Abstract
The increasing incidence and dissemination of multidrug-resistant Candida auris represents a serious global threat. The emergence of pan-resistant C. auris exhibiting resistance to all three classes of antifungals magnifies the need for novel therapeutic interventions. We identified that two HIV protease inhibitors, atazanavir and saquinavir, in combination with posaconazole exhibited potent activity against C. auris in vitro and in vivo. Both atazanavir and saquinavir exhibited a remarkable synergistic activity with posaconazole against all tested C. auris isolates and other medically important Candida species. In a time-kill assay, both drugs restored the fungistatic activity of posaconazole, resulting in reduction of 5 and 5.6 log10, respectively. Furthermore, in contrast to the individual drugs, the two combinations effectively inhibited the biofilm formation of C. auris by 66.2 and 81.2%, respectively. Finally, the efficacy of the two combinations were tested in a mouse model of C. auris infection. The atazanavir/posaconazole and saquinavir/posaconazole combinations significantly reduced the C. auris burden in mice kidneys by 2.04- (99.1%) and 1.44-log10 (96.4%) colony forming unit, respectively. Altogether, these results suggest that the combination of posaconazole with the HIV protease inhibitors warrants further investigation as a new therapeutic regimen for the treatment of C. auris infections.
Similar content being viewed by others
Introduction
Invasive fungal infections pose an underestimated threat to the public health1,2. Fungal infections are on the rise globally, particularly among immunocompromised individuals, reaching over 11 million infections and 1.5 million deaths annually3,4,5. The majority of invasive fungal infections is caused by Candida species which are associated with high mortality rates (up to 60%)2,6. The emerging fungal species, Candida auris has spread to more than 45 countries, causing serious hospital outbreaks, and significantly contributed to the worldwide health challenge of antifungal resistance7. The incidence and dissemination of multidrug-resistant C. auris-related infections are alarmingly growing and are associated with high mortality rates8. Thus, there is a desperate need to develop new antifungals for the treatment of multidrug-resistant C. auris infections.
Candida auris possesses an arsenal of virulence factors known to contribute to its pathogenicity. Among these, biofilm formation is a major virulence factor of C. auris which plays an important role in the nosocomial outbreaks of life-threatening invasive candidiasis9. Notably, C. auris infections are frequently associated with medical devices such as catheters, where it forms biofilms that adhere to the device surface, leading to device malfunction and serving as a source of bloodstream infection10. Furthermore, the formation of biofilms significantly contributes to the escalation of antifungal resistance, as these biofilms enable C. auris cells to tolerate higher concentrations of antifungal agents10,11. Consequently, novel therapeutic approaches are urgently needed to effectively manage C. auris infections, particularly those associated with biofilm formation9.
Posaconazole, a second-generation azole antifungal, has a potent and broad-spectrum antifungal activity against pathogenic fungi, including C. auris12. Posaconazole has a more favorable safety and tolerability profile when compared to the other azoles like fluconazole, itraconazole, and voriconazole. Long-term treatment with posaconazole has demonstrated a reduced risk of inducing side effects13,14. Furthermore, posaconazole’s extended half-life of 15–35 h, widespread tissue distribution, and gradual elimination contribute to its therapeutic effectiveness15. In addition to these attributes, posaconazole offers therapeutic advantages over fluconazole and voriconazole because it is less likely to be affected by mutations in the ergosterol gene (ERG11), which codes for lanosterol demethylase, the target enzyme of azoles11. Furthermore, it was reported that patients infected with fluconazole-resistant C. glabrata or C. tropicalis were able to recover when treated with posaconazole1. However, the emergence of pan-resistant C. auris, displaying resistance to all three classes of antifungals, underscores the urgency for the development of new therapeutics16.
