Abstract
SPARC is an extracellular Ca2+-binding, secreted glycoprotein that plays a dynamic role in the growth and development of organisms. This study aimed to describe the isolation, characterization, and expression analysis of HdhSPARC in Pacific abalone (Haliotis discus hannai) to infer its potential functional role. The isolated HdhSPARC was 1633 bp long, encoding a polypeptide of 284 amino acid residues. Structurally, the SPARC protein in abalone is comprised of three biological domains. However, the structure of this protein varied between vertebrates and invertebrates, as suggested by their distinct clustering patterns in phylogenetic analysis. In early development, HdhSPARC was variably expressed, and higher expression was found in veliger larvae. Moreover, HdhSPARC was highly expressed in juvenile abalone with rapid growth compared to their slower-growing counterparts. Among the testicular development stages, the growth stage exhibited higher HdhSPARC expression. HdhSPARC was also upregulated during muscle remodeling and shell biomineralization, as well as in response to different stressors such as heat shock, LPS, and H2O2 exposure. However, this gene was downregulated in Cd-exposed abalone. The present study first comprehensively characterized the HdhSPARC gene, and its spatio-temporal expressions were analyzed along with its responses to various stressors.
Similar content being viewed by others
Introduction
Aquaculture is one of the world's fastest-growing economic sectors1. Abalone are commercially important marine gastropod mollusks, that are widely distributed along tropical and temperate coastal regions2. Abalone is often referred to as the ‘ginseng of the ocean’ due to its health-promoting effects owing to its nutritional composition and bioactive molecules3. Currently, abalone production mainly relies on aquaculture, with fishery yields contributing very little to meet the demand for this resource. Abalone aquaculture completely depends on hatchery-produced seed4. There are thousands of genes and enzymes regulating the growth and development of abalone through different molecular mechanisms.
The extracellular matrix (ECM) acts as a reservoir for a complex combination of cytokines and growth factors with diverse structural and regulatory functions5. ECM proteins are known to be involved in a variety of activities, including muscle cell development, structure maintenance, and tissue repair, through the modulation of growth factors and cell–matrix signal transduction pathways6. SPARC (secreted protein acidic and rich in cysteine) is a Ca2+-binding glycoprotein found in extracellular matrix7 secreted by different cell types and plays a pleiotropic role8 throughout the different developmental stages of vertebrate and invertebrate species. This multifunctional secreted protein has a high affinity for cations, provides support to the extracellular matrix proteins, and mediates a wide range of growth factors and cytokines9 activities, including embryogenesis10, shell biomineralization11, muscle tissue regeneration12, and stress responses13, via cell–matrix interaction. SPARC deficiency in mice was previously reported to reduce glucose tolerance and insulin secretion14, which may influence the growth and development of aquaculture organisms. In contrast, overexpression of SPARC increases muscle strength, muscle mass, glucose transporter activity, and oxidative phosphorylation in mitochondria15. Inactivation of SPARC is also reported to impair gonad functions16. Moreover, although the exact function of SPARC in the early development (embryogenesis) of animals remains largely unknown, some studies have reported its involvement in embryo viability, tissue morphogenesis, and hematopoiesis19.
Tissue regeneration is a vital physiological development process that involves the growth and renewal of various cells and tissues to overcome biological disturbance and adaptation15. SPARC is a novel myokine18 secreted from the myocytes of muscle tissue. The SPARC molecule is known to have regenerative effects such as the regeneration of muscle tissue after physical or pathological damage through repair, cellular differentiation, and remodeling mechanisms16. In teleosts, SPARC is expressed in skeletal muscle during regeneration and development, as well as in satellite cells, suggesting that SPARC has a vital role in the skeletal muscle compartment19.
The shell of mollusks is an intricate natural structure that forms through a complex and highly controlled calcium metabolism process known as biomineralization20. In mollusks, several shell matrix proteins, including SPARC, have been isolated in the extrapallial fluid, which contains a variety of ions (Ca2+, Mg2+, K+, Na+, and HCO3–) that bind to CaCO3 crystals11. Previous studies have characterized the role of SPARC in shell formation in different mollusk species, including the Pacific oyster21, the pearl oyster22, and the sea snail23. A wide range of functional elements such as bone, shell, teeth, and coral reefs are the products of biomineralization process24.
In aquaculture systems and natural habitats, a variety of stressors including anthropogenic activities, pathogens, and climate change can affect vital physiological functions such as the growth and development of aquatic animals. Hydrogen peroxide (H2O2)25, cadmium (Cd)26, lipopolysaccharide (LPS)27, and heat stress20 are among the most common stressors that can alter SPARC expression and its related functional activity in aquaculture animals.
This study aimed to molecularly characterize the multifunctional matricellular glycoprotein SPARC in Pacific abalone (H. discus hannai) and investigate its transcriptional regulation during embryogenesis, postnatal development, remodeling and repair, and immune response through expression analysis. Regulation of SPARC in farmed abalone may contribute to the abalone aquaculture industry and related future research.
Results
HdhSPARC sequence analysis
The cDNA sequence encoding H. discus hannai SPARC was cloned from the mantle tissue and designated as HdhSPARC. The full-length sequence of HdhSPARC cDNA (GenBank Accession No. OM937904.1) was 1633 bp long, including a poly-A tail (Fig. S1), and its 5′- and 3′-untranslated regions (UTR) were 240 bp and 538 bp long, respectively. A putative polyadenylation signal (AATAAA) was identified in its nucleotide sequence 21 bp upstream of the poly-A tail. The ORF of the HdhSPARC cDNA sequence was 855 bp with 284 deduced amino acids.
The motif scan analyses detected a single amidation site X-G-[R/K]-[R/K] at the amino acid position 125–128 (GGRR) in the C-terminal region. A total of 18 phosphorylation sites were observed, of which seven were protein kinase C (PKC) phosphorylation sites, [S/T]-X-[R/K], at positions 107–109 (STK), 221–223 (SRR), 231–233 (SLK), 250–252 (SNR), 267–269 (SKK), and 271–273 (SDK); eleven were casein kinase II phosphorylation sites, [S/T]-X(2)-[D/E], 115–118 (SECE), 180–183 (SRDE), 214–217 (SPQD), 221–224 (SRRE), 244–247 (SMCD), and 255–258 (TLTD); three were cAMP- and cGMP-dependent protein kinase phosphorylation sites, [R/K](2)-X-[S/T], at position 150–153 (RKLT), 252–255 (RKIT), and 268–271 (KKIS); two N-myristoylation sites at position 132–137 (GCSNAR) and 260–265 (GACLGV); and two N-glycosylation sites at position 110–113 (NQTY) and 199–202 (NHTE) (Fig. S1).
