosporioides according to the method of poisoned food as described by Matrood and Rhouma9. Then, the results were expressed as percent inhibition of radial growth using the formula (3) Philippe et al.34:
where: Pl is the percentage inhibition, D is the mycelial growth in control Petri dishes, and d is the mycelial growth in the test Petri dishes.
A resumption of growth indicates a fungistatic effect and the absence of growth has a fungicidal effect.
Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) is the lowest concentration of tested plant extract (essential oil or aqueous extract) that completely inhibits the growth of A. alternata and C. gloeosporioides35.
In vivo fungistatic activity assay
The in vivo assay was conducted as previously described by Zhao et al.36. Healthy apple fruit cv. ‘Zina’(without physical injuries and infections) were harvested from fields cultivated with apple trees (Sbiba, Kasserine, Tunisia) based on size uniformity. Sampling fruits were soaked in 2% sodium hypochlorite for 3 min, then washed twice with distilled water, and air-dried. Apple fruits were wounded with a sterilized cork borer (6 mm diameter and 5 mm depth), for three wounds per fruit. Then, the fruits were dipped separately in prepared essential oils (4, 2, 1, 0.5, 0.25, and 0.125 µl/ml) and aqueous extracts (80, 60, 30, 20, and 10% v/v) concentrations of E. globulus, P. lentiscus, and J. phoenicea for 5 min. Two hours later, treated fruits were inoculated separately by spraying with 50 μL of A. alternata and C. gloeosporioides spore suspensions (106 spores.mL−1). Two controls were performed; one by inoculating the fruits with pathogen only (positive control), and the other with distilled water (negative control). Each treatment consisted of 27 fruits per replicate (3 replicates), and the experiment was performed twice. An average of nine treated fruits were placed in plastic containers on sterile wet paper. The containers were enclosed in a plastic bag to maintain high humidity (> 90%) and subsequently incubated for 7 days in a growth chamber at 25°C36.
After the incubation time, the lesion diameter (LD) (mm) and disease severity index (DSI) (%) were assessed. McKinney’s equation was used to calculate the disease severity index: DSI (%) = (Σvn)/(NV) × 100, where v represents the numeric value of the disease index (DI) scale, n is the number of plants assigned to the disease index scale, N is the total number of the plants and V is the numeric value of the highest disease index scale9. The disease index (DI) with a scale from 0 to 5; 0 = no rotting, 1 = 1–10%of the fruit surface, 2 = 11–20%of the fruit surface, 3 = 21–30%of the fruit surface, 4 = 31–50%of the fruit surface, and 5 = more than 50%of the fruit surface37. The efficacy of each treatment was rated based on its DSI: EE: Extremely effective (DSI = 0%), HE: Highly effective (DSI = 0.1–5%), E: effective (DSI = 5.1–25%), I: In-effective (DSI = 25.1–50%) and HI: Highly in-effective (DSI = 50.1–100%)38.
To compare the treatment’s effectiveness, the percentage values of infected wounds were transformed into percentages of protective (PP) and inhibitory growth potential (IGP), as follows39:
Statistical analysis
Each tested concentration of essential oils and aqueous extracts of the studied plants is tested three times. The fungistatic activity of both products was also repeated 3 times (n = 9), and the average of the tests is considered for statistical analysis. The variance analyses (ANOVA) are performed with the statistical software SPSS (version 20) to compare the effect of the different studied concentrations. For each case, the differences are considered significant at 5% (P ≤ 0.05) according to Tukey's multiple-range test.
Ethics declarations
The experiments did not involve endangered or protected species. All plant materials were collected and used in accordance with national and international standards and local laws and regulations. Furthermore, the sites where the plant material was harvested are not included in national or local parks or any other natural protected areas.
Results
Phytochemical screening of aqueous extracts
The phytochemical and bioactive properties of the aqueous extracts of studied plant species clearly show that E. globulus has the highest contents of total polyphenols (TPC) (348.17 mg EAG/g DW) compared to P. lentiscus and J. phoenicea which record respectively the following amount (319.88 mg EAG/g DW), (260.69 mg EAG/g DW) (Table 1).
The flavonoid contents in three aqueous plant extracts were determined to be in the range of 71.56 Ec/g DW for E. globulus, 65.23 mg Ec/g DW for J. phoenicea, and 72.82 Ec/g DW for P. lentiscus (Table 1).
The obtained results showed that plant extracts have high antioxidant activity. The anti-radical capacities recorded in our study are of decreasing order: 78.65% for E. globulus, followed by P. lentiscus records a value of 58.78% and 48.84% for J. phoenicea (Table 1).
Analysis of phenolic constituents of the leaf's aqueous content shows some variations by plant species (Table 1). According to the results, phenolic acids (quinic acid, gallic acid, chlorogenic acid, and caffeoylquinic acid) dominate the composition. The coumaric acid was only present in the extract of E. globulus, and P. lentiscus, while the synergic, and transfrulic acid were only detected in the extract of the Eucalyptus plant (Table 1).
The main detected flavonoids in our plants are quercetin, rutin, and catechin. Quercetin-3-o-galactoside is the most concentrated flavonoid in P. lentiscus extract (1864.89 ppm), followed by catechin (1013.98 ppm) in J. phoenicea and rutin (963.74 ppm) in E. globulus extract. It is to be noted that E. globulus extract included the most important number of components by its aqueous extract. The main detected flavonoids in the extract of P. lentiscus classified according to their concentration in the extract are as follows: quercetin, apeginin, kampherol, catechin, rutin, cirsiliol, and naringenin (Table 1).
Phytochemical characterization of essential oils
The organoleptic and physicochemical results of essential oils are shown in Table 2. All three plants produced liquid essential oils with the yellow color varying from very light for the J. phoenicea extract to darker coloration for P. lentiscus and greenish–yellow pigmentation for E. globulus. The different extracts presented pleasant odors with fresh and strong fragrances for E. globulus and J. phoenicea respectively whereas smelled a strong spicy flavor (Table 2).