Previously, we reported that lopinavir exhibited potent synergistic interactions with fluconazole, itraconazole and voriconazole against C. auris17. We also identified atazanavir and saquinavir as potent drugs that restored the activity of itraconazole against C. auris in vitro and in vivo18,19. Building upon our previous studies, we aimed herein to enhance the activity of posaconazole against C. auris using the HIV protease inhibitors (atazanavir and saquinavir). We also evaluated the activity of the combinations of posaconazole with atazanavir and saquinavir in inhibiting the C. auris biofilm. Finally, we investigated the in vivo efficacy of atazanavir/posaconazole and saquinavir/posaconazole combinations in a mouse model of C. auris infection.
Results
Posaconazole exhibits synergistic antifungal activity with atazanavir and saquinavir in vitro
Checkerboard assay was performed to corroborate the potential synergistic interactions for the combinations atazanavir/posaconazole and saquinavir/posaconazole against 17 C. auris isolates. Atazanavir and saquinavir were found to interact synergistically with posaconazole against 100% of C. auris isolates decreasing the minimum inhibitory concentrations (MICs) of posaconazole by 4–17 folds. Atazanavir/posaconazole and saquinavir/posaconazole combinations demonstrated synergistic fractional inhibitory concentration indices (FICIs) of (0.06–0.28) (Table 1). Additionally, these two combinations showed similar synergistic interactions against other medically important Candida species such as C. albicans, C. tropicalis, C. parapsilosis, C. krusei and C. glabrata with FICIs ranging between 0.12 and 0.38 (Table 2).
Atazanavir and saquinavir enhance the fungistatic activity of posaconazole against C. auris
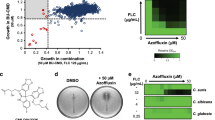
To investigate the impact of the two combinations on the growth kinetic of C. auris, a time-dependent killing assay was conducted against C. auris AR0390 for 48 h (Fig. 1). Single drug treatment of either atazanavir (16 µg/ml), saquinavir (16 µg/ml), or posaconazole (0.25 µg/ml) did not affect the fungal growth over time, where their growth pattern was similar to that of the mock (negative) control. However, the atazanavir/posaconazole combination reduced C. auris colony forming unit (CFU) by 3.78- and 5.02-log10 as compared to the mock (negative) control, after 24 and 48 h, respectively. On the other hand, saquinavir/posaconazole combination reduced C. auris CFU by 4.19- and 5.62-log10 as compared to the mock (negative) control, after 24 and 48 h, respectively (Fig. 1A). This was confirmed by the spotting assay which showed that C. auris had exponential growth in the presence and absence of atazanavir (16 µg/ml), saquinavir (16 µg/ml), posaconazole (0.25 µg/ml). Conversely, the growth of C. auris was inhibited significantly in the presence of the two combinations (Fig. 1B). Additionally, we used a posaconazole test strip in RPMI 1640 agar cultured with 106 C. auris cells to determine whether HIV protease inhibitors (atazanavir, and saquinavir) improve the activity of posaconazole against azole-resistant C. auris. As expected, atazanavir and saquinavir (32 µg/ml) decreased the MIC of posaconazole from 1 µg/ml to 0.38 and 0.19 µg/ml (2.6- and 5.2-folds reduction), respectively, against C. auris (Fig. 1C).
Time-kill of the HIV protease inhibitors (atazanavir (ATV), saquinavir (SQV)), and posaconazole (POS) alone and in combination against C. auris. (A) Time kill curve of C. auris AR0390. Data are presented as average log10 CFU/ml of C. auris AR0390 at the corresponding time points. Statistical difference was measured via two-way analysis of variance (ANOVA). An asterisk (*) denotes a statistically significant difference (P < 0.0001) from the POS-treated cells. (B) Spotting assay was used to visualize the growth kinetics of C. auris in the presence and absence of treatments at the 24-h time point. (C) Effects of atazanavir and saquinavir on azole-resistant C. auris. MIC test strips of posaconazole (ranged from 32–0.002 µg/ml) in the presence and absence of atazanavir and saquinavir at 32 µg/mL.
