Abstract
The microbial biotransformation using low-cost feedstock to produce biopolymers (degradable), an alternative to petrochemical-based synthesis plastics (non-degradable), can be a beneficial approach towards sustainable development. In this study, the dairy industry processes waste (whey) is used in polyhydroxyalkanoate (PHA) copolymer production. Initial screening suggested that Ralstonia eutropha produced higher PHA as compared to Bacillus megaterium. A central composite rotatable design-based optimization using two process variables (amino acid and tween-80) concentration remarkably influenced PHA co-polymer production under physiological conditions of pH (7), temperature (37 °C), and agitation rate of 150 rpm. High polyhydroxybutyrate (PHB) mass fraction yield of 69.3% was observed as compared to predicted yield of 62.8% from deproteinized whey as feed. The combination of tryptophan (50 mg L−1) and tween-80 (3 mL−1) enhanced R. eutropha mass gain to 6.80 g L−1 with PHB contents of 4.71 g L−1. Further, characterization of PHA and its copolymers was done by ESI–MS, FTIR, and TEM. On upscaling up to 3.0 L, the PHA contents and yields were noted as quite similar by R. eutropha. This study demonstrates that dairy waste processing waste can be potentially utilized as inexpensive feed for producing high content of biopolymers to develop a sustainable system of waste management.
Similar content being viewed by others
Introduction
There is a considerable need for biodegradable plastic nowadays to save nature. So, our lives as conventional non-biodegradable plastic are causing severe environmental threats. Production of biodegradable plastics is increasing daily, which is being used in different sectors like agriculture, pharmaceutical, cosmetics, and food industries. Therefore, researchers are focusing more on producing biodegradable plastics like PHA, which can be degraded by the microbial populations present in the soil or the environment1. The rapidly rising demand for biobased packaging locates PHA to bring down carbon footprints and save Mother Earth by reducing plastic pollution. 100% biodegradable, water-insoluble PHAs are obtained from prokaryotic microorganisms as an intracellular reserve material synthesized under nutrient-limiting conditions except for carbon which is the primary substrate for this PHA2. PHA polymer, produced by three different pathways in microorganisms as storage molecules, is present in different forms, like stiff, brittle, and rubbery, depending upon their chemical composition and these pathways requires three different precursors like acetyl CoA, malonyl CoA, acyl-ACP which are synthesized from sugar, oils and fatty acids, respectively (Proenca et al.; 2023). Unlike polylactic acid (PLA), PHA is UV stable3. Various kinds of microbial species can produce these inclusions in the cell’s cytoplasm. Bacteria can accumulate up to 90% of PHB on a cellular dry-weight basis in their cytoplasm4. Bacterial such as Bacillus, Ralstonia (formerly known as Cupriavidus, Wautersia, or Alcaligenes), Pseudomonas, Burkholderia, Comamonas, Thermus, and Escherichia are used in the production of PHA5. Due to PHA's attention-seeking properties resembling petroleum polymers except for biodegradability, it can be used in food packaging, medical, pharmaceutical, or other biotechnological applications6.
Right now high production cost of PHAs like P3HB (poly-3-hydroxybutyrate), PHBV (poly-3-hydroxybutyrate-co-3-hydroxy valerate), P3HP (poly-3-hydroxypropionate), P3HHx (poly-3-hydroxyhexanoate), P4HB (poly-4-hydroxybutyrate), and P5HV (poly-5-hydroxy valerate) compared to conventional polymers like polypropylene, polyethylene, and polyethylene terephthalate is the primary limiting for its broader application in the packaging industry. Therefore, PHAs can be produced either by using waste material as an organic substrate or by developing new fermentation, recovery, purification, and characterization techniques or by using such strains which are capable of biosynthesis of a high quantity of PHAs7,8. Previous studies have revealed that PHA can be produced by utilizing carbon-rich sources using suitable microorganisms from waste materials of agriculture-based industries, sugar manufacturing industries such as molasses3, oil refineries, marine industries, dairy, and food industries which produce colossal waste. Dairy industry waste is increasing in parallel with the increase in milk production and dairy product plants and therefore, their waste management is also an important area of interest9. Lower production costs can be achieved by using dairy industry waste which provides a huge amount of sweet whey rich in lactose and whey proteins. Although, microbes don't require nitrogen in a higher amount for PHA production as biosynthesis occurs in nitrogen-limiting conditions, is one of the above-mentioned alternative ways, but it results in lower yield than pure organic substrate10.