In the multiple sequence alignment, 14 conserved cysteine residues were found to be conserved when aligned with SPARC of H. discus hannai, H. discus discus, Pinctada fucata, Patella vulgata, Salmo salar, Xenopus tropicalis, and Homo sapiens (Fig. 1). The first 19 residues were detected as the signal peptide for this secreted protein. The SPARC domain was found in the C-terminal region of the sequence. Moreover, the N-terminal region of the HdhSPARC was more conserved than the C-terminal region for vertebrates and invertebrates. Among the species 13 cysteine residues were found conserved.
Multiple sequence alignment of HdhSPARC from deduced amino acid sequences of H. discus hannai (OM937904.1), H. discus discus (BAK22657.1), Pinctada fucata (AND99565.1) Patella vulgata (CCJ09602.1), Salmo salar (ACH70833.1), Xenopus tropicalis (AAT01218.1), Rattus norvegicus (NP_036788.1), and Homo sapiens (AAA60570.1). The blue letters indicate hydrophobic residues, red letters signify positively charged residues, magenta letters denote negatively charged residues, green letters represent polar residues, pink letters are for cysteine residues, orange letters for glycine residues, yellow letters for proline residues, cyan letters indicate aromatic residues, and white letters are for the unconserved residues. The green line indicates the signal peptide, and the red boxes indicate the conserved cysteine residues. The red line indicates the SPARC domain.
Structure of the HdhSPARC protein
Structurally, HdhSPARC exhibits three concordant functional domains, including the DUF domain (N-terminal region), the Kazal-type serine protease inhibitor domain, and the SPARC Ca-binding domain with EF-hand motif (Fig. 2a). The constructed three-dimensional structure was dominated by coils and helixes, with very few strands. The helix and strands were mostly found in the C-terminal region (Fig. 2b).
Phylogenetic analysis
The constructed phylogenetic tree based on the amino acid sequence using the maximum likelihood method formed five clusters (Mammalia, Amphibia, Actinopterygii, Gastropoda, and Branchiopoda), of which three were vertebrate species and two were invertebrate species. Among the two invertebrate clusters, the HdhSPARC clade with Hdd-SPARC and Pv-SPARC of gastropod clustered with high bootstrap value, whereas the branchiopod protein formed a separate cluster (Fig. 3). Phylogenetically, the invertebrate species were closer than the vertebrate species.
Phylogenetic tree of SPARC constructed via the maximum likelihood method after ClustalW alignment based on amino acid residues of different vertebrate and invertebrate species. The red circle in the tree indicate bootstrap probability. The green cluster indicates Mammalia, yellow for Amphibia, light green for Actinopterygii, blue for Gastropoda, and violet for Branchiopoda. The following protein sequences with their accession id were used to construct the phylogenetic tree: Homo sapiens (AAA60570.1), Pongo abelii (NP_001127042.1), Bos taurus (ABQ12988.1), Sus scrofa (AAX83050.1), Orcinus orca (XP_004280373.1), Pleurodeles waltl (AWQ28573.1), Rhinatrema bivittatum (AWQ28578.1), Typhlonectes compressicauda (AWQ28577.1), Xenopus laevis (CAA44350.1), Xenopus tropicalis (AAT01218.1), Oryzias latipes (AAT01217.1), Scophthalmus maximus (AGW25370.1), Epinephelus coioides (ACJ66296.1), Oncorhynchus mykiss (AAC99813.1), Salmo salar (ACH70833.1), H. discus discus (BAK22657.1), H. discus hannai (OM937904.1), Patella vulgata (CCJ09602.1), Pinctada fucata (BAK22656.1), Mytilus edulis (CAG2244558.1), Hyalella azteca (XP_018022874.1), Artemia franciscana (BAB20042.1), Daphnia magna (XP_045029097.1), Blattella germanica (CZQ50751.1), and Nilaparvata lugens (UPH52990.1).
Tissue-specific mRNA expression of HdhSPARC
Among the different tissues of Pacific abalone, HdhSPARC mRNA was most significantly (p < 0.05) expressed in the testis, followed by the mantle, muscle, and digestive gland tissue (Fig. S2). The lowest expression was observed in the ovary tissue. These findings were consistent with those of our semi-quantitative RT-PCR expression analysis (Fig. S3).
HdhSPARC expression in embryonic and larval development of Pacific abalones
HdhSPARC mRNA was dynamically expressed during the embryonic and larval development stages. After fertilization, HdhSPARC mRNA was minimally expressed until the blastula stage, and no significant differences were observed. However, after the blastula stage, the expression increased significantly (p < 0.05) in the trochophore stage, followed by the veliger and juvenile stages (Fig. 4a).
Development stage specific mRNA expression of HdhSPARC in Pacific abalone. (a) embryonic and larval developmental (FE, fertilized egg; 2-CL, 2-cell, 4CL, 4-cell, MOR, morula, BLS, blastula, TRO, trochophore; VLG, veliger; JUV, juvenile), (b) testicular development (PS, proliferative stage; GS, growth stage; SS, spawning stage; RS, resting stage). (nsp > 0.05; *p < 0.05; ***p < 0.001).
HdhSPARC mRNA was differentially expressed in testis tissue throughout the different development stages examined herein. The growth stage showed significantly higher (p < 0.05) expression. HdhSPARC expression decreased thereafter, with the lowest expression being observed in the resting stage (Fig. 4b).
HdhSPARC expression in Pacific abalones exhibiting different growth patterns
HdhSPARC showed upregulated expression according to growth pattern (stunted growth to rapid growth). Significantly higher expression (p < 0.05) was observed in rapid-growth abalone (Fig. 5). In contrast, the lowest expression was observed in the stunted growth abalone, but this expression level was not significantly different from that of the minimum and normal growth abalone.
HdhSPARC expression during muscle remodeling and shell biomineralization of Pacific abalone
Expression analysis in muscles injured Pacific abalone showed that HdhSPARC was significantly (p < 0.05) expressed on the first day of injury (Fig. 6a). After a rapid reduction at 6 days, the expression of HdhSPARC decreased slowly. However, the expression was higher throughout the remodeling period than in control and remodeled abalones. The expression in the control and remodeled abalone was not significant.
Figure 6b illustrates the expression of HdhSPARC mRNA during the shell biomineralization of Pacific abalone. In the first week, the expression was significantly higher than that of the control abalone. Afterward, a significant decrease in expression was observed in the second week, which was increased in the remaining experimental period but was not significant. Overall, the expression of HdhSPARC was significantly higher than that of control abalone during the study period.