The obtained physicochemical parameters demonstrated an acid pH of the three extracts, a close purity presented by the refraction index of about 1, 4, and a similar density value ranging from 0.87 g/cm3 for E. globulus and P. lentiscus to 0.83 g/cm3 for J. phoenicea. The acid index representing the stability degree of the essential oil was significantly different between the three species and the most important 2.84 mg KOH/g was that of E. globulus which by the way gave the best extraction yield of 3.14% by hydro-distillation (Tables 2 and 3).
The chemical composition of different extracts is presented in Table 4. The most common class of chemical compounds identified were sesquiterpenes hydrocarbons and monoterpenes hydrocarbons, while the esters were the lowest (Table 4). Results show that extracts are mainly composed of terpenoids and organic compounds witch concentrations vary between the three studied plants. According to the results, each plant EO extract is characterized by some specific components. For example, P. lentiscus presented four specific constituents, which are Terpinen-4-ol, β-Gurjunene, Epicadinol, and Isoledene. J. phoenicea displayed six distinct components, while E. globulus had nine unique ones (Table 4).
Fungistatic activity of essential oil
The disc diffusion method was used to emphasize the fungistatic potency of essential oils on the development of the studied fungus species. The results indicate that the essential oil of E. globulus has the potential to prevent the mycelial development of (A. alternata and C. gloeosporioides). Both fungi were classified as sensitive toward the essential oil of E. globulus (Table 5). At the concentration C1 (½), all plants showed potency against both pathogenic fungi with suppressive zone diameters between 15.12 and 22.65. This inhibition was confirmed by microscopic observation of the spore suspensions with and without essential oil disk (Table 5).
Effect of essential oils and aqueous extract on mycelium growth and spore germination inhibition
The effects of essential oils extracts of forest plant leaves at different dilutions on the spore germination and mycelium growth of C. gloeosporioides and A. alternate are shown in Table 6. The results showed significant inhibition of fungal spore germination (P < 0.05) by J. phoenicea followed by P. lentiscus and E. globulus at different concentrations. Data recorded after 7 days revealed that J. phoenicea EOs was most effective in inhibiting fungal spore germination from 50 to 100% at all applied concentrations (4, 2, 1, 0.5, and 0.25 µl/ml) (Table 6).
Regarding the fungal mycelium growth, E. globulus was the most efficient in inhibiting C. gloeosporioides and A. alternata growth by recording inhibition rates of 78.37% and 72.86%, respectively. The last J. phonenicea extracts are shown to be the least effective one in reducing in vitro mycelium growth of both fungi (Table 6).
C. gloeosporioides and A. alternata were exposed to different concentrations (C1–C5) of three aqueous extracts, after 7 days the mycelia growth of both fungi was measured and the spores’ germination rates were controlled (Table 7). Obtained results show that the inhibition of the mycelial growth of the fungus gradually increases with the concentration of the extract and reaches about 67% for C. gloeosporioides with the most concentrated extract “C5” (80% v/v) (Table 7).
For P. lentiscus and E. globulus, statistical analysis showed a significant difference (P = < 0.05) between the homogeneous group of the three concentrations C3, C4, and C5 and the group of C1 and C2. However, for J. phoenicea the only concentration that showed a significant effect on the inhibition of the growth of C. gloeosporioides was C5. When comparing all concentrations of the three extracts, we note that E. globulus provided the best decrease in fungal mycelial growth at all doses (Table 7).
Regarding A. alternata, the C5 concentration of E. globulus aqueous extract could inhibit the mycelia growth of the fungus with an inhibition percentage of 80%. However, the aqueous extract obtained from the species P. lentiscus didn’t show a promising result since it could only give an inhibition percentage of 50% with three different concentrations C3, C4, and C5. The statistical analysis clearly showed that in all studied species the C5 concentration significantly reduced the mycelial growth of A. alternate (P < 0.05). However, in the case of P. lentiscus, a significant difference was observed between C1 and C2. Likewise, we record a significant difference between C3, C4, and C5. When the fungistatic activity of the three extracts was compared, it was evident that the extract J. phoenicea was the least efficient in inhibiting the development of both fungi (A. alternata and C. gloeosporioides) (Table 7).
The fungus with an inhibition percentage of 80%. However, the aqueous extract obtained from the species P. lentiscus didn’t show a promising result since it could only give an inhibition percentage of 50% with three different concentrations C3, C4, and C5. The statistical analysis clearly showed that in all studied species the C5 concentration significantly reduced the mycelial growth of A. alternate (P < 0.05). However, in the case of P. lentiscus, a significant difference was observed between C1 and C2. Likewise, we record a significant difference between C3, C4, and C5. When the fungistatic activity of the three extracts was compared, it was evident that the extract J. phoenicea was the least efficient in inhibiting the development of both fungi (A. alternata and C. gloeosporioides) (Table 7).
Minimum inhibitory concentration
C. gloeosporioides and A. alternata exhibited high MICs to J. phoenicea essential oil with MICs > 7.514 μg mL−1.E. globulus essential oil had the lowest activity against both phytopathogens (Table 8).
In vivo fungistatic activity assay
The evaluation of essential oils and aqueous extract’s effectiveness in controlling A. alternata and C. gloeosporioides on apple fruits showed that the lesion diameter (LD) and disease severity index (DSI) were significantly lower compared to positive controls (P < 0.01).Generally, a significant decrease in DSI and LD values was observed with augmentation in the concentration (Tables 9 and 10).
Fruits treated separately with essential oils at a concentration of 4 µl/ml revealed a significant reduction in LD, as the values ranged from 0.83 mm (J. phoenicea) to 3.83 mm (E. globulus essential oil) for C. gloeosporioides (34.80 mm ≤ positive control ≤ 38.53 mm), and varied from to 0.67 mm (J. phoenicea) and 3.18 mm (E. globulus) for A. alternata (34.20 mm ≤ positive control ≤ 39.73 mm) (Table 9).The effect of essential oils on DSI was less observed at a concentration of 4 µl/ml, and the values ranged between1.83% (J. phoenicea) and 8% (E. globulus) for C. gloeosporioides (92.33% ≤ positive control ≤ 99.5%), and between 1.33% (J. phoenicea) and 4.83% (E. globulus) for A. alternata (76.5% ≤ positive control ≤ 96.83%) (Table 9). However, the apple fruits treated with essential oils at a concentration of 0.125 µl/mlshowed the highest LD and DSI (Table 9).