Various strategies have been demonstrated to improve biopolymer accumulation via screening of PHA-producers, optimization of process parameters (carbon/nitrogen source, pH, temperature, time, and agitation rate), and utilization of sugars as feed. Using inexpensive feed, such as biowaste, an alternative to costly pure sugars, can be helpful in enhancing PHA properties and bioprocess economy. In the present study, the PHA-producing potential of Bacillus megaterium and Ralstonia eutropha were evaluated from dairy processing waste under diverse physiological conditions. Furthermore, variables such as amino acid and tween-80 concentrations were optimized to enhance the PHA co-polymers production by R. eutropha as an efficient PHA-producers utilizing whey supernatant as carbon and nitrogen source under submerged fermentation.
Materials and methods
Cultures and growth conditions
PHA-producing cultures Bacillus megaterium (MTCC 428) and Ralstonia eutropha (MTCC 8320) were procured from Microbial Type Culture Collection, (MTCC) Chandigarh, India. These cultures were grown in a complex medium [peptone (10 g L−1), beef extract (10 g L−1), and ammonium chloride (5 g L−1)], and maintained on agar (2 0 g L−1) plates by every month sub-culturing.
Pretreatment of whey for feed preparation
The whey acquired during the manufacturing of paneer was pretreated through acidification to remove excess proteins prior to utilization as a carbon source (whey lactose), and the procedure details are illustrated in Supplementary Figure S111. The whey supernatant pH was adjusted to 7 (1 N NaOH/HCl) and used as feed for bacterial growth and PHA production.
Submerged culture PHA accumulation
PHA production by B. megaterium and R. eutropha was performed in 250 mL Erlenmeyer flasks with 100 mL of medium containing deproteinized whey (pH, 7) which was replaced with fructose (40, g L−1) for the control growth media. The production medium was prepared by adding mineral salt medium consisting of urea (0.8, g L−1), KH2PO4 (2.0 g L−1), Na2HPO4 (0.6, g L−1), MgSO4.7H2O (1.0, g L−1), yeast extract (0.1 g L−1), and trace element solution [1 mL/L of ZnSO4.7H2O (1.3, g L−1), CaCl2 (20, g L−1), FeSO4.7H2O (0.2, g L−1), (NH4)6Mo7O24.4H2O (0.6, g L−1), and H3BO3 (0.6, g L−1)], and deproteinized whey (pH, 7). Fully grown culture (5%, v/v) was inoculated to medium and incubated for up to 72 h for PHA accumulation at 37 °C under an agitation rate of 150 rpm. Further, the influence of carbon source, and process parameters (pH, temperature, and the agitation rate on PHA accumulation were evaluated for incubation of 48 h.
Supplementation of amino acids
To improve the growth and PHA accumulation, the supplementation of various amino acids [aromatic amino acids (tyrosine, tryptophan, and phenylalanine) and sulfur-containing amino acids (cystine, cysteine, and methionine)] were assessed at a concentration of 1 mg/1 mL of deproteinized whey. The resulting feed was inoculated with cultures (5%, v/v) to measure their influence on biomass and PHA production for incubation of 48 h at 37 °C under an agitation rate of 150 rpm.
Effect of different pH of production medium on growth and PHB production
In all experiments, pH was maintained at 7 but to check the effect of pH on the biomass and PHB yield pH of the production medium is adjusted to pH 5, 7, 9, and 11 using 1N NaOH/1N HCl. The inoculum was prepared as described previously. After preparation of the media, 5 ml of inoculums were inoculated in 100 ml of production medium. The flasks were incubated at 150 rpm at 37 °C. At 24 h, 48 h, and 72 h, biomass and PHB were measured in the culture broth.
Effect of different temperatures of fermentation on growth and PHB production
Production medium inoculated with two different strains in two separate 250 ml flasks. The flasks were incubated at 150 rpm at different temperature ranges such as 25 °C, 30 °C, 33 °C, 37 °C, and 40 °C. Biomass and PHB were determined in the culture broth at 24 h, 48 h, and 72 h.
