Abstract
This study aims to identify healthcare costs indicators predicting secondary surgery for degenerative lumbar spine disease (DLSD), which significantly impacts healthcare budgets. Analyzing data from the National Health Insurance Service-National Sample Cohort (NHIS-NSC) database of Republic of Korea (ROK), the study included 3881 patients who had surgery for lumbar disc herniation (LDH), lumbar spinal stenosis without spondylolisthesis (LSS without SPL), lumbar spinal stenosis with spondylolisthesis (LSS with SPL), and spondylolysis (SP) from 2006 to 2008. Patients were categorized into two groups: those undergoing secondary surgery (S-group) and those not (NS-group). Surgical and interim costs were compared, with S-group having higher secondary surgery costs ($1829.59 vs $1618.40 in NS-group, P = 0.002) and higher interim costs ($30.03; 1.86% of initial surgery costs vs $16.09; 0.99% of initial surgery costs in NS-group, P < 0.0001). The same trend was observed in LDH, LSS without SPL, and LSS with SPL (P < 0.0001). Monitoring interim costs trends post-initial surgery can effectively identify patients requiring secondary surgery.
Similar content being viewed by others
Introduction
Degenerative lumbar spine disease (DLSD) is one of the most common musculoskeletal conditions that affect the lower back and is characterized by the progressive deterioration of intervertebral discs, facet joints, and other structures in the lumbar region1,2. This degenerative process can cause a variety of symptoms, including back pain, leg pain, tingling, and weakness, which can significantly impact an individual's quality of life3,4. While various treatment options exist for DLSD, including conservative management such as physical therapy and medication, some patients may require surgical intervention to alleviate their symptoms and improve their quality of life5,6. Surgical procedures such as lumbar fusion or discectomy are often effective in providing relief, restoring spinal stability, and improving functional outcomes7,8,9,10. However, despite the success of initial surgical interventions, a subset of patients may experience recurrent symptoms or the progression of their condition over time. This may necessitate a second surgical procedure, commonly referred to as secondary surgery11,12,13. The need for secondary surgery in DLSD can arise due to various reasons, including adjacent segment disease (ASD), implant failure, persistent or recurrent symptoms, or disease progression14,15,16,17. Secondary surgeries often require more complex surgical techniques compared to the initial surgery, which can contribute to an increase in healthcare costs including surgical fees, hospitalization costs, and post-operative care18,19,20,21. The increasing incidence of secondary surgery has raised concerns regarding its impact on healthcare costs22. Therefore, screening secondary surgery for DLSD is essential not only for the efficient allocation of healthcare resources and rational medical expenditure but also for formulating appropriate policies regarding the medical costs associated with DLSD. Factors associated with secondary surgery are complex, but there has been no indicator showing the possibility of secondary surgery from the perspective of health insurance. The objective of this study is to propose indicators for screening patients requiring secondary surgery for DLSD, focusing on the aspect of increased healthcare costs, using data from the National Health Insurance Service-National Sample Cohort (NHIS-NSC) of the Republic of Korea (ROK).
Methods
Data source
The data for this study were derived from the National Health Insurance Database (NHID), which records personal information, demographics, and medical treatment data for all Korean citizens. In the ROK, all citizens have been beneficiaries of the NHIS for more than 20 years, and the NHIS covers both Western and Oriental medicine23,24,25. Because the NHIS follows a fee-for-service payment system, all nationwide inpatient and outpatient data on diseases and services (i.e., procedures and surgeries) are coded and registered in the National Health Insurance Corporation (NHIC) database and the Health Insurance Review & Assessment Service (HIRA) database23,24,25,26,27,28. The disease codes in the database adhere to the 10th version of the International Classification of Diseases (ICD-10), and procedure codes are standardized for billing purposes. Nearly all hospitals providing Western medicine and clinics providing Oriental medicine must follow the guidelines to obtain reimbursement. The detailed surgical and nonsurgical management were determined by the attending physicians23,25,29. By using the database, the NHIS-NSC was identified in 2017 for analysis while maintaining representativeness and protecting personal information28. The NHIS-NSC represents a representative sample cohort, consisting of 1,000,000 individuals (approximately 2.1% of the total Korean population) randomly selected from a population of 48,438,292 in 2006 (https://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do)28. Systematic stratified random sampling with proportional allocation within each stratum, including sex, age, location, and health insurance, was employed. To ensure privacy, the resident registration numbers were replaced with unique eight-digit personal IDs, enabling longitudinal follow-ups for all individuals until 2015. The cohort was updated annually during the follow-up period, and the size of the cohort was maintained. The records for each person in the NHIS-NSC can be traced back to 2002.