Aqueous extracts of E. globulus, P. lentiscus, and J. phoenicea at a concentration of 80% were significantly more effective in reducing the lesion diameter of C. gloeosporioides (3.83 and 5.50 mm for J. phoenicea and E. globulus, respectively) and A. alternata (4.75 and 6.80 mm for E. globulus and J. phoenicea, respectively) than other treatments (Table 10). In the same sense, the lowest DSI were obtained from the apple fruits treated with aqueous extracts at a concentration of 80% and the values ranged between 5% (P. lentiscus/A. alternata) and 15% (E. globulus/C. gloeosporioides) (Table 10).
The treatment effectiveness using essential oils and aqueous extracts of E. globulus, P. lentiscus, and J. phoenicea was variable (Tables 9 and 10). The treatment with essential oils at a concentration of 4 µl/ml showed its ability to protect the apple fruits against A. alternata and C. gloeosporioides (Table 9). The same results were obtained after treatments with aqueous extracts at a concentration of 80% (Table 10). Nevertheless, all treatments using essential oils at 0.125 µl/ml and aqueous extracts at 10% have shown their ineffectiveness in reducing the aggressively of A. alternata and C. gloeosporioides. Thus, the positive controls were highly sensitive to A. alternata and C. gloeosporioides attacks (Tables 9 and 10).
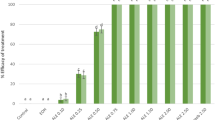
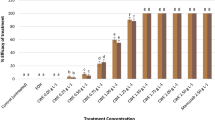
At high concentrations, essential oils have a strong inhibiting effect on the growth potential of A. alternata and C. gloeosporioides reaching up to 88.98%. Similarly, the protective potential was the highest in apple fruits treated separately with essential oils at 4 µl/ml (> 91.67%) (Table 11).
Aqueous extracts exert potent and long-lasting effects on A. alternata and C. gloeosporioides growth with inhibition rates above85.45% (for inhibitory growth potential) and 84.92% (for protective potential) (Table 12).
Discussion
Investigating numerous organoleptic and physicochemical properties enables us to better understand the usefulness of plant oils in daily life40. These characteristics, which offer a foundational assessment of an oil's fitness for use, are largely responsible for its commercial significance41,42.
The acid index of oil is a crucial physicochemical characteristic index that is used to assess its quality, age, digestibility, and appropriateness for industrial applications like paints34. This index is used to determine how much of the oil's glycerides have been broken down by lipase and other physical elements like light and heat43.
The refractive index represents the degree to which a light beam is deflected when it moves from one transparent medium to another. It increases, as there are more carbon atoms and as chains get longer. The refractive index indicates that the sample may contain an unsaturated long carbon chain44. Measurements of the refractive index are particularly useful for determining the purity of volatile and fixed oils45.
The obtained average extraction yield, in this work of E. globulus, is higher than that achieved by some researchers like Kumar et al.46 and Sameza et al.47, in which they found yields of 0.72 and 0.82%, respectively. Recent studies show that the yields of essential oils obtained from several species of Eucalyptus vary between 1.2 and 3%48. This variation between essential oil yields is probably attributed to the difference in the age of the leaves49 or to the time extraction of essential oils46.
The comparison of the essential oil yield of J. phoenicea revealed that it is equivalent to those produced by previous works in Tunisia, which range from 0.5 to 0.9%50,51. Lower yields of (0.39; 0.21; and 0.30) have been recorded in studies conducted in other Mediterranean nations such as Algeria, Greece, and Spain52. The yield of essential oil extracted from P. lentiscus was lower than that obtained by Zrira53 and Trabelsi et al.54 which is between 0.2 and 0.4% and higher than that obtained by Luigia et al.55 estimated at 0.07%. These differences in results can be explained by the fact that the yields of essential oils are influenced by several factors during their extraction: either factors related to the plant (species, variety, chemical race, geographical origin, etc.) or factors related to the experimental conditions (extraction process, extraction time, the part of the plant used, etc.)56,57. Moreover, these authors have shown that the location and duration used for drying leaf samples highly influence the yield of essential oil.
When comparing the organoleptic features of J. phoenicea to those found by Bouchenak et al.58, results revealed some conformity with a slight difference in density (ranging from 0.850 to 0.872).
Despite the high TPC on E. globulus obtained in this study, Zin et al.59 reported higher contents by the order of 432.63 μg EAG/mg DW).
Results obtained with P. lentiscus are close to those of Ebrahimzadeh et al.60 who obtained (289.5 mg EAG/g DW) of TPC. However, our result is higher than those reported by Atmani et al.61 (136.25 ± 18.9 mg EAG/g DW). TPCs on J. phoenicea are found superior to those determined in the studies of Soltani et al.62 with a content of 114.00 mg EAG/g DW and Hayouni et al.63 with a content of 167 mg EAG/g DW, and Keskes et al.64 with a content of 168 mg EAG/g DW.
Our results showed several phytochemicals including phenolic diterpenes, flavonoids, organic compounds, and phenolic acids that are known to be beneficial sources of natural antioxidants and could be isolated for a variety of medicinal, cosmetic, or agro-industrial applications65.
When comparing our J. phoenicea extract results with those cited in the literature, we note that according to Soltani et al.62, the flavonoid concentration of leaf extracts was quite high, with a value of 140.10 mg EC/g DW and Laouar et al.66 have been recorded extremely low values (l2.09 mg EQ/g DW). Studies by Zaouali et al.67 found a higher flavonoid concentration in the extract of the dry leaves of Lentiscus species (47.5 mg EQ/g DW). On the other hand, the flavonoid concentrations measured in E. globulus extracts are significantly higher than those reported in previous studies by Nicoláset al.68.