HdhSPARC mRNA expression in heat-stressed and LPS exposed Pacific abalone
In heat stress treatment, the expression of HdhSPARC mRNA increased for the first 3 h. Thereafter, the expression of 15 °C treatment slowly decreased in a time-dependent manner until 24 h of treatment. However, the expression of HdhSPARC mRNA at 25 °C and 30 °C exhibited increasing trends until six hours of heat treatment, when it reached its peak. After that, HdhSPARC expression decreased significantly in the 30 °C treatment, reaching levels that were even below that of the control treatment after 24 h (Fig. 7a). On the other hand, although the expression decreased gradually in the 25 °C treatment, this effect was not significant when compared with the 30 °C treatment. Upon LPS exposure, a gradual increase in HdhSPARC mRNA was observed with increasing post-exposure time. The expression increased slowly for the first six hours compared to the last twelve hours, and maximum expression was observed after 24 h of LPS exposure (Fig. 7b).
HdhSPARC mRNA expression in Cd- and H2O2-exposed Pacific abalone
A reverse relationship was observed between the Cd intensity and mRNA expression of HdhSPARC in Cd-exposed Pacific abalone. With increased post-exposure time, the intensity of the Cd signals increased (Fig. 8ai,aiii) when the expression levels decreased (Fig. 8aii). A significantly higher (p < 0.05) expression of HdhSPARC was observed after an hour of Cd exposure, whereas a lower expression level was observed at 24 h of Cd exposure. The time-dependent fluorescence signals of H2O2, mRNA expression, and fluorescence signal intensity are illustrated in Fig. 8b. HdhSPARC expression was found to increase with increasing post-exposure time (Fig. 8bi). The mRNA expression of HdhSPARC (Fig. 8bii) and intensity of the H2O2 signals (Fig. 8biii) were also significantly (p < 0.05) increased in a time-dependent manner, except for the first six hours, when the increase in expression was not significant.
Discussion
Several ECM proteins are known to be inherently involved in ECM deposition, cell–matrix interaction, and growth factor signaling and play pivotal roles in regulating multiple hallmarks of ontogenetic development10. SPARC is a multifunctional ECM protein with a high affinity for cations and hydroxyapatite that is found in both vertebrate and invertebrate species. To gain new insights into the cellular and molecular mechanisms that govern embryogenesis and postnatal development, muscle repairment, shell biomineralization, gonadal development, and immune response under different stress conditions, the matricellular protein SPARC was cloned and its expression was analyzed in Pacific abalone.
HdhSPARC has a putative signal peptide at its N-terminal region, thus confirming its nature as a secreted protein29. The signal peptide produces a mature peptide of 265 amino acids. A total of 18 cysteine residues were identified in the amino acid sequence, of which 13 residues were found to be identical when compared with other invertebrate and vertebrate species.
Structurally, the HdhSPARC protein comprises three biological structural domains: a DUF domain (I) at the N-terminal region, a Kazal-type serine protease inhibitor (central domain II), and a Ca2+-binding extracellular domain (III), which is an alpha-helix-rich region containing a high-affinity Ca2+-binding EF-hand. The N-terminal domain of SPARC is highly variable in invertebrates30, structurally acidic, thus binding Ca2+ with low affinity, and rich in glutamic acid in most cases9. Although the first domain (domain I) of HdhSPARC is dominated by glutamic acid and aspartic acid, the conserved domain did not exhibit this glutamic acid-rich region. The central domain (domain II), a Kazal-type serine protease inhibitor (Kazal-type 1, Kazal-type 2), is cysteine-rich and contains a highly conserved N-linked glycosylation site, which is an important trait for collagen affinity and protein functionality31. This domain plays vital roles, including endogenous protease regulation, blood coagulation, cell development, and the immune response32. The third domain (domain III) is an extracellular calcium-binding domain, including an EF-hand motif with high affinity towards Ca2+, and has been shown to bind collagen in a Ca2+-dependent manner33.
Amino acid-based multiple sequence alignment indicated that the SPARC protein of Haliotis discus was highly conserved compared to that of its vertebrate and invertebrate counterparts. The HdhSPARC showed the highest identity (99.65%) with the Hdd-SPARC, thus assuming the functional similarity of the protein. The results of our phylogenetic analysis based on protein sequence indicated that SPARC was present in both vertebrate and invertebrate species29. HdhSPARC clustered with its molluscan SPARC, which has a close functional relationship with the arthropod clade within the vertebrate and invertebrate clusters, indicating that invertebrate SPARC is functionally different from vertebrate SPARC.
The higher expression of HdhSPARC in different types of tissues (testis, mantle, muscle, and digestive gland) was indicative of multifunctional characteristics. Previous studies suggest that SPARC has functions in testis development34, shell formation by secreting glycoprotein from the mantle21, muscle development and regeneration35, metabolism, and immune response in the digestive gland36. However, this expression pattern can vary depending on the development stage, health status, and environmental conditions37.
In this study, we analyzed the expression patterns of HdhSPARC in the embryonic and larval development stages of Pacific abalone and found higher expression levels at the trochophore, veliger, and juvenile stages compared to the early cell division stages. The Pacific abalone undergoes a dynamic metamorphosis during its early development stages38, including shell biomineralization, muscle formation, formation of the basement membrane, and embryonic hematopoiesis. Gene ontology (GO) analysis (Fig. S4) also predicted that HdhSPARC is involved in developing anatomical features39. In the trochophore and veliger stages, complex physiological and morphological processes, including shell formation, cilia development, body shape development, and head-food differentiation, occur between the transition of these two stages40. A previous study on Caenorhabditis elegans suggested that SPARC is required for the fertility and viability of embryos. Knockdown of SPARC negatively affects inner ear and cartilage development in zebrafish, indicating that SPARC is required for cartilage morphogenesis10. In Patella vulgata, SPARC was localized in a ring around the larval shell field, indicating its involvement in shell development23. However, although the involvement of SPARC in early embryonic and larval development has previously been studied in different species, its precise function is largely unknown.
In this study, we investigated the mRNA expression of HdhSPARC in different testicular development stages and found that this gene was upregulated in PS and GS but down-regulated in SS and RS, indicating that HdhSPARC may have potential role in germ cell maturation of male organisms. Upregulated expression of SPARC was also previously reported in mice during fetal testis development41. HdhSPARC may be involved in the transport of Ca2+ and Fe2+ cations in the testes42. This was further supported by the results of our GO analyses (Fig. S4), which predicted that this gene was involved in calcium ion binding. Additionally, the 3D structure of the protein (Figure S3) contained an SF4 ligand (iron/sulfur cluster) that requires Fe2+ ions to become active. SPARC may also be involved in the formation of sperm bundles in the testis43. However, this has not yet been confirmed in abalone.