Study population
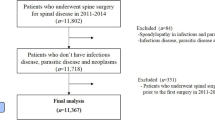
For this study, we utilized a cohort study design established in a previous study30. The study included patients diagnosed with lumbar disc herniation (LDH), lumbar spinal stenosis without spondylolisthesis (LSS without SPL), lumbar spinal stenosis with spondylolisthesis (LSS with SPL), and spondylolysis (SP)30,31. The disease codes for each diagnosis were as follows: (1) LDH, M51, M472; (2) LSS without SPL, M4800, M4805-8; (3) LSS with SPL, M431, M4315-9; (4) SP, M430, M4306-9. The selection of the surgical treatment cohort involved identifying patients who underwent specific surgical procedures between 2006 and 2008. The codes for each surgical procedure were as follows: (1) open discectomy, N1493; (2) laminectomy, N4199, N2499; (3) endoscopic lumbar discectomy, N1494; (4) spinal fusion, N0466, N1466, N0469, N2470, N1460, and N1469. A total of 4577 patients were selected in the surgical treatment cohort. Among them, patients with the following conditions were excluded: (1) patients with a history of spinal surgery within the past 3 years (n = 105), (2) patients who had utilized medical services with disease indicating spinal fracture, pathological fracture, spinal infection, malignancy, or inflammatory joint disease within the past 1 year (n = 207), (3) patients with concomitant rare diseases such as metabolic diseases, blood diseases, or congenital anomalies (n = 1), (4) patients admitted via the emergency room (n = 362), and (5) patients below 18 years of age (n = 21)30. After applying exclusion criteria, 3,881 patients remained in the surgical treatment cohort. After surgery, patients visited clinic for follow-up and may receive additional interventions, physiotherapy or medications depending on their specific needs following the surgery. All patients were followed up for at least 7 years. The patient flow diagram is presented in Fig. 1. Secondary surgery was defined as any kind of lumbar spinal surgery at any lumbar level being performed after initial surgery. However, since the exact lumbar level was not recorded in the registry, treatment failure after initial surgery could include both the index level and the other lumbar levels24,25,27,29,30,32. This study was conducted in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practice. The study protocol was approved by the Seoul National University Hospital ethics committee/institutional review board (2010-076-1164). The Seoul National University Hospital ethics committee/institutional review board approved the exemption of informed consent due to the retrospective nature of this study.
Flow diagram of patients. A total of 4577 patients who underwent surgery for lumbar disc herniation (LDH), lumbar spinal stenosis without spondylolisthesis (LSS without SPL), lumbar spinal stenosis with spondylolisthesis (LSS with SPL), and spondylolysis (SP) between 2006 and 2008 were registered in the surgery cohort. Among the registered patients, the following conditions resulted in exclusions: (1) patients with a history of spinal surgery within the past 3 years (n = 105), (2) patients who had utilized medical services for spinal fracture, pathological fracture, spinal infection, malignancy, or inflammatory joint disease within the past 1 year (n = 207), (3) patients with concomitant rare diseases such as metabolic diseases, blood diseases, or congenital anomalies (n = 1), (4) patients admitted via the emergency room (n = 362), and (5) patients below 18 years of age (n = 21). Finally, the surgical cohort consisted of 3881 patients and was followed up for at least 7 years.