According to Atmani et al.61, the leaves of P. lentiscus have an anti-free radical activity (DPPH) of 93%, which is significantly higher than that of the current study. Similarly, extracts of E. globulus had significant percentages of free radical catching DPPH (97.63 percent and 76.2 percent, respectively)69. However, the obtained results by Medini et al.70 on J. phoenicea harvested in Tunisia, have an antioxidant activity of the methanolic extracts, which is more important, compared to this work, their activity varies from 72.15 to 95.89%.
The HPLC phytochemical analysis revealed that the extract of E. globulus has significant antioxidant activity and a high concentration of phenols and flavonoids. These results are confirmed by those of Boulekbache-Makhlouf et al.71. However, catechins are the main flavonoid found in high amounts in J. phoenicea extract.
Leaves of several Eucalyptus species have shown significant biological activities such as antimicrobial, fungistatic, insecticidal, herbicide, acaricidal, and nematicidal72.
Moreover, crude extracts of Pistacia vera, P. terebinthus, and P. lentiscus leaves prevented the development of Pythiumultimum and Rhizoctoniasolani73.
Several studies have also reported that the essential oil from the aerial parts of P. lentiscus has significant antifungaland antibacterial properties73,74. Indeed, El Idrissi et al. 201675 reported that the essential oil of P. lentiscus inhibited the development of R. solani, F. sambucinum, and Candida albicans more than that of Penicillium.
Treatment with aqueous extracts and essential oils showed a valuable positive effect in laboratory conditions, confirming the findings of previous researchers. Indeed, Zhou et al.76 documented that the essential oils of Eucalyptus spp. effectively inhibited the growth of A. alternata and C. gloeosporioides in vitro. Ghaffar et al.77 and Salem et al.78 revealed the effectiveness of Eucalyptus spp. oils in controlling Alternaria spp. and Colletotrichum spp. under in vitro conditions. More so, Pedrotti et al.79 reported the ability of Eucalyptus sp. to reduce the mycelial growth and colonization of A. alternaria, and to inhibit the sporulation and spore germination. El Idrissi et al.75 documented that P. lentiscus oils showed the strongest antibacterial and antibacterial activities under laboratory conditions. Kordali et al.73 depicted that Pistacia spp. oils greatly lowered mycelial growth and spore germination. As stated by Pepeljnjak et al.80; Sati and Joshi 201081 and Bais et al.82, treatment with aqueous extracts and essential oils of Juniperus spp. significantly decreased the fungi mycelial growth.
This study proved that essential oils (at 4 µl/ml) and aqueous extracts (at 80%) of E. globulus, P. lentiscus,andJ. phoenicea have an effective bio-fungicides potential against A. alternata and C. gloeosporioides on apple fruits. Similar to our results, previous reports confirmed the efficacy of aqueous extracts and essential oils against the pathogenicity of A. alternata and C. gloeosporioides37,83. Ikeura et al.84 illustrated the effectiveness of aqueous extracts and essential oils in controlling Penicillium expansum on apple fruits. Steglińska et al.85 documented that plant extracts of Eucalyptus sp. effectively inhibited the growth of Colletotrichum spp. and Alternaria spp. under in vivo conditions.
The antifungal potential of several plant extracts was linked to their phytochemical composition and bioactive compounds like phenolic acids. Phenolic can trigger the plant's natural defense responses, leading to increased production of antifungal compounds and strengthening the plant's cell walls2. The hydroxyl groups of phenolics disrupt the cell membranes of Colletotrichum spp. and Alternaria spp., causing leakage of vital cellular components and ultimately leading to cell death7. Moreover, some phenolics can hinder the function of key enzymes involved in the metabolism of Colletotrichum spp. and Alternaria spp., disrupting their growth and reproduction3,46.
The phytochemical composition of essential oil of Eucalyptus globulus exhibits a remarkable multi-layered defense against fungal pathogens. Monoterpenes (1,8-cineole, α-pinene, and limonene) dominate the composition86,87. These compounds display potent radical scavenging activity, effectively neutralizing reactive oxygen species (ROS) that can damage fungal cell membranes and disrupt vital cellular processes88. This antioxidant shield directly protects the fungal cells from oxidative stress, potentially weakening their virulence87,89. In addition to antioxidant defenses, the essential oil has powerful fungicidal activity90. Limonene and α-pinene cause disruption of fungal cell membranes through their lipophilic properties and lead to cell leakage and collapse91. 1,8-cineole inhibits fungal growth and spore germination, effectively cutting off the reproductive pathways and preventing further colonization87,92. Phenolic compounds exemplified by α-terpineol, add another layer of defense with their inherent antifungal properties, further bolstering the overall efficacy against fungal pathogens93.
The essential oil extracted from Pistacia lentiscus exhibits a fascinating interplay between its phytochemical composition, antioxidant activity, and potent fungicidal effects, ultimately creating a multi-pronged defense against phytopathogens67. Monoterpenes possess antioxidant properties, particularly myrcene, and α-terpineol, which scavenge harmful ROS that can damage fungal cell membranes and disrupt vital processes94. This protective shield indirectly weakens the phytopathogens, making them more susceptible to other essential oils56,57. The lipophilic nature of α-pinene and limonene allows them to infiltrate and disrupt fungal cell membranes, causing leakage and ultimately leading to cell collapse95. Furthermore, sesquiterpenes such as α-caryophyllene inhibit fungal enzymes and metabolic pathways56,57,96. The antioxidant action of myrcene and α-terpineol weakens the phytopathogen's defenses, making them more susceptible to membranous assaults97. This interplay amplifies the individual potencies of each compound, creating a robust and multifaceted defense system. Additionally, the presence of phenolic compounds like gallic acid and its derivatives can contribute to antifungal activity through their direct interaction with fungal membranes and proteins56,57,98,99.