The growth of Pacific abalone was significantly influenced by HdhSPARC. The low expression of this gene in individuals with stunted growth coupled with a significantly higher expression in rapid-growth juvenile Pacific abalone suggested that HdhSPARC may be involved in the growth of Pacific abalone. In mice, the level of intracellular SPARC was reported to increase with the administration of growth hormone (GH)44. Moreover, SPARC-deficient mice also exhibited reduced insulin secretion (a potent growth-promoting hormone)45, thus confirming that HdhSPARC may be related to the growth of Pacific abalone. SPARC regulates growth development by cell proliferation, migration, and differentiation through MEK/ERK, TAK1 and p38 signaling pathways46.
As a myokine, SPARC was previously predicted to participate in muscle damage repair in muscle satellite cells12. The present study found that the mRNA expression of HdhSPARC was increased during muscle regeneration in Pacific abalone, indicating that it plays a functional role in muscle tissue repair and regeneration, although the molecular mechanism is unknown yet. However, mice with null-Sparc also generate muscle, indicating that a lack of SPARC does not necessarily affect muscle development47. So, it can be hypothesized that presence of SPARC is compensatory but not compulsory in damage tissue repairment. Deficiency of SPARC may not affect tissue repairment pathway, other muscle development genes may be active and complete the muscle development pathway. There are lots of genes responsible for damaged tissue replacement including SPARC.
SPARC is a common biomineralization-related protein in vertebrates and mollusks and is considered a shell matrix protein due to its shell-biomineralizing functionality48. The expression levels of HdhSPARC mRNA increased after shell puncture, indicating that HdhSPARC may be involved in shell formation in the Pacific abalone. SPARC regulates the crystallization of CaCO3 through the binding of Ca2+ by both its amino- and carboxyl-terminal domain and induces vaterite formation in the calcite crystallization system11. Previously, the expression of SPARC mRNA was reported to increase in extrapallial fluid (i.e., an aqueous fluid located between the outer mantle epithelium and the inner face of the shell) in Pinctada fucata after shell-notching22, suggesting its participation in the shell repair process. The mantle is believed to be involved in prismatic layer ingrowth and thickening of the nacreous layer49, and HdhSPARC was highly expressed in the mantle during the shell biomineralization of Pacific abalone.
SPARC is a secreted heat-shock protein that is induced by heat shock and other forms of stress, with its secondary structure remaining unchanged up to 50 °C50. The HdhSPARC expression of the heat-stressed abalones was higher than that of the control abalone during the experiment, indicating that the secretion of Pacific abalone SPARC was increased. These findings were consistent with those of a previous study that analyzed SPARC expression during heat-shock treatment in chick embryos51. Another study revealed that the expression of SPARC was associated with other heat shock proteins such as SHP70 and HSP4734. Therefore, the higher expression of HdhSPARC during the heat shock treatment indicates its role in the protection of tissues or cells during a transient stressful state.
Furthermore, the present study observed that HdhSPARC is LPS-inducible and therefore LPS-exposed abalones exhibited a significantly higher HdhSPARC expression level compared to the control. Given that LPS is a pathogen-associated immune stimulant52, the higher expression of HdhSPARC suggests that this secreted protein also possesses immune functions. Although the specific reason for the induction of SPARC by LPS is unknown in abalone, previous studies suggest that LPS-induced SPARC is required for endothelial cell proliferation at the damage site27 and has been linked to changes in cell morphology and the modeling of the germinal center microenvironment53.
The heavy metal Cd is toxic to most aquatic animals, including marine invertebrates, and can affect growth and developmental, reproductive, hematological, and immunological functions54. The present study found a reverse relationship between Cd absorption and HdhSPARC expression in Pacific abalone. Although Cd absorption was previously reported to induce the generation of reactive oxygen species (ROS) that cause oxidative stress and eventually impair cell and tissue functions55, exposure of cells or tissues to high levels of Cd was also previously reported to decrease the expression level of SPARC26. In this study, exposure experiments were conducted using a high (6 mgL−1) CdCl2 dose. However, the specific molecular mechanism through which Cd decreases the expression level of HdhSPARC in Pacific abalone remains unknown.
H2O2 is an ecological factor with potential biological and chemical reactivity that can trigger oxidative stress and affects the growth, survival, and metabolic rate of aquatic organisms56. In this study, the expression level of HdhSPARC was upregulated in the digestive gland with increasing post-induction time after H2O2 injection. Fluorescence analyses detected a gradual increase of H2O2 intensity after induction, which can cause lipid peroxidation57. Given that the lack of SPARC increases the accumulation of reactive oxygen species, protein oxidation, lipid peroxidation, and DNA damage58, HdhSPARC may play a role in preventing lipid peroxidation in Pacific abalone.
Collectively, our findings revealed that HdhSPARC has a dynamic role that starts at the very beginning of Pacific abalone development. HdhSPARC is expressed differentially depending on body development, growth phases, reproductive period, and diverse environmental conditions, thus confirming that the variations in its expression are not only stage-specific but also organ-specific. Understanding the regulatory nature of HdhSPARC through expression analysis would enable the development of strategies for the rearing of abalone with slow and stunted growth to increase HdhSPARC expression levels, which may improve growth rates. HdhSPARC expression can also serve as an indicator of environmental stress and injury, thus enabling the detection of abnormal conditions and the implementation of corrective measures if necessary. Therefore, the data acquired in this study provide insights into the effects of different stressors on HdhSPARC expression and could thus be applied to the Pacific abalone aquaculture industry.
Materials and methods
Ethical statement
The present study is reported in accordance with the ARRIVE guidelines. The experimental protocols used in this study were approved by the Animal Care and Use Committee of Chonnam National University (approval number: CNU IACUC-YS-2022-8) and according to Article 14th of the Korean Animal Protection Law of the Korean government. The present study was conducted following the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. To minimize pain and discomfort during the sample collection from the abalones, anesthesia (5% MgCl2) was applied following the standard operating procedure of Chonnam National University.
Experimental organisms and sample collection
A total of 215 reproductively mature Pacific abalone (male and female) with a mean body weight of 122.6 ± 0.59 g and a mean shell length of 88.2 ± 0.39 mm were randomly collected for different experiments from the abalone sea cage culture site at Wando-gun and transported to the Tou-Jeong Soosan abalone hatchery in Dolsan-eup, Yeosu, South Korea, where they were cultured for one month with sufficient food and water supply to recover from the transportation-related stress.
Tissue collection for gene cloning and expression analysis
A total of two mature Pacific abalone (male and female) were sacrificed for molecular cloning purposes after anesthetizing with 5% MgCl2, and hemocytes, heart, testis, ovary, pleuropedal ganglion, cerebral ganglion, gill, muscle, mantle, and digestive gland were collected. Immediately after collection, tissue samples were washed with PBS (phosphate-buffered saline, 0.1 M), flash-frozen in liquid nitrogen (LN2), and stored at − 80 °C until total RNA extraction.