Statistics
We analyzed direct medical costs for Western and Oriental medicine in two groups: those who had secondary surgery (S-group) and those who did not (NS-group). Costs only considered medical expenses and did not account for societal costs. Initial costs for surgical treatment were incurred during hospitalization for surgery. In the S-group, interim costs covered expenses between initial and secondary surgeries, including consultation fees, procedures, physiotherapy, and medications. In the NS-group, interim costs included expenses after the initial surgery. Costs related to the secondary surgery were specific to the secondary surgery purpose. We compared costs between groups using the Mann–Whitney U test. To find the optimal cutoff for interim costs predicting secondary surgery, we selected the value maximizing sensitivity and specificity based on Youden's index. Statistical analysis was done using SAS version 9.4, with significance set at P < 0.05.
Results
Baseline characteristics of the cohort
The characteristics of patients are described in Table 1. The most common disease was LDH (47.85%) followed by LSS without SPL (36.12%), LSS with SPL (13.63%), and SP (2.4%). Open discectomy was the most common surgical technique in all diseases. The initial surgical methods for each diagnosis are shown in Table 2. Fusion surgery was performed in 3.82%, 12.91%, 37.24%, and 43.01% of patients with LDH, LSS without SPL, LSS with SPL, and SP, respectively. The distribution of secondary surgery methods for each diagnosis is presented in Table 3. Secondary surgery was performed in 14.81%, 15.62%, 11.34%, and 6.45% of patients with LDH, LSS without SPL, LSS with SPL, and SP, respectively. Open discectomy was the most common secondary surgical method, and the fusion surgery was more frequently performed than initial surgery in LDH and LSS without SPL; 9.09% (vs 3.82%) and 17.35% (vs 12.91%), respectively.
Medical costs by diagnosis in each group
The surgery costs and interim costs of the patients are presented in Table 4. The initial surgery costs were $1618.40 (range, 11.31–16,803.78), while the secondary surgery costs were $1829.59 (range, 9.89–19,988.60), which were higher than the initial surgery costs (P = 0.002). In LDH, LSS without SPL, and SP, the secondary surgery costs were higher than the initial surgery costs. However, the initial surgery costs were higher than the median secondary surgery costs in LSS with SPL. Before secondary surgery, the S-group incurred higher interim costs ($30.03; 1.86% of initial surgery costs) compared to the NS-group ($16.09; 0.99% of initial surgery costs). Higher interim costs before secondary surgery were observed in LDH (1.62% vs 0.99% of initial surgery costs), LSS without SPL (2.04% vs 1.06% of initial surgery costs), and LSS with SPL (1.36% vs 0.47% of initial surgery costs) in S-group than NS-group (P < 0.0001, < 0.0001, and < 0.0001, respectively). A comparison of initial, secondary, and interim costs for each diagnosis is presented in Fig. 2.
The comparison of interim costs and surgical costs based on diagnosis. Excluding patients of lumbar spinal stenosis with spondylolisthesis (LSS with SPL), secondary surgery costs of S-group were higher than initial surgery costs of NS-group. In all diagnosis, secondary surgery group (S-group) spent higher interim costs compared to non-secondary surgery group (NS-group).
The cutoff interim costs between S-group and NS-group
The cutoff interim costs for screening secondary surgery based on the surgical methods in each diagnosis of DLSD are presented in Table 5. For LDH, if interim costs after initial surgery were greater than $8.24 (0.63% of initial surgery costs), a secondary surgery could be predicted with sensitivity of 0.80 and specificity of 0.37. The cutoff value for predicting secondary surgery was $20.63 (1.58% of initial surgery costs; sensitivity of 1.00 and specificity of 0.51) for laminectomy as initial surgery and $16.83 (1.29% of initial surgery costs; sensitivity of 0.68 and specificity of 0.72) for endoscopic discectomy as initial surgery. The cutoff values were $25.16 (1.35% of initial surgery costs; sensitivity of 0.67 and specificity of 0.58) in LSS without SPL. For decompression as initial surgery, the cutoff value was $23.32 (1.25% of initial surgery; sensitivity of 0.71 and specificity of 0.56). The cutoff value was $28.42 (0.75% of initial surgery costs; sensitivity of 0.73 and specificity of 0.64) in LSS with SPL. The cutoff value for anterior fusion as initial surgery was $88.41 (2.34% of initial surgery costs; sensitivity of 1.00 and specificity of 0.96), and the cutoff value for posterior fusion as initial surgery was $20.50 (0.54% of initial surgery; sensitivity of 0.88 and specificity of 0.60). For decompression as initial surgery, the cutoff value was $28.69 (0.76% of initial surgery; sensitivity of 0.74 and specificity of 0.60).