Juniperus phoenicea essential oil boasts a rich of volatile compounds, each playing a crucial role in its antifungal arsenal. Juniperus phoenicea essential oil boasts a diverse cast of monoterpenes like α-pinene, sabinene, and β-pinene. Their lipophilic nature allows infiltrating and disrupting fungal cell membranes, causing leakage and ultimately celling death100,101. Sesquiterpenes (α-cedrene and β-caryophyllene) inhibit crucial fungal enzymes and metabolic pathways102. Phenolic compounds (thymol and carvacrol) are wielding their potent antioxidant properties to neutralize ROS generated by phytopathogens. These ROS can damage fungal cells and weaken their defenses, making them more susceptible to the membranous assaults of the monoterpenes and sesquiterpenes100. In an accordant study, the potential of thymol and carvacrol enhanced the disruption of fungal membranes by monoterpenes, acting as synergistic co-solvents103.
The true masterpiece lies in the intricate synergy between these components. The antioxidant shield of these essential oils provided by phenolic compounds weakens fungal defenses, allowing monoterpenes and sesquiterpenes to suppress pathogens. This combined assault amplifies the individual powers of each compound, creating a robust and multifaceted defense system56,57,103.
Conclusion
From the aforementioned results, the present investigation shows that the aqueous and EO extract of E. globulus leaves exhibit good efficiency in inhibiting the growth and spore germination of tested pathogenic fungi. These extracts have a brilliant future in C. gloeosporioides and A. alternata management to substitute synthetic fungicides. Due to the limited number of commercially natural compounds, it is of great interest to deepen our knowledge about the molecular mechanisms and discover new bio-pesticide and bio-stimulant agents. In addition, the impact of plant extracts application on the consumer’s acceptability and fruit sensory characteristics need to be carefully considered. These obtained results from laboratory experiments, could be supplemented also by other in vivo studies, both in controlled greenhouse conditions and in open fields to practically evaluate the use of these extracts in the frame of an Integrated Crop Management System and introduce them into practical use in preventive conservation.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Vitoratos, A., Bilalis, D., Karkanis, A. & Efthimiadou, A. Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Hortic. Agrobo. 41, 86–92 (2013).
Bensaci, O. A. et al. The use of mycoendophyte-based bioformulations to control apple diseases: Toward an organic apple production system in the Aurès (Algeria). Plants 11, 3405 (2022).
Kumar, S. et al. Anti-oxidant and anti-microbial properties of mutton nuggets incorporated with blends of essential oils. J. Food Sci. Technol. 55, 821–832 (2018).
Sanzani, S. M., Reverberi, M. & Geisen, R. Mycotoxins in harvested fruit and vegetables: Insights in producing fungi biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 122, 95–105 (2016).
Escrivá, L., Oueslati, S., Font, G. & Manyes, L. Alternaria Mycotoxins in food and feed: An overview. J. Food Qual. 156, 1–20 (2017).
Den Hollander, D. et al. Cytotoxic effects of alternariol, alternariol monomethyl-ether, and tenuazonic acid and their relevant combined mixtures on human enterocytes and hepatocytes. Front. Microbiol. 13, 849243 (2022).
Tripathi, A. N., Tiwari, S. K. & Behera, T. K. Postharvest diseases of vegetable crops and their management: Postharvest technology—Recent advances. New Perspect. Appl. 10, 1–18 (2022).
Rebouh, N. Y. et al. Influence of three cultivation technologies to control Fusarium spp. in winter wheat (Triticum aestivum L.) production under Moscow conditions. Res. Crop. 21, 17–25 (2020).
Matrood, A. A. A. & Rhouma, A. Evaluating eco-friendly botanicals as alternatives to synthetic fungicides against the causal agent of early blight of Solanum melongena. J. Plant Dis. Prot. 128, 1517–1530 (2021).
Zemmouri, B. et al. Modelling human health risks frompesticide use in innovative legume-cereal intercropping systems in Mediterranean conditions. Ecotoxicol. Environ. Saf. 238, 113590 (2022).
Wisniewski, M., Droby, S., Norelli, J., Liu, J. & Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 122, 3–10 (2016).
Temirbekova, S. K. et al. Evaluation of wheat resistance to snow mold caused by Microdochium nivale (Fr) Samuels and IC Hallett under abiotic stress influence in the central non-black Earth Region of Russia. Plants 11, 699 (2022).
Rebouh, N. et al. Environmentally friendly wheat farming: Biological and economics efficiency of three treatments to control fungal diseases in winter wheat (Triticum aestivum L.) under field conditions. Plants 10, 1566 (2022).
Tohidi, B., Rahimmalek, M. & Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 220, 153–161 (2017).
Hajji-Hedfi, L. et al. Biological activities and chemical composition of Pistacia lentiscus in controlling Fusarium wilt and root-knot nematode disease complex on tomato. Eur. J. Plant Pathol. 155, 281–291 (2019).
Noumi, E. et al. Thymus musilii Velen. methanolic extract: in vitro and in silico screening of its antimicrobial, antioxidant, anti-quorum sensing, antibiofilm, and anticancer activities. Life. 13, 62 (2023).
Elgat, W. A. A. A. et al. Eucalyptus camaldulensis, Citrus aurantium, and Citrus sinensis essential oils as antifungal activity against Aspergillus flavus, Aspergillus niger, Aspergillus terreus, and Fusarium culmorum. Processes 8, 1003 (2020).
Shuping, D. S. S. & Eloff, J. N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement Altern. Med. 14, 120–127 (2017).
Parthiban, K. T. et al. Physico-chemical characterization of seed oil from Jatropha curcas L. genetic resources. J. Econ. Nat. Environ. 3, 163–167 (2011).
Apolonio-Rodríguez, I., Franco-Mora, O. & Salgado-Siclán, M. L. In vitro inhibition of Botrytis cinerea with extracts of wild grapevine (Vitis spp.) leaves. Rev. Mexicana Fitopatol. 35, 170–185 (2017).
Al-Mayahi, M. Z. & Fayadh, M. H. The effects of garlic extract, its application methods and their interaction on growth and yield of potato, Solanum tuberosum (L.) cv. Latonia. Adv. Agric. Bot. 7, 59–69 (2015).