Effect of different agitation speeds during fermentation on growth and PHB production
Here, the production medium made up of whey was inoculated with two different bacterial strains in two separate flasks. Then the flasks were incubated at 37 °C at different ranges of agitation speeds such as 50 rpm, 100 rpm, 150 rpm, and 200 rpm. Biomass and PHB were measured in the culture broth at different time intervals such as 24 h, 48 h, and 72 h.
Effect of different times of fermentation on growth and PHB production
To check the effect of time of fermentation on biomass growth and PHB production, a previously prepared production medium containing whey was inoculated with 5 ml bacterial inoculums of different strains in a 250 ml flask separately and the cell culture was incubated at 150 rpm at 37 °C. Biomass and PHB were estimated in the culture broth at different time intervals such as 48 h, 60 h, and 72 h. The production medium in each flask contained whey only.
Experimental design and validation
To determine the interaction impact of two physical process factors (amino acid and tween-80) concentration on PHA accumulation, a two-factor central composite rotary design (CCRD) was employed using software (Design Expert 12.0, Stat-Ease Inc., USA). At various levels of two parameters, 13 sets of experiments were created by design experts (Table 1). Point prediction was used to adjust each factor's level for maximum performance. Experimental testing was done to determine the model’s effectiveness using the combination of several optimal parameters that generated the highest reaction, or maximum PHA content.
Analytical measurements
DCW content of the biomass was measured by cell drying procedures to achieve a constant weight at 80 °C for incubation of 24 h12. Whey protein and total sugar contents were measured by procedures of Lowery13, and 3,5-dintrosalicylic acid (DNSA)14, respectively. PHA accumulation was assessed using sodium hypochlorite (50 mL) and chloroform (50 mL) dispersion procedure from retrieved cells (1.0 g dry cell weight)15. The obtained crude PHA was precipitated in a non-solvent solution [70% methanol, v/v] and recovered via filtration (Whatman No. 1 paper) followed by drying for incubation of 5 h at 70°C15. PHA contents of cultures were measured by chloroform extraction followed by spectrophotometrically at 235 nm estimation of crotonic acid as described earlier16.
PHA characterization
The Fourier transform infrared (FTIR) spectra of PHA granules were recorded by Bruker spectrometer (Thermo Nicolet, MA, USA). Electrospray ionisation mass spectrometry (ESI–MS) analysis was evaluated by a Finnigan LCQ ion trap mass spectrometer (Thermo Finnigan LCQ Fleet, San Jose, CA, USA)17. The intracellular PHA granules of the cells were recorded by transmission electron microscopy (TEM) using glutaraldehyde (2%) procedures as described earlier18.
Up-scaling of PHA production
The culture was grown in a 7.5-L Bentchtop bioreactor (BioFlo/Celligen 115, New Brunswick, USA) to study the up-scaling of PHA production with a working volume of 3-L under optimized conditions.
Results and discussion
PHA production from whey hydrolysate
The whey composition analysis reveals that it contains approximately 6.8% of total sugars (lactose, glucose, and galactose) and traces of essential growth factors (calcium and phosphorus) Pure sugars have been widely reported as a feed for PHA production. Therefore, low-cost biowaste materials such as whey can improve process economics19. In this study, B. megaterium and R. eutropha as PHA-produces were employed to produce PHA under submerged culture from whey hydrolysate as a carbon source. The PHA production details of these cultures from various carbon sources are presented in Table 2. The cell biomass of B. megaterium and R. eutropha improved to 4.54 and 6.05 g/L by using whey hydrolysate, which is greater than 3.55 and 3.94 g/L that was obtained after using pure sugars like glucose and fructose as carbon sources. These carbon sources resulted in PHA yields of up to 2.93 g/L for B. megaterium and 3.84 g/L for R. eutropha. Here, the PHA contents in cell biomass noted entirely consist in the range of 61.4–65.3% for B. megaterium and 61.1–63.5% for R. eutropha. Previously, a PHA production of 1.1 g/L by Paracoccus homiensis from cheese whey mother liquor (CWML)20, 3.32 g/L by Bacillus mycoides DFC1 from glucose21, and 4.01 g/L by Alcaligenes latus (ATCC 29714) from sugar beet juice supplemented with minerals22, 1.69 g/L by Bacillus firmus NII 0830 from acid pretreated rice straw hydrolysate23, and 2.7 g/L by Hydrogenophaga pseudoflava from whey lactose24 were obtained. Growth factors (organic acids, vitamins, and minerals) present in whey positively supported cell biomass and PHA production. Higher cell mass and increased PHA productivity clearly indicate that these PHA-producers assimilated nitrogen from whey efficiently and the uptake rate across cell membrane may be higher due to its non-ionic form and less pH dependency during transport across the membrane. Based on the high production of cell biomass (6.05 g/L) and PHA (3.84 g/L) by R. eutropha over B. megaterium, R. eutropha was selected for further studies.