Fertilization and sample collection during the early larval development (ELD) stages
Three-year-old reproductively mature 12 females and four male’s abalone were induced for artificial fertilization. Among them, three females and two males were responded to spawn. After spawning, artificial fertilization was conducted following the procedure described previously. After fertilization, samples of fertilized eggs, 2-cell and 4-cell stages, blastula, trochophore larvae, veliger larvae, and juveniles were collected through microscopic observation. The samples were immediately flash-frozen in LN2 and stored at − 80 °C until total RNA extraction.
Culture of juvenile abalone and growth type sample collection
After approximately 120 h of fertilization, when they were ready for settlement, approximately 10,000 larvae were transferred to three culture tanks, each with diatoms precoated on transparent and flat plastic film (30 × 30 cm, 0.2–0.3 mm thickness). According to the size of the rearing tank, several plastic films were joined and placed at the tank bottom for convenient management. The early juvenile abalone fed on diatoms adhered to plastic films and artificial feed consisting of powder fish meal, kelp powder, micronutrients, and effective microorganisms. In South Korea, powder feed and several artificial granular feeds of different sizes are commercially available for abalone nursing at the farm level. Abalone fed with artificial feed tends to grow faster than those fed with only algae. After one year of culturing in the tank, juvenile abalone samples exhibiting different growth patterns [rapid growth, normal growth (average growth), minimal growth (growth rate less than average), and stunted growth (seems to stop growth)] were collected based on length and weight data. Muscle tissue samples were collected from each growth type of juvenile abalone, flash-frozen in LN2, and stored at − 80 °C until total RNA extraction.
Tissue collection from muscle-injured tissue of Pacific abalone
A total of 18 mature Pacific abalones (three-year-old) with a mean weight of 121.4 ± 0.53 g and a mean shell length of 84.11 ± 0.51 mm were used for the muscle tissue remodeling experiment. Briefly, 3 × 1 mm of muscle tissue was carefully removed from each abalone using a tissue collecting needle after anesthetizing with 5% MgCl2, after which they were cultured in a rearing tank with continuous aeration, water, and food supply. Muscle tissue samples from injured abalone were collected at a one-week interval. Tissue samples from control abalone and remodeled abalone were also collected at the beginning of the experiment and after injury repair. The samples were washed with PBS immediately after collection, flash-frozen in LN2, and stored at − 80 °C until total RNA extraction.
Tissue collection during shell biomineralization
Ten-month-old 20 juvenile Pacific abalones were used for the shell biomineralization experiment. The shell of each abalone was punctured (1 mm round orifice) near the mantle tissue using a perforator and then cultured in a rearing tank with continuous aeration, water, and food supply. Mantle tissues from three abalone were collected weekly. Mantle tissue from the control abalone was also collected at the beginning of the experiment. Immediately after collection, the tissues were washed with PBS, flash-frozen in liquid nitrogen, and stored at − 80 °C until total RNA extraction.
Tissue collection of testicular developmental stages
Testis tissues were collected from five two-year-old abalones for each gonadal development stage [proliferative stage (PS), growth stage (GS), spawning stage (SS), and resting stage (RS)], and the samples were immediately flash-frozen and stored at − 80 °C until total RNA extraction.
Tissue samples from Pacific abalones subjected to heat stress
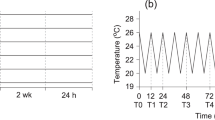
To characterize the changes in HdhSPARC expression in Pacific abalones in response to stress, a heat treatment was performed at 15 °C, 25 °C, and 30 °C. For this experiment, 3-year-old Pacific abalone with a mean weight of 121.06 ± 0.41 g and a shell length of 82.8 ± 0.52 mm were collected from the sea cage abalone culture area in Wando-gun and transported to the Tou-Jeong Soosan abalone hatchery in Dolsan-eup, Yeosu, South Korea. To recover from transportation-related stress and to adjust to the hatchery environment, abalone were cultured in tanks for about 30 days with a sufficient food supply. Thereafter, 12 abalones were kept in three different aquariums for 24 h each. For the 25 and 30 °C treatment tanks, the water temperature was gradually increased (2–4 °C/h) to avoid sudden heat shock using a digital temperature controller. During this experiment, we did not provide any food for the abalone. From each treatment and control abalone (19 °C), digestive gland tissue was collected after 3, 6, 12, and 24 h, washed with PBS (0.1 M), flash-frozen in LN2, and stored at − 80 °C until total RNA extraction. The temperature in the control tank was 20.4 °C.
Lipopolysaccharide (LPS) exposures treatment
The changes in SPARC expression in response to LPS were evaluated using LPS from Escherichia coli O55:B5 (Sigma-Aldrich, Saint Louis, MO, USA). LPS was injected at a concentration of 10 µg/g-BW into the muscle of 16 abalone, while an equal amount of PBS was injected into the muscle of three control abalone. Digestive gland tissue from five abalones was collected, washed with PBS (0.1 M), flash-frozen in LN2, and stored at − 80 °C until total RNA extraction.
H2O2 exposure treatment
Next, an H2O2 induction experiment was performed on two-year-old Pacific abalones to examine the effects of oxidative stress on HdhSPARC expression in Pacific abalones. Prior to the treatment, the abalones were reared for 2 weeks in the culture tanks with continuous water supply and aeration. For the experiments, 50 μL of H2O2 solution (0.3 mg/mL) was injected into the muscle of twelve abalones, and an equal amount of PBS (0.1 M) was injected into three control abalones. Digestive gland tissue samples from injected abalones were collected at 3, 6, 12, 24, and 48 h, washed with PBS (0.1 M), flash-frozen in LN2, and stored at − 80 °C until total RNA extraction.
CdCl2 exposure treatment
Acclimatized 2-year-old Pacific abalones were subjected to CdCl2 (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 6 mgL−1 in an aquarium. Tissue samples from the digestive gland (n = 5) were collected at 1, 6, 12, and 24 h, washed with PBS (0.1 M), flash-frozen in LN2, and stored at − 80 °C until total RNA extraction.
Total RNA extraction and first-stand cDNA synthesis
Extraction of total cellular RNA from the collected tissue samples was performed using an ISOSPIN Cell and Tissue RNA Kit (Nippon Gene, Tokyo, Japan). First-strand cDNA synthesis was conducted from the extracted total RNA using oligo(dt) primers (Sigma) and a superscript III first-strand cDNA synthesis kit (Invitrogen, USA). The RACE cDNA (3′- and 5′-RACE) was synthesized from the extracted total RNA using a SMARTer® RACE 5′/3′ Kit (Takara Bio Inc., Japan). Total RNA extraction and cDNA synthesis were conducted following the manufacturer’s protocol.