Li, P. et al. Rapid identification and simultaneous quantification of multiple constituents in nao-shuan-tong capsule by ultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. Sci. 53, 886–897 (2015).
Ren, J. N. et al. Study on the optimization of the decolorization of orange essential oil. Food Sci. Biotechnol. 27, 929–938 (2018).
Zhou, W., Sun, Y., Zou, L., Zhou, L. & Liu, W. Effect of galangal essential oil emulsion on quality attributes of cloudy pineapple juice. Front. Nutr. 8, 751405 (2021).
AFNOR NF ISO 279. Essential oils. Determination of relative density at 20 degrees Celsius. Reference method (1999).
AFNOR. NF ISO 279, (T 75-112). Recueil des normes. Les huiles essentielles. Tome 1. Echantillonnage et méthodes d’analyse (2000).
Sharma, P. R., Joshi, R., Sharma, S. K. & Hsiao, B. S. A simple approach to prepare carboxycellulose nanofibers from untreated biomass. Biomacromol. 18, 2333–2342 (2014).
Lagha-Benamrouche, S., Addar, L., Bouderhem, H., Tani, S. & Madani, K. Caractérisation chimiques des écorces d’oranges, identification par GC-MS et évaluation du pouvoir antioxydant de leurs huiles essentielles. Nat. Techn. 16, 1–8 (2017).
Perczak, A. et al. Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch. Microbiol. 201, 1085–1097 (2019).
Mironescua, M. & Georgescub, C. Preliminary researches on the effect of essential oils on molds isolated from surfaces. J. Agro. Aliment. Proc. Technol. 14, 30–33 (2008).
Ponce, A. G., Fritz, R., Del Valle, C. E. & Roura, S. I. Antimicrobial activity of essential oils on native microbial population of organic Swiss chard. Lebensmittel-Wissenschaft. Tech. 36, 679–684 (2003).
Moreiraa, M. R., Ponceb, A. G., Del Vallea, C. E. & Roura, S. I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT Food Sci. Tech. 38, 565–570 (2005).
Droby, S. et al. Influence of CaCl2 on Penicillium digitatum, grapefruit peel tissue, and biocontrol activity of Pichia guilliermondii. Phytopathology 87, 310–315 (1997).
Philippe, S. et al. Chemical composition and antifungal activity of essential oil of fresh leaves of Ocimum gratissimum from Benin against six mycotoxigenic fungi isolated from traditional cheese wagashi. Res. J. Biol. Sci. 1, 22–27 (2012).
Rekha, S. R., Kulandhaivel, M. & Hridhya, K. V. Antibacterial efficacy and minimum inhibitory concentrations of medicinal plants against wound pathogens. Biomed. Pharmacol. J. 11, 237–246 (2018).
Zhao, S. et al. Antifungal effects of lycorine on Botrytis cinerea and possible mechanisms. Biotechnol. Lett. 43, 1503–1512 (2021).
Aslam, M. F., Irshad, G., Naz, F. & Khan, M. A. Evaluation of the antifungal activity of essential oils against Alternariaalternata causing fruit rot of Eriobotrya japonica Urk. J. Biochem. 47, 511–521 (2022).
Rhouma, A. et al. Combining melon varieties with chemical fungicides for integrated powdery mildew control in Tunisia. Eur. J. Plant Pathol. 165, 189–201 (2023).
Rhouma, A., Khrieba, M. I., Salih, Y. A., Rhouma, H. & Bedjaoui, H. Efficacy of fungicides for control of powdery mildew on grapevines in ChottSidi Abdel Salam oasis, southeastern Tunisia. J. Oasis Agric. Sustain. Dev. 3, 1–7 (2021).
Akubugwo, I. E., Chinyere, G. C. & Ugbogu, A. E. Comparative studies on oils from some common plant seeds in Nigeria. Pak. J. Nutr. 7, 570–573 (2008).
Bamgboye, I. A. & Adejumo, O. I. Physicochemical properties of Roselle seed oil. Nutr. Food Sci. 40, 186–192 (2010).
Parthiban, R., Sivarajan, M. & Sukumar, M. Ethanol production from banana peel waste using Saccharomyces cerevisiae. In International Conference on Sustainable Energy and Intelligent Systems. 177–183 (2011).
Siddeeg, A. & Xia, W. Oxidative stability, chemical composition and organoleptic properties of seinat (Cucumis melo var. tibish) seed oil blends with peanut oil from China. J. Food Sci. Technol. 52, 8172–8179 (2015).
Bosha, J. A., Anaga, A. O. & Asuzu, I. U. Chemical composition of the marc of a wild tropical plant Tacca involucrata (Schumach and Thonn, 1827). Food Nutr. Sci. 6, 135–140 (2015).
Barkatullah, M. I., Rauf, A. & Ur-Rahman, I. Physicochemical characterization of essential and fixed oils of Skimmia laureola and Zanthoxylum armatum. Middle-East J. Med. Plants Res. 1, 51–58 (2012).
Kumar, P., Mishra, S., Malik, A. & Satya, S. Compositional analysis and insecticidal activity of Eucalyptus globulus (family: Myrtaceae) essential oil against housefly (Musca domestica). Acta Tropica 122, 212–218 (2012).
Sameza, M. L. et al. Potential use of Eucalyptus globulus essential oil against Phtophthora colocasiae the causal agent of taro leaf blight. Eur. J. Plant Pathol. 140, 243–250 (2014).
Sebei, K. et al. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 48, 1–5 (2015).
Ben Hassine, D., Ben Ismail, H., Jribi, C., Khouja, M. L. & Abderrabba, M. Eucalyptus oleosa F. Muell essential oil: Extraction, chemical composition and antimicrobial activity International symposium on medicinal and aromatic plants. ISHS Acta. Hortic. 99, 7 (2012).
Vitti, D. M. S. S. et al. Do all tannins have similar nutritional effects, a comparison of three Brazilian fodder legumes. Anim. Feed. Sci. Technol. 119, 345–361 (2005).
Williams, Y. J. et al. A vaccine against rumen methanogens can alter the composition of archaeal populations. Appl. Environ. Microbiol. 75, 1860–1866 (2009).