Influence of process parameters on PHA production by R. eutropha
To improve the PHA production by R. eutropha from whey hydrolysate, the physiological process parameters were evaluated at pH (5 – 11), temperature (25–40 °C), agitation rate (50–200 rpm), and incubation period (24–72 h) (Fig. 1). The optimum pH of 7 was noted for the high cell biomass (6.05 g/L) and PHA (3.84 g/L) (Fig. 1a). Under an acidic pH of 5, a significant decline in cell biomass to 4.45 g/L and PHA yield to 2.85% was noted by R. eutropha. In contrast, higher alkaline pH of up to 11 exhibited consistent cell biomass and PHA yields. These findings revealed that PHA accumulation by R. eutropha is favorable under neutral and higher alkaline conditions. Previously, a lower PHA production was recorded of 0.84 ± 0.14 g/L by Pichia sp. TSLS24 using Zobell marine agar medium (ZMA) supplemented with 2 gL−1 of sucrose to enrich the growth of yeast under alkaline pH 925. The temperature has a notable influence on PHA production that can be associated with the survival strategy adopted by organisms over bioconversion at declined/elevated temperatures to the optimum conditions26. The optimum temperature of 37 °C was observed for efficient cell biomass and PHA production of 6.05, and 3.84 g/L, respectively (Fig. 1b). At 25 °C, a significant decline in cell biomass and PHA yield up to ~ 33%. In contrast, the lowest PHA yield and contents of 1.86 g/L and 53.6% were recorded at a higher incubation temperature of 40 °C, respectively. The optimum agitation rate of 150 rpm was noted for biomass and high PHA accumulation (Fig. 1c). The cell biomass R. eutropha increased with an increase in the incubation period from 1.86 g/L at 24 h to 6.65 g/L at 72 h (Fig. 1d). The optimum incubation of 72 h was noted for high PHA yield and contents of 3.84 g/L and 63.9%, respectively. At a higher incubation of 96 h, the partial decline in PHA contents to 57.8% can be associated with the diversion of accumulated PHA to depolymerization for survival benefits for R. eutropha27.
(a) Effect of different pH on dry cell mass and PHB production of two bacterial strains; (b) Effect of different temperatures on dry cell mass and PHB production of two bacterial strains; (c) Effect of different agitation speeds on dry cell mass and PHB production of two bacterial strains; (d) Comparison of Dry Cell Mass content and PHB production of Bacillus megaterium and Ralstonia eutropha as a function of incubation time.