Cloning of SPARC mRNA sequence in Pacific abalone
Cloning of partial sequence
Reverse transcription polymerase chain reaction (RT-PCR) was conducted for partial sequence cloning of HdhSPARC using muscle tissue cDNA, gene-specific forward and reverse primers, and GoTaq® DNA polymerase (Promega, Madison, WI, USA). The gene-specific primer used in the present study was designed from the known H. discus discus SPARC mRNA sequence (GenBank Accession No. AB600274.1), available at NCBI database. All primers used in this study are listed in Table S1 (see supplementary files). The reaction mixture for RT-PCR was prepared to a total volume of 50 μL, containing cDNA (1 μL), 20 pmol forward (HdhSPARC RT Fw) and reverse (HdhSPARC RT Rv) primers (1 μL each), GoTaq reaction buffer (colorless) (10 μL), dNTP mix (1 μL), DNA polymerase (0.25 μL), and ultra-pure water (35.75 μL). The RT-PCR thermal cycling conditions consisted of an initial denaturation step for 3 min at 95 °C, followed by 35 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 58 °C, extension for 45 s at 72 °C, and a final extension for 5 min at 72 °C. After completion, the PCR products were subjected to gel electrophoresis using 1.2% agarose, after which bands were purified using the Wizard® SV Gel and PCR Clean-Up System kit (Promega, Madison, WI, USA). Afterward, the purified DNA was ligated into the pGEM®-T Easy Vector (Promega, Madison, WI, USA) and transformed into DH5α chemically competent E. coli cells (Enzynomics, Korea). The positive clones were selected for plasmid DNA purification using a Hybrid-QTM Plasmid Rapidprep mini kit (GeneAll, Seoul, Korea) and sequenced at Macrogen (Seoul, Korea).
Cloning of RACE (5′- and 3′) sequence
The rapid amplification of cDNA ends polymerase chain reaction (RACE-PCR) was performed using the SMARTer® RACE 5′/3′ kit (Takara Bio Inc., Japan) for 5′- and 3′-RACE to obtain the full-length sequence of HdhSPARC. A set of gene-specific primers (5′- and 3′-RACE) was designed from the previously obtained partial sequence of HdhSPARC. Reaction mixtures for RACE PCR (3′- and 5′-RACE) were prepared using 2.5 μL cDNA (3′- or 5′-RACE), 1 μL RACE primers, 1 μL SeqAmp DNA polymerase, 5 μL universal primer mix (UPM), 25 μL SeqAmp buffer, and 15.5 μL ultra-pure water. 30 cycles of touchdown PCR were carried out for RACE-PCR. The thermal cycle conditions were set following the kit manufacturer's instructions. Thereafter, the PCR products were subjected to gel electrophoresis, and positive bands were purified using the NucleoSpin® Gel and PCR Clean-up Kit (Macherey–Nagel GmbH & Co. KG, Germany). The purified products were ligated into a linearized pRACE vector and transformed into Stellar-competent cells (E. coli HST08). Positive clones were purified and sequenced at Macrogen as previously described for the partial sequence. Finally, the 5′-RACE sequence, the initially cloned partial cDNA fragment, and the 3′-RACE sequence were combined and trimmed to obtain the full-length sequence.
Sequence analysis of cloned H. discus hannai SPARC (HdhSPARC)
The complete nucleotide and protein sequences of cloned HdhSPARC were analyzed using several online tools. The ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder/) online tool was used to predict potential protein-encoding segments from the nucleotide sequence. The molecular weight and isoelectric point (pI) of the HdhSPARC protein were calculated using the ProtParam online tool (https://web.expasy.org/protparam/). The protein structure and gene ontology of HdhSPARC were predicted using the online protein structure prediction server I-TASSER-MTD (https://zhanggroup.org/I-TASSER-MTD/). The functional domains of the HdhSPARC protein were identified using the Motif Scan web tool (https://myhits.sib.swiss/cgi-bin/motif_scan). Conserved motifs in the SPARC protein of Pacific abalone and other species were identified using the GenomeNet (https://www.genome.jp/tools/motif/) online tool. Multiple sequence alignments based on SPARC protein sequences of abalone and other species were obtained using MEGA (version 11.0.13) and visualized using Jalview (version 2.11.1.7).
Phylogenetic analysis
The amino acid sequence from HdhSPARC was aligned with other SPARC protein sequences using the ClustalW online tool. A phylogenetic tree was constructed and edited using the MEGA software (version 11.0.13) with the maximum likelihood algorithm. The protein sequences used in constructing phylogenetic trees are presented in Table S2 (see supplementary files).
Three-dimensional modelling of HdhSPARC protein
The three-dimensional (3D) structure of the HdhSPARC protein was predicted using the previously mentioned protein structure and functional prediction program C-I-TASSER server. The 3D structure was visualized using the UCSF ChimeraX software (v. 1.2.5).
Quantitative real-time PCR (qRT-PCR) analysis
To quantify the relative mRNA expression of HdhSPARC, qRT-PCR analysis was performed in different tissues of Pacific abalone. Before qRT-PCR analysis, primer efficiency was tested to avoid experimental errors.
All qRT-PCR assays were conducted according to the 2 × qPCRBIO SyGreen Mix Lo-Rox kit (PCR Biosystems Ltd., UK) manual. Each qRT-PCR reaction mixture was prepared in a total volume of 20 μL, containing cDNA template (1 μL), 10 pmol gene-specific forward and reverse primers (1 μL each), SyGreen Mix (10 μL), and double distilled water (10 μL). Triplicate reactions were performed for target and reference genes in each tissue sample. The PCR amplification conditions consisted of a preincubation step at 95 °C for 2 min, followed by 40 cycles of denaturation, annealing, and extension at 95 °C for 30 s, 60 °C for 20 s, and 72 °C for 30 s. The melting temperature was set as the instrument's default setting. At the end of each cycle, a fluorescence reading was recorded for quantification. A LightCycler® 96 System (Roche, Germany) was used for amplification and data analysis. The relative gene expression was determined using the 2−ΔΔCT method with the Pacific abalone β-actin gene (accession no. MW387000) as an internal reference. All primers used in qRT-PCR analysis are summarized in Table S1 (see supplementary files).
Fluorescence-based detection of intracellular H2O2 and Cd
The intracellular hydrogen peroxide and cadmium levels in digestive gland tissue samples were detected using an intracellular hydrogen peroxide assay kit (MAK164, Sigma-Aldrich) and Leadmium™ Green AM dye (Invitrogen, Thermo Fisher Scientific), with slight protocol modifications as described previously59. Sample preparation and intensity measurement were performed as described by Hossen et al.60. Briefly, samples were stained with a fluorescent peroxide sensor and incubated for 15 min at 37 °C in the dark. The stained cells were visualized under a fluorescence microscope (green filter: 450–490 nm, Eclipse E600, Nikon, Tokyo, Japan). The fluorescence intensity of a minimum of 100 cells was measured using ImageJ software version 1.8.0_172.