Oukadir, Z., Abdellaoui, A., Lyoussi, B. & Senhaji Rhazi, N. Phytochemical, antioxidant and antibacterial study of essential oils of the leaves and fruits of Juniperus Phoenicea. Arab. J. Med. Aromatic. Plants. 7, 321–340 (2021).
Zrira, S., Elamrani, A. & Benjilali, B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco—A seasonal variation. Flavour. Fragr. J. 18, 475–480 (2003).
Trabelsi, H. et al. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 131, 434–440 (2012).
Luigia, L., Scardino, A. & Vasapollo, G. Identification and quantification of anthocyanins in the berries of Pistacia lentiscus L., Phillyrea latifolia L. and Rubia peregrina L. Innov. Food Sci. Emerg. Technol. 8, 360–364 (2007).
Sehaki, C. et al. Profiling of essential oils from the leaves of Pistacia lentiscus collected in the Algerian region of Tizi-Ouzou: Evidence of chemical variations associated with climatic contrasts between littoral and mountain samples. Molecules 27, 4148 (2022).
Sehaki, C., Jullian, N., Ayati, F., Fernane, F. & Gontier, E. A review of Pistacia lentiscus polyphenols: Chemical diversity and pharmacological activities. Plants 12, 279 (2023).
Bouchenak, O. et al. Caractérisation phytochimique et évaluation de l’activité antimicrobienne des huiles essentielles de Juniperus phoenicea. Rev. Agr. 11, 51–57 (2020).
Zin, Z., Abdul Hamid, A., Osman, A. & Saari, N. Antioxidative activities of chromatographic fractions obtained from root, fruit and leaf of Mengkudu (Morinda citrifolia L.). Food Chem. 94, 169–178 (2006).
Ebrahimzadeh, M. A., Pourmorad, F. & Bekhradnia, A. R. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 7, 3188–3192 (2008).
Atmani, D. et al. Antioxidant capacity and phenol content of selected algerian medicinal plants. Food Chem. 112, 303–309 (2009).
Soltani, Y., Ali-Bouzidi, M., Toumi, F. & Benyamina, A. Activités antioxydantes des extraits de trois organes de Juniperus phoenicea L. de l’Ouest algérien. Phytoth 16, 142–148 (2017).
Hayouni, A., Abedrabba, M., Bouix, M. & Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 105, 1126–1134 (2007).
Keskes, H. et al. In vitro anti-diabetic, anti-obesity and antioxidant proprieties of Juniperus phoenicea L. leaves from Tunisia. Asian Pac. J. Trop Biomed. 4, 649–655 (2014).
Nino, J., Nishat, A. & Tripathi, Y. C. Phytochemical screening and evaluation of polyphenols, flavonoids and antioxidant activity of Prunus cerasoides D. Don leaves. J. Pharm. Res. 10, 502–508 (2016).
Laouar, A. et al. Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCl4 induced hepatic damage in rats. Asian Pac. J. Trop. Med. 10, 263–269 (2017).
Zaouali, Y., Bel Hadj Yahya, I., Jaouadi, R., Messaoud, C. & Boussaid, M. Sex-related differences in essential oil composition, phenol contents and antioxidant activity of aerial parts in Pistacia lentiscus L. during seasons. Ind. Crops Prod. 121, 151–159 (2018).
Nicolás, G., Juan, E., Miguel, P. & Katherina, F. Extraction of polyphenols from Eucalyptus nitens and Eucalyptus globulus: Experimental kinetics, modeling and evaluation of their antioxidant and antifungical activities. Ind. Crops Prod. 109, 737–745 (2017).
Milia, E. et al. Leaves and fruits preparations of Pistacia lentiscus L.: a review on the ethnopharmacological uses and implications in inflammation and infection. Antibiotics 10, 425 (2021).
Medini, H., Elaissi, A., Khouja, M. L. & Chemli, R. Phytochemical screening and antioxidant activity of Juniperus phoenicea ssp. phoenicea L. extracts from two Tunisian localities. J. Exp. Biol. Agric. Sci. 1, 77–82 (2013).
Boulekbache-Makhlouf, L., Meudec, E., Mazauric, J. P., Madani, K. & Cheynier, V. Qualitative and semi-quantitative analysis of phenolics in Eucalyptus globulus leaves by high performance liquid chromatography coupled with diode array detection and mass-spectrometry. J. Phytoch. Anal. 24, 162–170 (2013).
Batish, D. R., Singh, H. P., Kohli, R. K. & Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 256, 2166–2174 (2008).
Kordali, S., Cakir, A., Zengin, H. & Duru, M. E. Antifungal activities of the leaves of three Pistacia species grown in Turkey. Fitoterapias 74, 164–167 (2003).
Gardeli, C., Papageorgiou, V., Mallouchos, A., Theodosis, K. & Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 107, 1120–1130 (2008).
El Idrissi, M., Barbouchi, M., Choukrad, M. B. & Louzi, L. Chemical composition and antimicrobial activity of essential oils isolated from leaves and twigs of Pistacia lentiscus L. Growing wild in Morocco. World J. Pharm. Pharm. Sci. 5, 516–524 (2016).
Zhou, L. J. et al. Antifungal activity of Eucalyptus oil against rice blast fungi and the possible mechanism of gene expression pattern. Molecules 21, 621 (2016).
Ghaffar, A. et al. Chemical composition and in-vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules 20, 20487–20498 (2015).
Salem, M. Z. M., Zidan, Y. E., Mansour, M. M. A., El Hadidi, N. M. N. & Abo Elgat, W. A. A. Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. Int. Biodeterior. Biodegrad. 106, 88–96 (2016).
Pedrotti, C. et al. Antifungal activity of essential oil from Eucalyptus staigeriana against Alternaria alternata causing of leaf spot and black rot in table grapes. An. Acad. Bras. Cienc. 94, e20200394 (2022).
Pepeljnjak, S., Kosalec, I., Kalodera, Z. & Blazević, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis, Cupressaceae). Act. Pharmaceutica. 55, 417–422 (2005).