Effect of amino acids supplementation on PHA Production by R. eutropha
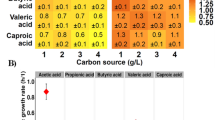
Amino acids play a crucial role in cell biomass synthesis via their involvement in proteins manufacture. The bulk of chemical reactions that take place in cells are catalyzed by proteins. Several of the structural components of a cell are supplied by them28. Therefore, six amino acids (1 mg/mL) were supplemented to medium, including cystine, cysteine, methionine and tyrosine, tryptophan, and phenylalanine to evaluate their influence on the PHA contents and composition. On the supplementation of these amino acids, R. eutropha accumulated PHA in the ranges of 60.2–63.2% (Fig. 2). Here, PHA yields varied from 3.76–4.42 g/L. Among these amino acids, tryptophane supplementation to whey hydrolysate proved more beneficial for R. eutropha for accumulating high PHA content and yield of 62.1% and 4.42 g/L, respectively. Previously, supplementation of known amino acids (50 mg/L) in glycerol medium resulted in much lower PHB contents in the ranges of 8.86–26.2% by recombinant E. coli29. Further, by increasing amino acid contraction to 150 mg/L, cysteine was found suitable to enhance PHA yield up to 30.8% by E. coli. On the other hand, methionine, and isoleucine negatively influenced PHA with a remarkable decline in E. coli contents to 19.1 and 16.6% under similar conditions, respectively29. In contrast, R. eutropha in this study showed much better PHA contents up to 62.5% and 62% in the presence of cysteine and methionine, respectively (Fig. 2). The enhancement in PHA content by R. eutropha on supplementation of tryptophan might be associated with the fact that tryptophan production consumes more ATP than the biosynthesis of other amino acids, which requires less ATP8. When whey is supplemented with acetic acid (16%), butyric acid (26%), and lactic acid (58%), bacteria synthesized PHB only, while whey with valeric acid (4%), lactic acid (6%), butyric acid (13%), propionic acid (19%), and acetic acid (58%) bacteria have synthesized a copolymer made of (40%) PHV along with PHB (60%)30. Haloferax mediterranei, for example, uses whey to yield 66% CDW PHA (0.11 g L−1 h−1). High PHA producers, such as Ralstonia eutropha can accumulate PHAs up to 80% of its dry cell weight when grown on glucose31 & can make SCL-PHA and PHA biopolymers made up of 3HB, 3HV, and 4HB subunits32.
Design of experiments and model fitting for PHA accumulation from whey hydrolysate supplemented with various concentrations of Tween 80 and tryptophan
The effective operating conditions for improving PHA content were examined using the response surface approach for R. eutropha from whey hydrolysate. Two process variables as, tryptophan and tween 80 concentrations, were selected at various levels, and 13 sets of experiments were then created using the central composite rotatable design (CCRD). The outcomes of the experimental trials are represented in Table 1. The cell biomass and PHA varied in the ranges of 5.41–6.81, and 3.56–4.90 g/L, respectively. The maximum PHA accumulation recorded of 4.90 g/L with yields of 77.4% by R. eutropha at concentrations of 37.5% for tryptophan, and 6.6% for the tween-80.
Tryptophan and tween-80 showed a beneficial effect on PHA accumulation, as seen in Fig. 3a. The 3D surface and contour plot clearly evidenced that increasing the concentrations of tween-80 up to 6.6% v/v and tryptophan up to 37.5% improved PHA accumulation by R. eutropha (Figs. 3a-c). The biomass of R. eutropha was raised by the amino acid concentration. In contrast, the biomass was adversely lowered by the increase in the tween-80 concentration, as indicated in the 3D plot's boundary (Fig. 3b). Cell biomass was found to be at its highest value of 6.81 g/L at 50 mg/L of tryptophan and 3% of tween-80, according to a contour plot illustrating the interaction impact of these two concentrations (Fig. 3c). A lower PHA accumulation can be correlated by a substantial portion of acetyl-CoA alteration to metabolic pathways that compete with synthesis of PHA, such as the formation of acetate, fatty acids, and amino acids8. An increasing concentration of up to 50 mg/L of tryptophan raised cell biomass. In contrast, a significant decline in biomass was noted on increasing tween-80 concentration up to 6% (Fig. 3d). However, there was no synergistic effect on PHA accumulation noted as the concentration of tryptophan increased. This might be explained by the PHA synthase enzyme being inhibited by its substrate8.
(a) 3D surface plot showing the relationship between amino acid and tween 80 concentration on the PHB yield (gL−1) in Ralstonia eutropha in the optimized sample; (b) 3D surface plot showing the relationship between amino acid and tween 80 concentration on the cell mass (gL−1) of Ralstonia eutropha in the optimized sample; (c) Contour plot showing the interactive effect of amino acid and tween-80 concentration on PHB content (gL−1) in Ralstonia eutropha in the optimized sample; (d) Contour plot showing the interactive effect of amino acid and tween-80 concentration on cell mass (gL−1) of Ralstonia eutropha in the optimized sample.