Statistical analysis
The mRNA expression values were statistically analyzed and expressed as the mean ± standard error. Changes in relative mRNA expression were calculated via nonparametric ANOVA analysis using the GraphPad Prism software (version 9.3.1). Statistical significance was set at a p-value less than 0.05. All graphs were generated using Microsoft Excel and GraphPad Prism 9.3.1 software.
Data availability
All data generated in this study is included in this article. The nucleotide and protein sequence of SPARC is available at https://www.ncbi.nlm.nih.gov/nuccore/OM937904.1. The nucleotide and protein sequences used in the present study were collected from NCBI (https://www.ncbi.nlm.nih.gov/) and are presented in Table S2.
References
Sim, B. R. et al. Influence of intensive net cage farming on hydrodynamic and geochemical environmental conditions and the mass mortality of abalone in South Korea. Mar. Pollut. Bull. 169, 112555 (2021).
An, H. S., Lee, J. W., Kim, H. C. & Myeong, J. I. Genetic characterization of five hatchery populations of the pacific abalone (Haliotis discus hannai) using microsatellite markers. Int. J. Mol. Sci. 12, 4836–4849 (2011).
Shi, L., Hao, G., Chen, J., Ma, S. & Weng, W. Nutritional evaluation of Japanese abalone (Haliotis discus hannai Ino) muscle: Mineral content, amino acid profile and protein digestibility. Food Res. Int. 129, 108876 (2020).
Hanif, M. A. et al. Characterization and expression analysis of mollusk-like growth factor: A secreted protein involved in Pacific abalone embryonic and larval development. Biology 11, 1445 (2022).
Hynes, R. O. The extracellular matrix: Not just pretty fibrils. Science 326, 1216–1219 (2009).
Melouane, A., Carbonell, A., Yoshioka, M., Puymirat, J. & St-Amand, J. Implication of SPARC in the modulation of the extracellular matrix and mitochondrial function in muscle cells. PLoS ONE 13, e0192714 (2018).
Kucukdereli, H. et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. U.S.A. 108, 440–449 (2011).
Kos, K. & Wilding, J. SPARC: A key player in the pathologies associated with obesity and diabetes. Nat. Rev. Endocrinol. 6, 225–235 (2010).
Brekken, A. & Sage, E. SPARC, a matricellular protein: At the crossroads of cell–matrix communication. Matrix Biol. 19, 816–827 (2001).
Rotllant, J. et al. Sparc (Osteonectin) functions in morphogenesis of the pharyngeal skeleton and inner ear. Matrix Biol. 27, 561–572 (2008).
Song, X., Liu, Z., Wang, L. & Song, L. Recent advances of shell matrix proteins and cellular Orchestration in marine molluscan shell biomineralization. Front. Mar. Sci. 6, 41 (2019).
Lee, J. H. & Jun, H.-S. Role of myokines in regulating skeletal muscle mass and function. Front. Physiol. 10, 42 (2019).
Kudo, H., Hirayoshi, K., Kitagawa, Y., Imamura, S. & Nagata, K. Two collagen-binding proteins, osteonectin and HSP47, are coordinately induced in transformed keratinocytes by heat and other stresses. Exp. Cell Res. 212, 219–224 (1994).
Hu, L. et al. SPARC promotes insulin secretion through down-regulation of RGS4 protein in pancreatic β cells. Sci. Rep. 10, 17581 (2020).
Ghanemi, A., Yoshioka, M. & St-Amand, J. Secreted protein acidic and rich in cysteine as a regeneration factor: Beyond the tissue repair. Life 11, 38 (2021).
Bradshaw, A. D. & Sage, E. H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 107, 1049–1054 (2001).
Ceinos, R. M. et al. Critical role of the matricellular protein SPARC in mediating erythroid progenitor cell development in zebrafish. Cells Tissues Organs 197, 196–208 (2013).
Aoi, W. et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 62(6), 882–889 (2013).
Jørgensen, L. H. et al. Secreted protein acidic and rich in cysteine (SPARC) in human skeletal muscle. J. Histochem. Cytochem. 57, 29–39 (2009).
Huang, J., Zhang, C., Ma, Z., Xie, L. & Zhang, R. A novel extracellular EF-hand protein involved in the shell formation of pearl oyster. Biochim. Biophys. Acta 1770, 1037–1044 (2007).
Feng, D., Li, Q., Yu, H., Kong, L. & Du, S. Identification of conserved proteins from diverse shell matrix proteome in Crassostrea gigas: Characterization of genetic bases regulating shell formation. Sci. Rep. 7, 45754 (2017).
Xie, J. et al. Influence of the extrapallial fluid of Pinctada fucata on the crystallization of calcium carbonate and shell biomineralization. Cryst. Growth Des. 16, 672–680 (2016).
Werner, G. D., Gemmell, P., Grosser, S., Hamer, R. & Shimeld, S. M. Analysis of a deep transcriptome from the mantle tissue of Patella vulgata Linnaeus (Mollusca: Gastropoda: Patellidae) reveals candidate biomineralising genes. Mar. Biotechnol. 15, 230–243 (2013).
Jackson, D. J. et al. The Magellania venosa biomineralizing proteome: A window into brachiopod shell evolution. Genome Biol. Evol. 7, 1349–1362 (2015).
Shibata, S. & Ishiyama, J. Secreted protein acidic and rich in cysteine (SPARC) is upregulated by transforming growth factor (TGF)-β and is required for TGF-β-induced hydrogen peroxide production in fibroblasts. Fibrogenesis Tissue Repair. 6, 6 (2013).
Larson, J. et al. SPARC gene expression is repressed in human urothelial cells (UROtsa) exposed to or malignantly transformed by cadmium or arsenite. Toxicol. Lett. 199, 166–172 (2010).
Peng, X., Luo, Z., He, S., Zhang, L. & Li, Y. Blood–brain barrier disruption by lipopolysaccharide and sepsis-associated encephalopathy. Front. Cell. Infect. Microbiol. 11, 768108 (2021).
Sauk, J. J., Norris, K., Kerr, J. M., Somerman, M. J. & Young, M. F. Diverse forms of stress result in changes in cellular levels of osteonectin/SPARC without altering mRNA levels in osteoligament cells. Calcif. Tissue Int. 49, 58–62 (1991).
Torres-Núñez, E. et al. Molecular cloning and characterization of the matricellular protein Sparc/osteonectin in flatfish, Scophthalmus maximus, and its developmental stage-dependent transcriptional regulation during metamorphosis. Gene 568, 129–139 (2015).
Koehler, A. et al. Molecular evolution of SPARC: Absence of the acidic module and expression in the endoderm of the starlet sea anemone, Nematostella vectensis. Dev. Genes Evol. 219, 509–521 (2009).