Atazanavir/posaconazole and saquinavir/posaconazole combinations inhibit biofilm formation of C. auris
Due to the strong antifungal activity against C. auris, the two combinations were selected to investigate the biofilm inhibition activity. As shown in Fig. 2, atazanavir, and saquinavir, at a low concentration of 16 µg/ml, in combination with sub-inhibitory concentration of posaconazole (0.03 µg/ml) showed a significant inhibition of the C. auris biofilm formation by 66.2%, and 81.2%, respectively, compared to the mock (negative) control.
Anti-biofilm activity of the HIV-protease inhibitors/posaconazole combinations. The inhibitory effect of atazanavir/posaconazole (ATV/POS) (16/0.03 µg/mL), and saquinavir/ posaconazole (SQV/POS) (16/0.03 µg/mL) combinations on the formation of C. auris AR0390 biofilms was determined using OD600 (A), which can be visualized using crystal violet (B). Statistical difference was measured via One-way Analysis of Variance (ANOVA) with the post hoc Dunnett’s test for multiple comparisons. An asterisk (*) denotes a statistically significant difference (P < 0.0001) from the DMSO-treated (control).
Atazanavir and saquinavir enhance posaconazole’s efficacy in a mouse model of C. auris infection
A mouse model of disseminated C. auris infection was used to investigate the antifungal activity of the two combinations (atazanavir/posaconazole, saquinavir/posaconazole) in vivo. First, the posaconazole dose was optimized to determine the highest dose that does not result in significant reduction in the kidney fungal burden. Mice were treated with different doses of posaconazole (0.25, 0.5, 1, 3, 5 mg/kg) and the optimal dose of posaconazole was determined as 3 mg/kg, which did not result in significant difference as compared to the untreated control (Fig. S1). Next, we tested the in vivo efficacy of the two combinations on this mouse model in the presence of the bioavailability enhancer, ritonavir (to mimic the 3:1 atazanavir: ritonavir and the 10:1 saquinavir: ritonavir ratio used clinically)20. The doses chosen of atazanavir/ritonavir (90/30 mg/kg) and saquinavir/ritonavir (200/20 mg/kg) was equal or lower than the dose required in mice to achieve plasma concentration, which is equivalent to the therapeutic levels in humans21,22. Treatments were administered orally for 2 days. The next day, mice were euthanized and the C. auris burden in their kidneys was determined. Neither posaconazole alone, nor the HIV protease inhibitors (atazanavir–ritonavir, or saquinavir–ritonavir) treatments was capable of reducing the fungal burden of C. auris when compared to the untreated control. In contrast, compared to the vehicle control, the posaconazole/atazanavir-ritonavir and posaconazole/saquinavir-ritonavir combinations significantly reduced the burden of C. auris in the murine kidneys, producing 2.04 (99.1%) and 1.44-log10 CFU (96.4%) reduction. Moreover, when compared to posaconazole treatment, posaconazole/atazanavir-ritonavir and posaconazole/saquinavir-ritonavir combinations generated 1.40 (96%) and 0.8-log10 CFU (84.2%) reduction, respectively (Fig. 3).
Reduction in fungal load in mice kidneys at 48 h post-infection with C. auris AR0390. Treatments were administered orally: posaconazole (3 mg/kg), atazanavir-ritonavir (ATV-RTV; 90–30 mg/kg), saquinavir-ritonavir (SQV-RTV; 200–20 mg/kg), posaconazole/atazanavir-ritonavir (POS/ATV-RTV; 3/90-30 mg/kg), posaconazole/saquinavir-ritonavir (POS/SQV-RTV; 3/200-20 mg/kg) for 2 days. Animals were humanely euthanized after 48 h, and the fungal burden was determined. The data are presented as average CFU in mice kidneys. The data were analyzed using a one-way ANOVA with post-hoc Dunnett's test. An asterisk (*) denotes a statistically significant difference of mice treated with the corresponding treatments as compared to the vehicle-treated mice (P < 0.05). A pound (#) indicates a statistically significant difference of mice treated with POS/ATV-RTV combination as compared to POS alone (P < 0.05).