Up-scaling of PHA production
The up-scaled production profile of PHA production by R. eutropha from whey hydrolysate is presented in Fig. 4. At the log phase of growth of 12 h, the biomass growth increased to 5.65 g/L at 48 h of incubation. At 3-L of culturing, the maximum PHA content and yields were noted 4.19 g/L, and 74.2%, respectively. During PHA accumulation, the substrate consumption was observed at 79.0% from the initial feed of 40 g/L. The kinetic measurements reveal that PHA yield (YP/x) in terms of cell biomass was recorded at 0.72 with a productivity of 0.19 g/L/h. Previously, 0.024 g/L/h PHA productivity was observed in Bacillus sp. from media containing glucose, yeast extract, peptone and a few inorganic salts33 which is lower than the present finding. Similarly, 0.071 g/L/h PHA productivity in Pseudomonas chlororaphis grown on animal derived waste34.
Characterization of produced PHA by R. eutropha
PHA granule formations occurred in cells as PHA oligomers create a micelle-like form or plasma membrane buddings off, leaving a granule coated in a monolayer of lipids35. The visualization of R. eutropha TEM micrographs depicts rod-shaped cells containing white inclusions of PHA granules. Overall, PHA granules are generated both at the cytoplasmic membrane and dispersed evenly throughout the cytoplasm, which is consistent with the previous report on PHA granules formation by Bacillus cereus from pea-shells hydrolysate18.
The controlled PHA chemical decomposition can occur in several ways via acetic acid salts that generate oligomers with the same composition and sequence distribution of monomer units36. To validate the existence of P(3-HB) monomers, ESI/MS analysis was performed to determine the chemical structure of PHA produced by R. eutropha from whey hydrolysate. The ESI–MS spectrum of oligomers produced by PHA breakdown in the presence of potassium acetate is shown in Fig. 5. The peak-to-peak mass increment in the ESI–MS spectrum was noted of 86 Da, which is equivalent to the mass of the 3-hydroxybutyrate (3-HB) repeating unit. Other series of ions recorded correspond to the sodium adduct of poly (3-HB) with crotonate and carboxyl end groups. The molar mass of the 3-hydroxyhexanoate (HH, 114 Da), and 3-hydroxyvalerate (HV, 100 Da) co-monomeric units differed by 14 Da across surrounding signals (3-HB, 86 Da) within the clusters. The ESI mass spectrum of PHA polymers (HB, HV, and/or HH subunits) revealed a dispersion of singly charged sodium adducts of the distinct PHA polymer chains (terminating with unsaturated and carboxylic end groups) due to their strong sensitivity for alkali metals (particularly sodium). The present investigation showed PHA copolymers i.e., HB-HH, HB-HV in whey supplemented media which is in correlation with previous findings of Kowalzuck et al., who reported HB-HV oligomers in oxidized polyethylene wax supplemented media using R. eutropha37. The composition of the PHAs obtained from C. testosteroni during growth on variety of vegetable oils showed 3-hydroxyoctanoic acid and/or 3-hydroxydecanoic acid38.
The characterization of produced PHA was validated by FTIR spectra (Fig. 6). The peaks at 1021 cm−1 represent the ester bond. The stretching of the C–O and -CH bond present in the ester group was noted at peaks ~ 1700 and ~ 1300 cm−1, respectively39. The peak at 1377 cm−1 indicates the occurrence of a symmetric bending of the -CH3 group. Further, the asymmetric bending of –CH2 and –CH groups correlated to the peaks at 1448 and 2942 cm−1, respectively. The peak at 3314 cm−1 correlated to a terminal –OH group8.
Conclusion
In this study, PHA-producers B. megaterium and R. eutropha feasibility to produce PHAs from spilled whey as low-cost raw materials feed was demonstrated. Initial screening suggested that R. eutropha can efficiently produce PHAs up to twofold higher compared to B. megaterium. The supplementation of amino acids and tween 80 in feed showed remarkable enhancement in PHAs production ~ 50-fold under optimized conditions in medium optimization over uses of whey alone as a feed.
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
13 May 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-61698-9
References
Shen, M. et al. Are biodegradable plastics a promising solution to solve the global plastic pollution?. Environ. Pollut. 263, 114469 (2020).
Koller, M. & Rodríguez-Contreras, A. Techniques for tracing PHA-producing organisms and for qualitative and quantitative analysis of intra- and extracellular PHA. Eng. Life Sci. 15, 558–581 (2015).