Discussion
Frequency and causes of secondary surgery in patients with degenerative lumbar spine disease
For LDH, the secondary surgery rate is reported to be 10% at 2 years, 15% at 5 years, and 20% at 10 years11,32. The most common cause of secondary surgery is known to be the recurrence of disc protrusion16. Factors such as age, gender, body mass index (BMI), smoking, and diabetes are known to contribute to the secondary surgery of LDH33,34. For LSS, secondary surgery is reported to occur at a rate of 11% to 18% between 8 and 10 years13,35,36. The main causes of secondary surgery are known to be the recurrence of stenosis due to disease progression or technical issues during surgery, accounting for about 50%14,37. Other causes include inadequate decompression, persistent pain, and complications resulting from the initial surgery38,39,40. Secondary surgery rates for degenerative SPL have been reported to range from 10 to 38% in previous literature12,24,41. Patients may undergo secondary surgery due to various reasons following the initial surgery, including facet joint hypertrophy, persistent pain, infection, and progression of degenerative changes15,41,42. The main complications that require secondary surgery in degenerative SPL are ASD and same segment disease (SSD). The risk factors associated with the occurrence of ASD and SSD are age, gender, BMI, facet tropism, disc height, and spinal instability15,43.
The need to predict the occurrence of secondary surgery in degenerative lumbar spine disease
The prevalence of DLSD is increasing worldwide and it has placed a burden on healthcare budgets44,45. The growing burden of healthcare costs related to DLSD is a consequence of various factors, including an aging population, the increasing prevalence of the condition, the need for long-term management and treatment, advancements in medical technologies, the overall increase in use of medical resources, and increased number of secondary surgery46,47,48. In ROK, just like in other countries, the medical costs associated with DLSD are increasing26 and DLSD is placing a burden on the health insurance finances26. In this study, patients who underwent secondary surgery were found to incur significantly higher interim costs before secondary surgery compared to patients who did not undergo secondary surgery. In addition, the medical costs associated with secondary surgery were higher than the medical costs of the initial surgery. While many factors are known to be associated with the risk of secondary surgery for DLSD, there are no financial indicators for predicting secondary surgery33,38,41,49. In this study, interim costs after initial surgery showed promise in predicting the occurrence of secondary surgery in DLSD. Specifically, the study presented the cutoff interim costs that can predict secondary surgery based on the surgical methods for each diagnosis. Therefore, by tracking the post-surgical medical costs associated with DLSD, it may be possible to predict the occurrence of secondary surgery. Therefore, although it is an indirect indicator, the surrogate (interim costs) may indicate the number of patients having the possibility of secondary surgery. Screening secondary surgery in DLSD is a crucial factor in managing healthcare insurance budgets and can provide valuable information for the development of efficient healthcare policies.
Limitations
Firstly, our pilot study used a sample cohort, which, while representing the national population, may not fully represent all cases of lumbar spine disease. Secondly, we hypothesized that higher medical costs could be linked to poor clinical outcomes. However, medical resource utilization varied among patients and doctors, and the study did not consider the impact of time on surgical outcomes50. Thirdly, the medical cost claims data lacked comprehensive clinical and imaging details. These limitations restricted our analysis of individual patient conditions, including the direct relationship between secondary and primary surgeries, and hindered our ability to fully assess patient-specific factors affecting surgical outcomes and subsequent healthcare costs. Fourthly, our analysis relied on medical cost data submitted to NHIS and did not consider factors like the patient's quality of life decline or losses due to unemployment. Additionally, non-insurance treatments were not included in the analysis.
Conclusion
Among patients who underwent surgery for DLSD, those who underwent secondary surgery tend to have higher interim costs than those who did not undergo secondary surgery. Furthermore, secondary surgeries generally involve higher medical expenses than the initial surgery. Therefore, tracking the trend of medical costs increases in patients with DLSD who have undergone surgery can serve as an indicator for screening the need for secondary surgery.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoy, D. et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 73, 968–974. https://doi.org/10.1136/annrheumdis-2013-204428 (2014).