Sati, S. C. & Joshi, S. Antibacterial potential of leaf extracts of Juniperus communis L. from Kumaun Himalaya. Afr. J. Microbiol. Res. 4, 1291–1294 (2010).
Bais, S., Gill, N. S., Rana, N. & Shandil, S. A phytopharmacological review on a medicinal plant: Juniperus communis. Int. Sch. Res. Notices 63472, 1–6 (2014).
Wang, D., Wang, G., Wang, J., Zhai, H. & Xue, X. Inhibitory effect and underlying mechanism of cinnamon and clove essential oils on Botryosphaeria dothidea and Colletotrichum gloeosporioides causing rots in postharvest bagging-free apple fruits. Front. Microbiol. 14, 1109028 (2023).
Ikeura, H., Somsak, N., Kobayashi, F., Kanlayanarat, S. & Hayata, Y. Application of selected plant extracts to inhibit growth of Penicillium expansum on apple fruits. Plant Pathol. J. 10, 79–84 (2011).
Steglińska, A. et al. Antimicrobial activities of plant extracts against Solanumtuberosum L. phytopathogens. Molecules 27, 1579 (2022).
Harkat-Madouri, L. et al. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crops Prod. 78, 148–153 (2015).
Čmiková, N. et al. Chemical composition and biological activities of Eucalyptus globulus essential oil. Plants 12, 1076 (2023).
Bachir, R. G. & Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2, 739–742 (2012).
Boukhatem, M. N., Boumaiza, A., Nada, H. G., Rajabi, M. & Mousa, S. A. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (Orangina fruit juice). Appl. Sci. 10, 5581 (2020).
Marzoug, H. N. B. et al. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 16, 1695–1709 (2011).
Sharma, A. D., Farmaha, M. & Kaur, I. Preparation and characterization of O/W nanoemulsion with eucalyptus essential oil and study of in vitro antibacterial activity. Nanomed. Res. J. 5, 347–354 (2020).
Sheikh, Z. et al. Repellent efficacy of Eucalyptus globulus and Syzygium aro-maticum essential oils against Malaria vector, Anopheles stephensi (Diptera: Culicidae). Iran. J. Public Health 50, 1668–1677 (2021).
Adak, T. et al. Nanoemulsion of eucalyptus oil: An alternative to synthetic pesticides against two major storage insects (Sitophilus oryzae (L.) and Tribolium castaneum (Herbst)) of rice. Ind. Crops Prod. 143, 111849 (2020).
Benhammou, N. B. et al. Fatty acid composition and antioxidant activity of Pistacia lentiscus L. fruit fatty oil from Algeria. J. Food Meas. Charact. 12, 1408–1412 (2018).
Abdeldjelil, M. C., Bensegueni, A., Messaï, A., Agabou, A. & Benazzouz, H. Medicinal use of Pistacia lentiscus fixed oil in Constantine province, northeast Algeria. J. Nat. Prod. Plant Resour. 4, 48–51 (2014).
Aissi, O., Boussaid, M. & Messaoud, C. Essential oil composition in natural populations of Pistacia lentiscus L. from Tunisia: Effect of ecological factor and incidence on antioxidant and antiacetylcholinesterase activities. Ind. Crops Products 91, 379–382 (2016).
Bouyahya, A. et al. Could volatile compounds from leaves and fruits of Pistacia lentiscus constitute a novel source of anticancer, antioxidant, antiparasitic and antibacterial drugs?. Ind. Crops Prod. 128, 62–69 (2019).
Haouli, A., Seridi, R., Djemli, S., Bourdjiba, O. & Frih, H. Contribution to the analysis of Pistacia lentiscus extracted oil. Am. Eur. J. Agric. Environ. Sci. 15, 1075–1081 (2015).
Harrat, M., Benalia, M., Gourine, N. & Yousfi, M. Variability of the chemical compositions of fatty acids, tocopherols and lipids antioxidant activities, obtained from the leaves of Pistacia lentiscus L. growing in Algeria. Mediterr. J. Nutr. Metab. 11, 199–215 (2018).
Ben Mansour, R. et al. In vivo gastroprotective effect and biological potentialities of six Tunisian medicinal plants using multivariate data treatment. Plant Biosyst. 156, 152–163 (2020).
Aouadi, M. et al. Antioxidant, anthelmintic and anti-bacterial activities of red juniper (Juniperus phoenicea L.) essential oil. J. Essent. Oil Res. 34, 163–172 (2021).
Terfaya, B., Makhloufi, A., Mekboul, A., Benlarbi, L. & Abdelouahi, D. In vitro antifusarial activity of a tar extracted from the Juniperus phoenicea L Wild in Southwest of Algeria. Phytothérapie 19, 243–249 (2021).
Mansour, R. B. et al. Insights on Juniperus phoenicea essential oil as potential anti-proliferative, anti-tyrosinase, and antioxidant candidate. Molecules 28, 7547 (2023).
Acknowledgements
We thank Aliat Toufik from Higher National School of Forests, Khenchela, Algeria for assisting taxonomic identification of forest species.
Funding
This publication has been supported by the RUDN University Scientific Projects Grant System, project N° "202724-2-000".
Author information
Authors and Affiliations
Contributions
L.H.H. Conceptualization, A.R., methodology, L.H.H., software, W.H., validation, N.Y.R., formal analysis, R.J. Y.Z; investigation, S.B., N.Y.R., resources, L.H.H., A.R., data curation, L.H.H., A.R , R.J., writing—original draft preparation, L.H.H., A.R and N.Y.R., writing—review and editing, L.H.H., A.R., N.Y.R., visualization, L.H.H., A.R., supervision, L.H.H., N.Y.R., project administration, L.H.H., N.Y.R. All authors have read, reviewed and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hajji-Hedfi, L., Rhouma, A., Hlaoua, W. et al. Phytochemical characterization of forest leaves extracts and application to control apple postharvest diseases. Sci Rep 14, 2014 (2024). https://doi.org/10.1038/s41598-024-52474-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52474-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.