Tripathi, A. et al. Recovery and characterization of polyhydroxyalkanoates. Rec. Adv. Biotechnol. 2, 266–302 (2016).
Menzel, G., Harloff, H.-J. & Jung, C. Expression of bacterial poly(3-hydroxybutyrate) synthesis genes in hairy roots of sugar beet (Beta vulgaris L.). Appl. Microbiol. Biotechnol. 60, 571–576 (2003).
Chee, J. Y. et al. Bacterially produced polyhydroxyalkanoate (PHA): Converting renewable resources into bioplastic. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microbial Biotechnol. 2, 1395–1404 (2010).
Kalia, V. C., Singh Patel, S. K., Shanmugam, R. & Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 326, 124737 (2021).
Bosco, F. & Chiampo, F. Production of polyhydroxyalcanoates (PHAs) using milk whey and dairy wastewater activated sludge. J. Biosci. Bioeng. 109, 418–421 (2010).
Tripathi, A. D. et al. Effect of nutritional supplements on bio-plastics (PHB) production utilizing sugar refinery waste with potential application in food packaging. Preparat. Biochem. Biotechnol. 49, 567–577 (2019).
Adesra, A., Srivastava, V. K. & Varjani, S. Valorization of dairy wastes: Integrative approaches for value added products. Indian J. Microbiol. 61, 270–278 (2021).
Berwig, K. H., Baldasso, C. & Dettmer, A. Production and characterization of poly(3-hydroxybutyrate) generated by Alcaligenes latus using lactose and whey after acid protein precipitation process. Bioresour. Technol. 218, 31–37 (2016).
Koller, M. et al. Potential of various archae- and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol. Biosci. 7, 218–226 (2007).
Patel, S. K. S., Gupta, R. K., Kalia, V. C. & Lee, J.-K. Synthetic design of methanotroph co-cultures and their immobilization within polymers containing magnetic nanoparticles to enhance methanol production from wheat straw-based biogas. Bioresour. Technol. 364, 128032 (2022).
Porwal, S. et al. Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour. Technol. 99, 5444–5451 (2008).
Patel, S. K. S., Purohit, H. J. & Kalia, V. C. Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int. J. Hydrogen Energy 35, 10674–10681 (2010).
Tripathi, A. D. Statistical optimization of parameters affecting polyhydroxybutyrate(PHB) recovery by dispersion method from alcaligenes cells and its characterization. J. Bioprocess Biotech 05, 1 (2015).
Law, J. H. & Slepecky, R. A. Assay of poly-β-hydroxybutyric acid. J. Bacteriol. 82, 33–36 (1961).
Johnston, B. et al. The molecular level characterization of biodegradable polymers originated from polyethylene using non-oxygenated polyethylene wax as a carbon source for polyhydroxyalkanoate production. Bioengineering 4, 73 (2017).
Patel, S. K. S., Kumar, P., Singh, M., Lee, J.-K. & Kalia, V. C. Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour. Technol. 176, 136–141 (2015).
Du, C., Sabirova, J., Soetaert, W., Ki, S. & Lin, C. Polyhydroxyalkanoates production from low-cost sustainable raw materials. Curr. Chem. Biol. 6, 14–25 (2012).
Mozejko-Ciesielska, J. et al. Cheese whey mother liquor as dairy waste with potential value for polyhydroxyalkanoate production by extremophilic Paracoccus homiensis. Sustain. Mater. Technol. 33, e00449 (2022).
Narayanan, A. & Ramana, K. V. Polyhydroxybutyrate production in Bacillus mycoides DFC1 using response surface optimization for physico-chemical process parameters. 3 Biotech 2, 287–296 (2012).
Wang, B., Sharma-Shivappa, R. R., Olson, J. W. & Khan, S. A. Production of polyhydroxybutyrate (PHB) by Alcaligenes latus using sugarbeet juice. Ind. Crops Prod. 43, 802–811 (2013).
Sindhu, R., Silviya, N., Binod, P. & Pandey, A. Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem. Eng. J. 78, 67–72 (2013).
Koller, M., Atlić, A., Gonzalez-Garcia, Y., Kutschera, C. & Braunegg, G. Polyhydroxyalkanoate (PHA) biosynthesis from whey lactose. Macromol. Symp. 272, 87–92 (2008).