Son, S., Yoo, B. R., Kim, H. J., Song, S. K. & Ahn, Y. Efficacy of transforaminal endoscopic lumbar discectomy in elderly patients over 65 years of age compared to young adults. Neurospine 20, 597–607. https://doi.org/10.14245/ns.2346192.096 (2023).
Ravindra, V. M. et al. Degenerative lumbar spine disease: Estimating global incidence and worldwide volume. Glob. Spine J. 8, 784–794. https://doi.org/10.1177/2192568218770769 (2018).
Ambrosio, L. et al. The effect of transitioning to remote working in patients affected by chronic low back pain: A cross-sectional study. Neurospine 20, 692–700. https://doi.org/10.14245/ns.2346510.255 (2023).
Jacobs, W. C. H., Rubinstein, S. M., Koes, B., van Tulder, M. W. & Peul, W. C. Evidence for surgery in degenerative lumbar spine disorders. Best Pract. Res. Clin. Rheumatol. 27, 673–684. https://doi.org/10.1016/j.berh.2013.09.009 (2013).
Kluba, T., Dikmenli, G., Dietz, K., Giehl, J. P. & Niemeyer, T. Comparison of surgical and conservative treatment for degenerative lumbar scoliosis. Arch. Orthop. Trauma Surg. 129, 1–5. https://doi.org/10.1007/s00402-008-0673-z (2009).
Lee, N. et al. Comparison of outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg. 101, 216–226. https://doi.org/10.1016/j.wneu.2017.01.114 (2017).
Wilson, C. A., Roffey, D. M., Chow, D., Alkherayf, F. & Wai, E. K. A systematic review of preoperative predictors for postoperative clinical outcomes following lumbar discectomy. Spine J. 16, 1413–1422. https://doi.org/10.1016/j.spinee.2016.08.003 (2016).
Rushton, A., Zoulas, K., Powell, A. & Staal, J. B. Physical prognostic factors predicting outcome following lumbar discectomy surgery: Systematic review and narrative synthesis. BMC Musculoskelet. Disord. 19, 326. https://doi.org/10.1186/s12891-018-2240-2 (2018).
Zhang, X. et al. Perioperative clinical features and long-term prognosis after oblique lateral interbody fusion (OLIF), OLIF with anterolateral screw fixation, or OLIF with percutaneous pedicle fixation: A comprehensive treatment strategy for patients with lumbar degenerative disease. Neurospine 20, 536–549. https://doi.org/10.14245/ns.2244954.477 (2023).
Heindel, P. et al. Reoperation rates after single-level lumbar discectomy. Spine (Phila Pa 1976) 42, e496–e501 https://doi.org/10.1097/brs.0000000000001855 (2017).
Ghogawala, Z. et al. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J. Neurosurg. Spine 1, 267–272. https://doi.org/10.3171/spi.2004.1.3.0267 (2004).
Gerling, M. C. et al. Risk factors for reoperation in patients treated surgically for lumbar stenosis: A subanalysis of the 8-year data from the SPORT trial. Spine (Phila Pa 1976) 41, 901–909 https://doi.org/10.1097/brs.0000000000001361 (2016).
Thomé, C. et al. Outcome after less-invasive decompression of lumbar spinal stenosis: A randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J. Neurosurg. Spine 3, 129–141. https://doi.org/10.3171/spi.2005.3.2.0129 (2005).
Sato, S. et al. Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: minimum 5-year follow-up. Spine J. 15, 1536–1544. https://doi.org/10.1016/j.spinee.2015.02.009 (2015).
McGirt, M. J. et al. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery 64, 338–344 https://doi.org/10.1227/01.Neu.0000337574.58662.E2 (2009) (discussion 344–345).
Chang, C.-C. et al. Comparison of cortical bone trajectory to pedicle-based dynamic stabilization: An analysis of 291 patients. Neurospine 20, 308–316. https://doi.org/10.14245/ns.2244888.444 (2023).