Kaufmann, B. et al. Structural variability of BM-40/SPARC/osteonectin glycosylation: Implications for collagen affinity. Glycobiology 14, 609–619 (2004).
Kwon, H. et al. Characterization of a Kazal-type serine protease inhibitor from black rockfish Sebastes schlegelii and its possible role in hepatic immune response. Fish Shellfish Immunol. 74, 485–490 (2018).
Sasaki, T., Hohenester, E., Göhring, W. & Timpl, R. Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J. 17, 1625–1634 (1998).
Wilson, M. J., Bowles, J. & Koopman, P. The matricellular protein SPARC is internalized in Sertoli, Leydig, and germ cells during testis differentiation. Mol. Reprod. Dev. 73, 531–539 (2006).
Jørgensen, L. H. et al. SPARC interacts with actin in skeletal muscle in vitro and in vivo. Am. J. Pathol. 187, 457–474 (2017).
Nian, Q. et al. SPARC in hematologic malignancies and novel technique for hematological disease with its abnormal expression. Biomed. Pharmacother. 153, 113519 (2022).
Bozinovic, G., Sit, T. L., Hinton, D. E. & Oleksiak, M. F. Gene expression throughout a vertebrate’s embryogenesis. BMC Genom. 12, 132 (2011).
Liu, M. et al. Environmental factors have a major effect in shaping the gene expression of Siberian larch in the Altai Mountains of China. Plant Genom. 15, e20240 (2022).
Lee, H. G. et al. Lipid profiling of pacific abalone (Haliotis discus hannai) at different developmental stages using ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Anal. Methods Chem. 2022, 5822562 (2022).
Di, G. et al. Proteomic analysis of trochophore and veliger larvae development in the small abalone Haliotis diversicolor. BMC Genom. 18, 809 (2017).
Wilson, M. J., Liaw, L. & Koopman, P. Osteopontin and related SIBLING glycoprotein genes are expressed by Sertoli cells during mouse testis development. Dev. Dyn. 233, 1488–1495 (2006).
Vernon, R. B. & Sage, H. The calcium-binding protein SPARC is secreted by Leydig and Sertoli cells of the adult mouse testis. Biol. Reprod. 40, 1329–1340 (1989).
Nixon, B. et al. Formation and dissociation of sperm bundles in monotremes. Biol. Reprod. 95, 1–11 (2016).
Mathes, S., Fahrner, A., Luca, E. & Krützfeldt, J. Growth hormone/IGF-I-dependent signaling restores decreased expression of the myokine SPARC in aged skeletal muscle. J. Mol. Med. 100, 1647–1658 (2022).
Atorrasagasti, C. et al. SPARC is required for the maintenance of glucose homeostasis and insulin secretion in mice. Clin. Sci. 133, 351–365 (2019).
Chang, M.-C. et al. bFGF stimulated plasminogen activation factors, but inhibited alkaline phosphatase and SPARC in stem cells from apical Papilla: Involvement of MEK/ERK, TAK1 and p38 signaling. J. Adv. Res. 40, 95–107 (2022).
Petersson, S. J. et al. SPARC is upregulated during skeletal muscle regeneration and inhibits myoblast differentiation. Histol. Histopathol. 28, 1451–1460 (2013).
Maurer, P. & Hohenester, E. Structural and functional aspects of calcium binding in extracellular matrix proteins. Matrix Biol. 15, 569–580 (1997).
Jolly, C. et al. Zona localization of shell matrix proteins in mantle of Haliotis tuberculata (Mollusca, Gastropoda). Mar. Biotechnol. 6, 541–551 (2004).
Chlenski, A. et al. Secreted protein acidic and rich in cysteine is a matrix scavenger chaperone. PLoS ONE 6, e23880 (2011).
Neri, M., Descalzi-Cancedda, F. & Cancedda, R. Heat-shock response in cultured chick embryo chondrocytes. Osteonectin is a secreted heat-shock protein. Eur. J. Biochem. 205, 569–574 (1992).
Qiao, K. et al. Molecular characterization, purification, and antioxidant activity of recombinant superoxide dismutase from the Pacific abalone Haliotis discus hannai Ino. World J. Microbiol. Biotechnol. 36, 115 (2020).
Aly, N. A. R. et al. The role of lymphoid tissue SPARC in the pathogenesis and response to treatment of multiple myeloma. Front. Oncol. 12, 1009993 (2022).
Tarasco, M. et al. Anti-osteogenic activity of cadmium in Zebrafish. Fishes 4, 11 (2019).
Zhao, Y. Q., Liu, G. D., Hou, C. C., Han, Y.-L. & Zhu, J.-Q. Effect of cadmium exposure on antioxidant enzyme catalase in different tissues of Acrossocheilus fasciatus. Mol. Cell. Toxicol. 12, 255–263 (2016).
Sánchez-Quiles, D. & Tovar-Sánchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 48, 9037–9042 (2014).
Chen, X., Kang, R. & Tang, D. Ferroptosis by lipid peroxidation: The tip of the iceberg?. Front. Cell. Dev. Biol. 9, 646890 (2021).
Said, N., Frierson, H. F., Sanchez-Carbayo, M., Brekken, R. A. & Theodorescu, D. Loss of SPARC in bladder cancer enhances carcinogenesis and progression. J. Clin. Invest. 123, 751–766 (2013).
Lu, L. L. et al. Enhanced root-to-shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfredii. J. Exp. Bot. 59, 3203–3213 (2008).
Hossen, S. et al. Molecular cloning and functional characterization of catalase in stress physiology, innate immunity, testicular development, metamorphosis, and cryopreserved sperm of Pacific abalone. Antioxidants 12, 109 (2023).
Acknowledgements
The authors are grateful to the Ministry of Oceans and Fisheries of South Korea for providing research funds.
Funding
This research was supported by a grant (20180375) funded by the Ministry of Oceans and Fisheries, South Korea.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.H.K. and M.A.H.; research designed, M.A.H.; methodology, M.A.H., S.H. and K.H.K.; formal analysis; M.A.H.; visualization, M.A.H. and S.H.; investigation, M.A.H. and S.H.; data curation, K.H.K., M.A.H. and C.C.Y.; validation, M.A.H., K.H.K., C.C.Y.; resources, K.H.K.; writing-original draft preparation, M.A.H. and K.H.K.; and writing-review and editing, K.H.K., C.C.Y.; project administration, funding acquisition, and supervision, K.H.K. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanif, M.A., Hossen, S., Choi, C.Y. et al. Cloning, characterization, and spatio-temporal expression patterns of HdhSPARC and its responses to multiple stressors. Sci Rep 14, 2224 (2024). https://doi.org/10.1038/s41598-024-51950-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51950-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.