Thu, N. T. T. et al. Evaluation of polyhydroxyalkanoate (PHA) synthesis by Pichia sp. TSLS24 yeast isolated in Vietnam. Sci. Rep. 13, 3137 (2023).
Mascarenhas, J. Production and characterization of polyhydroxyalkanoates (pha) by bacillus megaterium strain jha using inexpensive agro-industrial wastes. (2019). https://doi.org/10.13140/RG.2.2.36677.60641.
Sasidharan, R. S., Bhat, S. G. & Chandrasekaran, M. Biocompatible polyhydroxybutyrate (PHB) production by marine Vibrio azureus BTKB33 under submerged fermentation. Ann. Microbiol. 65, 455–465 (2015).
Kelly, B. & Pearce, E. L. Amino assets: How amino acids support immunity. Cell Metab. 32, 154–175 (2020).
Mahishi, L. H. & Rawal, S. K. Effect of amino acid supplementation on the synthesis of poly(3-hydroxybutyrate) by recombinant phaSa+Escherichia coli. World J. Microbiol. Biotechnol. 18, 805–810 (2002).
Colombo, B., Pepè Sciarria, T., Reis, M., Scaglia, B. & Adani, F. Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour. Technol. 218, 692–699 (2016).
Povolo, S., Toffano, P., Basaglia, M. & Casella, S. Polyhydroxyalkanoates production by engineered Cupriavidus necator from waste material containing lactose. Bioresour. Technol. 101, 7902–7907 (2010).
Jindal, P. & Tiwari, D. P. Biosynthesis of PHA and it’s copolymers—A review. 4, (2013).
Israni, N. & Shivakumar, S. Evaluation of upstream process parameters influencing the growth associated PHA accumulation in Bacillus sp. Ti3. 74, (2015).
Muhr, A. et al. Novel description of mcl-PHA biosynthesis by pseudomonas chlororaphis from animal-derived waste. J. Biotechnol. 165, 45–51 (2013).
Tripathi, A. D. & Srivastava, S. K. Kinetic study of biopolymer (PHB) synthesis in Alcaligenes sp. in submerged fermentation process using TEM. J. Polym. Environ. 19, 732–738 (2011).
Kawalec, M., Sobota, M., Scandola, M., Kowalczuk, M. & Kurcok, P. A convenient route to PHB macromonomers via anionically controlled moderate-temperature degradation of PHB. J. Polym. Sci. A Polym. Chem. 48, 5490–5497 (2010).
Radecka, I. et al. Oxidized polyethylene wax as a potential carbon source for PHA production. Materials 9, 367 (2016).
Thakor, N., Trivedi, U. & Patel, K. C. Biosynthesis of medium chain length poly(3-hydroxyalkanoates) (mcl-PHAs) by Comamonas testosteroni during cultivation on vegetable oils. Bioresour. Technol. 96, 1843–1850 (2005).
Kumar, P., Ray, S., Patel, S. K. S., Lee, J.-K. & Kalia, V. C. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int. J. Biol. Macromol. 78, 9–16 (2015).
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number ISP23-101. The Authors are grateful to Institute of Eminence (IoE), Development Grant (6031) for providing fund in carrying out this research work.
Funding
Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia-Project Number ISP23-101.
Author information
Authors and Affiliations
Contributions
T.D.P., A.D.T., P.S., A.P., S.H. and S.K.S.P. conceptualized the experiment. T.D.P., A.D.T. and A.A. did the methodology and validation. T.D.P., A.D.T. conducted the formal analysis and investigation. T.D.P., A.D.T., and S.K.S.P. wrote and prepared the original draft. A.D.T., P.S., A.P., S.H., and S.K.S.P. conducted the writing, review, and editing. A.A. helped in visualization. A.D.T. and A.A. supervised the experiment. S.H. and D.K.M. edited the manuscript. T.D.P., A.D.T., P.K., and A.A. performed analytical work. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Author Contributions section in this Article was incomplete. Full information regarding the correction made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patil, T.D., Ghosh, S., Agarwal, A. et al. Production, optimization, scale up and characterization of polyhydoxyalkanoates copolymers utilizing dairy processing waste. Sci Rep 14, 1620 (2024). https://doi.org/10.1038/s41598-024-52098-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52098-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.