Discussion
Candida auris is a widespread pathogenic yeast causing severe invasive candidiasis in critically ill and immunocompromised patients23. Due to its multidrug resistance, severity of infections, and nosocomial outbreaks with high mortality rate, C. auris represent an alarming paradigm shift for Candida infections24. Moreover, the Centers for Disease Control and Prevention (CDC) has classified C. auris as the highest level of threat on the most recent report of antibiotic resistance threat in the United States25. Thus, a robust response is needed to develop new antifungals and treat such infections.
Fluconazole is the mainstay of antifungal therapy for disseminated candidiasis. Nonetheless, posaconazole is recommended as an alternative treatment option for patients who are infected with fluconazole or itraconazole-resistant Candida26. Posaconazole was discovered to be more effective than fluconazole, itraconazole, and voriconazole against Candida isolates based on its MIC values because of the long side chain of posaconazole, which increases its binding affinity to the target27,28. Therefore, in this study we evaluated the ability of the HIV protease inhibitors to synergize and potentiate the activity of posaconazole against C. auris.
Here, we found that posaconazole interacted synergistically with both HIV protease inhibitors (atazanavir, and saquinavir) against all 17 C. auris isolates. Additionally, the synergistic combinations exhibited a fungistatic activity reducing the C. auris CFU by 5 and 5.6-logs after 48 h as compared to the mock (negative) control in a time kill assay. Furthermore, the synergistic interaction between atazanavir/saquinavir and posaconazole has proven effective against all other clinically important Candida species, providing an additional clinical advantage to these combinations.
Based on these results and our prior findings, it is evident that both atazanavir and saquinavir exhibit more potent synergistic interactions when combined with itraconazole and posaconazole compared to fluconazole and voriconazole. This suggests a potential therapeutic advantage of using HIV protease inhibitors in combination with itraconazole and posaconazole, as mutations in the target gene (ERG11) appear to exert a lesser influence on the binding and activity of these particular antifungal agents11,18,19.
Biofilm formation is one of the major virulence factors of C. auris29. C. auris possesses high ability to form a strong adhesive biofilm on non-living structures, such as indwelling medical devices or implants10. Additionally, it was reported that the expression of efflux pump transporters, including both ATP-binding cassette (ABC) and Major facilitator superfamily (MFS), increased during the maturation stage of biofilm formation3. HIV protease inhibitors were reported to impair biofilm formation and disrupt the mature biofilm of some fungal species such as C. albicans and Trichosporon30,31. Therefore, we investigated the biofilm inhibition activity of atazanavir/posaconazole and saquinavir/posaconazole combinations. Both combinations significantly inhibited the C. auris biofilm formation.
Finally, we evaluated the synergistic combinations atazanavir/posaconazole and saquinavir/posaconazole in a C. auris disseminated infection mouse model. Posaconazole alone has previously demonstrated its efficacy in numerous mouse models of disseminated candidiasis32. However, the in vivo efficacy of posaconazole was not reported against C. auris before. Thus, we were encouraged to test the two combinations against mouse model of C. auris infection. First, we evaluated the efficacy of different doses of posaconazole to determine the optimal dose that does not result in a significant reduction of the fungal burden. We found that posaconazole’s dose of 3 mg/kg was the highest dose that did not significantly reduced C. auris CFU as compared to the untreated control. This dose was used in combination with either atazanavir or saquinavir to test the in vivo efficacy of the combinations against C. auris. Ritonavir was added to each combination as a pharmacokinetic enhancer33,34. Atazanavir–ritonavir and saquinavir–ritonavir did not reduce the fungal CFU, similar to previous studies18,19. On the other hand, the two combinations atazanavir/posaconazole and saquinavir/posaconazole, in the presence of ritonavir, significantly reduced the C. auris CFU burden in mice kidneys, generating 2.04 (99.1%) and 1.44-log10 CFU (96.4%) reduction, compared to the mock (negative) control group. Remarkably, the in vivo efficacy of the atazanavir/posaconazole combination (with 2.04 -log10 reduction) surpassed the in vivo efficacy of atazanavir/itraconazole combination (with 1.15-log10 reduction) against C. auris18. Additionally, the in vivo efficacy of the saquinavir/posaconazole combination (with 1.44-log10 reduction) surpassed the in vivo efficacy of saquinavir/itraconazole combination (with 0.85-log10 reduction) against C. auris19.