Montenegro, T. S. et al. Clinical outcomes in revision lumbar spine fusions: An observational cohort study. J. Neurosurg. Spine 35, 437–445. https://doi.org/10.3171/2020.12.Spine201908 (2021).
Kim, S. S. & Michelsen, C. B. Revision surgery for failed back surgery syndrome. Spine 17, 957 (1992).
Bonano, J. et al. Economic impact of revision operations for adjacent segment disease of the subaxial cervical spine. JAAOS Glob. Res. Rev. 6, 58 (2022).
Wang, H., Yu, H., Zhang, N. & Xiang, L. Incidence, risk factors, and management of postoperative hematoma following anterior cervical decompression and fusion for degenerative cervical diseases. Neurospine 20, 525–535. https://doi.org/10.14245/ns.2245066.533 (2023).
Rajaee, S. S., Kanim, L. E. & Bae, H. W. National trends in revision spinal fusion in the USA: Patient characteristics and complications. Bone Jt. J. 96-b, 807–816 https://doi.org/10.1302/0301-620x.96b6.31149 (2014).
Kim, C. H. et al. The selection of open or percutaneous endoscopic lumbar discectomy according to an age cut-off point: Nationwide Cohort Study. Spine (Phila Pa 1976) 40, E1063–E1070 https://doi.org/10.1097/brs.0000000000001053 (2015).
Kim, C. H. et al. Reoperation rate after surgery for lumbar spinal stenosis without spondylolisthesis: A nationwide cohort study. Spine J. 13, 1230–1237. https://doi.org/10.1016/j.spinee.2013.06.069 (2013).
Kim, C. H. et al. Reoperation rate after surgery for lumbar herniated intervertebral disc disease: nationwide cohort study. Spine (Phila Pa 1976) 38, 581–590 https://doi.org/10.1097/BRS.0b013e318274f9a7 (2013).
Lee, C. H., Chung, C. K., Kim, C. H. & Kwon, J. W. Health care burden of spinal diseases in the Republic of Korea: Analysis of a nationwide database from 2012 through 2016. Neurospine 15, 66–76. https://doi.org/10.14245/ns.1836038.019 (2018).
Kim, C. H. et al. Increased volume of surgery for lumbar spinal stenosis and changes in surgical methods and outcomes: A nationwide cohort study with a 5-year follow-up. World Neurosurg. 119, e313–e322. https://doi.org/10.1016/j.wneu.2018.07.139 (2018).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC). South Korea. Int. J. Epidemiol. 46, e15. https://doi.org/10.1093/ije/dyv319 (2017).
Kim, C. H. et al. Increased volume of lumbar surgeries for herniated intervertebral disc disease and cost-effectiveness analysis: A nationwide cohort study. Spine (Phila Pa 1976) 43, 585–593 https://doi.org/10.1097/brs.0000000000002473 (2018).
Kim, C. H. et al. Direct medical costs after surgical or nonsurgical treatment for degenerative lumbar spinal disease: A nationwide matched cohort study with a 10-year follow-up. PLoS One 16, e0260460. https://doi.org/10.1371/journal.pone.0260460 (2021).
Martin, B. I. et al. Indications for spine surgery: validation of an administrative coding algorithm to classify degenerative diagnoses. Spine (Phila Pa 1976) 39, 769–779 https://doi.org/10.1097/BRS.0000000000000275 (2014).
Kim, C. H. et al. The long-term reoperation rate following surgery for lumbar herniated intervertebral disc disease: A Nationwide Sample Cohort Study with a 10-year follow-up. Spine (Phila Pa 1976) 44, 1382–1389 https://doi.org/10.1097/brs.0000000000003065 (2019).
Huang, W., Han, Z., Liu, J., Yu, L. & Yu, X. Risk factors for recurrent lumbar disc herniation: A systematic review and meta-analysis. Medicine (Baltimore) 95, e2378 https://doi.org/10.1097/md.0000000000002378 (2016).
Zhu, F. et al. Moderate to severe multifidus fatty atrophy is the risk factor for recurrence after microdiscectomy of lumbar disc herniation. Neurospine 20, 637–650. https://doi.org/10.14245/ns.2346054.027 (2023).