In conclusion, the atazanavir/posaconazole and saquinavir/posaconazole combinations displayed not only in vitro synergistic antifungal activity but also anti-biofilm activity against C. auris. Moreover, our study found that the combinations efficiently reduced C. auris burden in mice kidneys. Thus, atazanavir and saquinavir can be considered as promising agents for enhancing the potency of posaconazole against azole-resistant C. auris.
Materials and methods
Candida auris strains, reagents and chemicals
C. auris strains were obtained from the BEI Resources (Manassas, VA, USA), and CDC (Atlanta, GA, USA). Media and reagents were provided from the following chemical vendors: crystal violet (Acros Organics, New Jersey, USA), 3-(N-Morpholino) propane sulfonic acid (MOPS) (Fisher Bioreagents, Fairlawn, NJ, USA), phosphate-buffered saline (PBS) (Corning, Manassas, VA, USA), RPMI 1640 (Gibco, Grand, Island, NY, USA), yeast peptone dextrose (YPD) broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), and YPD agar (DOT Scientific Inc, Burton, MI, USA). Drugs were obtained commercially as follows: atazanavir and saquinavir (Ambeed, Arlington Heights, IL, USA), chloramphenicol (Sigma-Aldrich, St. Louis, MO, USA), cyclophosphamide (Cayman Chemical, Ann Arbor, MI, USA), posaconazole (Biosynth Carbosynth, San Diego, CA, USA), and ritonavir (TCI America, Portland, OR, USA).
Minimum inhibitory concentration and checkerboard assay
The MICs values of posaconazole, the HIV protease inhibitors (atazanavir and saquinavir) were evaluated against C. auris isolates following the CLSI guidelines35. The combinations of posaconazole with atazanavir or saquinavir were determined against 17 C. auris isolates using the checkerboard method, as described elsewhere36. Similarly, the two combinations were evaluated against other Candida species including C. albicans, C. tropicalis, C. parapsilosis, C. krusei and C. glabrata. The FICI was calculated and interpreted as follows: FICI of > 4 was classified as antagonism, FICI of > 0.5–4: indifference, and FICI of ≤ 0.5: synergism37. To confirm the ability of atazanavir and saquinavir to improve the activity of posaconazole against C. auris, we used posaconazole test strips. C. auris AR0390 was cultured in RPMI 1640 agar in the presence of atazanavir, saquinavir or dimethyl sulfoxide (DMSO). Next, the posaconazole test strip was added, and the agar plate was incubated at 35 °C for 24 h. The concentration at which a zone of inhibition intercepted with the posaconazole strip used as the MIC.
Time-kill kinetics and spotting assays
A time-kill assay was used to investigate the killing kinetics of the atazanavir/posaconazole and saquinavir/posaconazole combinations against C. auris AR0390, as described before17,38,39. Briefly, exponential phase C. auris cells were diluted to ~ 104 CFU/ml in RPMI 1640 medium and incubated with either atazanavir (16 µg/ml), saquinavir (16 µg/ml), posaconazole (0.25 µg/ml), atazanavir/posaconazole combination, or saquinavir/posaconazole combination. DMSO-treated cells were used as a negative (mock) control. Aliquots were taken at specific time points (0, 6, 12, 24, 30, 36, and 48 h), diluted and counted to determine the number of viable cells. The data are presented as the average of three independent experiments. For the spotting assay, aliquots from the time point of 24-h were plated onto YPD agar plates and incubated at 35 °C for 24 h before the plates were scanned.
C. auris biofilm formation assay
The C. auris biofilm formation assay was performed as described previously40,41,42. To evaluate the effect of the atazanavir/posaconazole and saquinavir/posaconazole combinations in preventing biofilm formation, HIV-protease inhibitors (atazanavir and saquinavir) diluted in RPMI medium (at 16 μg/ml) with and without posaconazole (0.03 μg/ml) were added to 96-well plates containing 106 CFU/ml of C. auris AR0390 cells, and the plates were incubated at 35 °C for 24 h. To assess the biofilm inhibition of the C. auris AR0390 isolate, adherent biofilms were stained for 30 min with 100 μl of 0.1% (wt/vol) crystal violet. After crystal violet was removed, the cells were washed 3 times with PBS and the plates were allowed to dry. The resultant biofilm biomasses were then quantified by dissolving the crystal violet-stained biofilms in 100 μl of ethanol before recording absorbance values (OD600).
C. auris infection mouse model
The Virginia Tech Animal Care and Use Committee reviewed and approved the mouse study, which was conducted strictly in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The mouse studies are in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. To assess the efficacy of the two combinations atazanavir/posaconazole and saquinavir/posaconazole in vivo against C. auris, we used a mouse model of disseminated C. auris infection as reported previously43. First, we conducted a dose–response study for posaconazole to determine the dose that can be used in combination with either atazanavir or saquinavir. Female CD-1 mice were injected intraperitoneally (I.P.) with cyclophosphamide (200 mg/kg) and (150 mg/kg) 4 days and 1 day, respectively before infection. On the infection day, mice were injected I.P with 4.5 × 107 cells/mouse of C. auris AR0390. Two hours later, mice were randomly distributed into 6 groups and administered different doses of posaconazole (0.25, 0.5, 1, 3, 5 mg/kg) orally once daily for two days. One day later, mice were humanely euthanized using CO2, and their kidneys were extracted, homogenized, serially diluted, and plated onto YPD agar supplemented with chloramphenicol. YPD plates we then incubated for 24 h at 35 °C for the CFU determination.
We determined the optimal dose of posaconazole as 3 mg/kg to be used in the following experiment. Next, we assessed the in vivo efficacy of the two combinations, with adding ritonavir as pharmacokinetic enhancer. Mice were rendered neutropenic by cyclophosphamide injection as described above. On the challenge day, mice were injected I.P. with 5.67 × 107 cells/mouse of C. auris AR0390. Two hours after infection, mice were randomly allocated into groups and all treatments were given orally as follows: (A) Control, (B) posaconazole (3 mg/kg), (C) atazanavir–ritonavir (90–30 mg/kg), D) saquinavir–ritonavir (200–20 mg/kg), (E) posaconazole/atazanavir–ritonavir (3/90-30 mg/kg), (F) posaconazole/saquinavir–ritonavir (3/200-20 mg/kg). In these groups, posaconazole was administered once daily while atazanavir and saquinavir were administered with ritonavir twice daily for 2 days. Twelve-hours after the last dose, mice were euthanized and the C. auris burden in the mice kidneys was determined as described above. The data was analyzed via one-way analysis of variance (ANOVA) with post-hoc Dunnett’s test for multiple comparisons.
Statistical analyses
The statistical tests and significance were determined and indicated in each figure legend using GraphPad Prism 8 software.
Ethical approval
All mice experiments were approved, and performed according to the regulations of the Virginia Tech Animal Care and Use Committee.
Data availability
All data generated or analyzed during this study are included in the supplementary information files.
References
Skiest, D. J. et al. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin. Infect. Dis. 44(4), 607–614 (2007).
Bongomin, F. et al. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi (Basel) 3(4), 57 (2017).
Kean, R. et al. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere 3(4), 10–1128 (2018).
Naveen, K. V. et al. Human fungal infection, immune response, and clinical challenge-a perspective during COVID-19 pandemic. Appl. Biochem. Biotechnol. 194(9), 4244–4257 (2022).
Ademe, M. Immunomodulation for the treatment of fungal infections: opportunities and challenges. Front. Cell Infect. Microbiol. 10, 469 (2020).
Turner, S. A. & Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 4(9), a019778 (2014).
Akinbobola, A. B. et al. Environmental reservoirs of the drug-resistant pathogenic yeast Candida auris. PLoS Pathog. 19(4), e1011268 (2023).
Sticchi, C. et al. Increasing number of cases due to Candida auris in north Italy, July 2019-December 2022. J Clin Med 12(5), 1912 (2023).
Lara, H. H. et al. Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater Interfaces 12(19), 21183–21191 (2020).
Horton, M. V. & Nett, J. E. Candida auris infection and biofilm formation: Going beyond the surface. Curr. Clin. Microbiol. Rep. 7(3), 51–56 (2020).
Rodrigues, C. F., Alves, D. F. & Henriques, M. Combination of posaconazole and amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms 6(4), 123 (2018).
Dekkers, B. G. J. et al. Therapeutic drug monitoring of posaconazole: An update. Curr. Fungal Infect. Rep. 10, 51–61 (2016).
Chau, M. M. et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy, 2014. Intern. Med. J. 44(12b), 1364–1388 (2014).
Raad, I. I. et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 42(12), 1726–1734 (2006).
Li, Y. et al. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin. Pharmacokinet. 49(6), 379–396 (2010).
O’Brien, B. et al. Pan-resistant Candida auris: New York subcluster susceptible to antifungal combinations. Lancet Microbe 1(5), e193–e194 (2020).
Eldesouky, H. E., et al., Potent synergistic interactions between lopinavir and azole antifungal drugs against emerging multidrug-resistant Candida auris. Antimicrob. Agents Chemother, 2020. 65(1).
Elgammal, Y., Salama, E. A. & Seleem, M. N. Atazanavir resensitizes Candida auris to azoles. Antimicrob. Agents Chemother. 67(5), e0163122 (2023).
Elgammal, Y., Salama, E. A. & Seleem, M. N. Saquinavir potentiates itraconazole’s antifungal activity against multidrug-resistant Candida auris in vitro andin vivo. Med Mycol 61(9), myad081 (2023).
Zeldin, R. K. & Petruschke, R. A. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J. Antimicrob. Chemother. 53(1), 4–9 (2004).
Pettoello-Mantovani, M. et al. Saquinavir-mediated inhibition of human immunodeficiency virus (HIV) infection in SCID mice implanted with human fetal thymus and liver tissue: An in vivo model for evaluating the effect of drug therapy on HIV infection in lymphoid tissues. Antimicrob. Agents Chemother. 41(9), 1880–1887 (1997).
Prot, M. et al. Long-term treatment with lopinavir-ritonavir induces a reduction in peripheral adipose depots in mice. Antimicrob. Agents Chemother. 50(12), 3998–4004 (2006).
Sanyaolu, A. et al. Candida auris: An overview of the emerging drug-resistant fungal infection. Infect. Chemother. 54(2), 236–246 (2022).
Lone, S. A. & Ahmad, A. Candida auris-the growing menace to global health. Mycoses 62(8), 620–637 (2019).
Prevention, C.F.D.C.A., Increasing Threat of Spread of Antimicrobial-resistant Fungus in Healthcare Facilities. March 20, 2023.
Langner, S., Staber, P. B. & Neumeister, P. Posaconazole in the management of refractory invasive fungal infections. Ther. Clin. Risk Manag. 4(4), 747–758 (2008).
Vazquez, J. A. Role of posaconazole in the management of oropharyngeal and esophageal candidiasis. Ther. Clin. Risk Manag. 3(4), 533–542 (2007).
Ianas, V., Matthias, K. R. & Klotz, S. A. Role of posaconazole in the treatment of oropharyngeal candidiasis. Infect. Drug Resist. 3, 45–51 (2010).
Sherry, L. et al. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 23(2), 328–331 (2017).
Lohse, M. B. et al. Combination of antifungal drugs and protease inhibitors prevent candida albicans biofilm formation and disrupt mature biofilms. Front. Microbiol. 11, 1027 (2020).
Cordeiro, R. A. et al. The HIV aspartyl protease inhibitor ritonavir impairs planktonic growth, biofilm formation and proteolytic activity in Trichosporon spp. Biofouling 33(8), 640–650 (2017).
Marine, M., Pastor, F. J. & Guarro, J. Efficacy of posaconazole in a murine disseminated infection by Candida tropicalis. Antimicrob. Agents Chemother. 54(1), 530–532 (2010).
Achenbach, C. J. et al. Atazanavir/ritonavir-based combination antiretroviral therapy for treatment of HIV-1 infection in adults. Fut. Virol. 6(2), 157–177 (2011).
Buss, N. et al. Saquinavir and ritonavir pharmacokinetics following combined ritonavir and saquinavir (soft gelatin capsules) administration. Br. J. Clin. Pharmacol. 52(3), 255–264 (2001).
CLSI. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed. CLSI standard M27. Clinical and Laboratory Standards Institute, Wayne, PA.
Eldesouky, H. E. et al. Ospemifene displays broad-spectrum synergistic interactions with itraconazole through potent interference with fungal efflux activities. Sci. Rep. 10(1), 6089 (2020).
Eldesouky, H. E. et al. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimicrob. Agents 52(6), 754–761 (2018).
Eldesouky, H. E. et al. Aprepitant, an antiemetic agent, interferes with metal ion homeostasis of Candida auris and displays potent synergistic interactions with azole drugs. Virulence 11(1), 1466–1481 (2020).
Hagras, M. et al. Oxadiazolylthiazoles as novel and selective antifungal agents. Eur. J. Med. Chem. 189, 112046 (2020).
Eldesouky, H. E. et al. Reversal of azole resistance in Candida albicans by sulfa antibacterial drugs. Antimicrob. Agents Chemother. 62(3), 10–1128 (2018).
Mohammad, H. et al. Identification of a phenylthiazole small molecule with dual antifungal and antibiofilm activity against Candida albicans and Candida auris. Sci Rep 9(1), 18941 (2019).
Eldesouky, H. E. et al. Repurposing approach identifies pitavastatin as a potent azole chemosensitizing agent effective against azole-resistant Candida species. Sci. Rep. 10(1), 7525 (2020).
Salama, E. A. et al. Lopinavir and ritonavir act synergistically with azoles against Candida auris in vitro and in a mouse model of disseminated candidiasis. Int. J. Antimicrob. Agents 62(3), 106906 (2023).
Acknowledgements
We would like to express our gratitude to CDC, and BEI Resources for generously providing the fungal strains utilized in this study. Furthermore, we extend our appreciation to Dr. Nader S. Abutaleb and Nour M. Alkashef of Virginia Tech for their assistance with the animal experiments.
Author information
Authors and Affiliations
Contributions
Y.E. performed the experiments, analyzed the data, and wrote the original draft of the manuscript. E.A.S. assisted in the experiments, and formal analysis. M.N.S. funded and supervised the project. All the authors read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elgammal, Y., Salama, E.A. & Seleem, M.N. Enhanced antifungal activity of posaconazole against Candida auris by HIV protease inhibitors, atazanavir and saquinavir. Sci Rep 14, 1571 (2024). https://doi.org/10.1038/s41598-024-52012-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52012-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.