Jansson, K. A., Németh, G., Granath, F. & Blomqvist, P. Spinal stenosis re-operation rate in Sweden is 11% at 10 years—A national analysis of 9,664 operations. Eur. Spine J. 14, 659–663. https://doi.org/10.1007/s00586-004-0851-9 (2005).
Park, S.-J. et al. Indirect decompression using oblique lumbar interbody fusion revision surgery following previous posterior decompression: comparison of clinical and radiologic outcomes between direct and indirect decompression revision surgery. Neurospine 19, 544–554. https://doi.org/10.14245/ns.2244242.121 (2022).
Martin, B. I. et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976) 32, 382–387 https://doi.org/10.1097/01.brs.0000254104.55716.46 (2007).
Weinstein, J. N. et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 35, 1329–1338 https://doi.org/10.1097/BRS.0b013e3181e0f04d (2010).
Radcliff, K. et al. Risk for adjacent segment and same segment reoperation after surgery for lumbar stenosis: a subgroup analysis of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976) 38, 531–539 https://doi.org/10.1097/BRS.0b013e31827c99f0 (2013).
Hazard, R. G. Failed back surgery syndrome: surgical and nonsurgical approaches. Clin. Orthop. Relat. Res. 443, 228–232. https://doi.org/10.1097/01.blo.0000200230.46071.3d (2006).
Blumenthal, C. et al. Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J. Neurosurg. Spine 18, 340–346. https://doi.org/10.3171/2013.1.Spine12537 (2013).
Gerling, M. C. et al. Risk factors for reoperation in patients treated surgically for degenerative spondylolisthesis: A subanalysis of the 8-year data from the SPORT trial. Spine (Phila Pa 1976) 42, 1559–1569 https://doi.org/10.1097/brs.0000000000002196 (2017).
Okuda, S. et al. Risk factors for adjacent segment degeneration after PLIF. Spine (Phila Pa 1976) 29, 1535–1540 https://doi.org/10.1097/01.brs.0000131417.93637.9d (2004).
Geurts, J. W., Willems, P. C., Kallewaard, J. W., van Kleef, M. & Dirksen, C. The impact of chronic discogenic low back pain: Costs and patients’ burden. Pain Res. Manag. 2018, 4696180. https://doi.org/10.1155/2018/4696180 (2018).
Martin, B. I. et al. Expenditures and health status among adults with back and neck problems. Jama 299, 656–664. https://doi.org/10.1001/jama.299.6.656 (2008).
Vassilaki, M. & Hurwitz, E. L. Insights in public health: Perspectives on pain in the low back and neck: Global burden, epidemiology, and management. Hawaii J. Med. Public Health 73, 122–126 (2014).
Hurwitz, E. L., Randhawa, K., Yu, H., Côté, P. & Haldeman, S. The Global Spine Care Initiative: A summary of the global burden of low back and neck pain studies. Eur. Spine J. 27, 796–801. https://doi.org/10.1007/s00586-017-5432-9 (2018).
Alonso-García, M. & Sarría-Santamera, A. The economic and social burden of low back pain in Spain: A national assessment of the economic and social impact of low back pain in Spain. Spine (Phila Pa 1976) 45, E1026–E1032 https://doi.org/10.1097/brs.0000000000003476 (2020).
Nolte, M. T. et al. Change in patient-reported outcome measures as predictors of revision lumbar decompression procedures. Neurospine 18, 863–870. https://doi.org/10.14245/ns.2142230.115 (2021).
Jacob, C. et al. Claims data analysis on the annual frequency and incremental cost of reoperations in instrumental spinal surgeries in Germany. Value Health 17, A376. https://doi.org/10.1016/j.jval.2014.08.2591 (2014).
Acknowledgements
The authors appreciate the Medical Research Collaborating Center for statistical analysis and consultation.
Funding
This study was supported by Ministry of National Defence of Republic of Korea (800-20230466) and Doosan Yonkang foundation (800-20210527). This study was also supported by grant (30-2023-0120) from the Seoul National University Hospital research fund.
Author information
Authors and Affiliations
Contributions
C.H.K. contributed to the study's conception and design. Material preparation was performed by J.H.L., Y.H.C., S.B.P., and K.T.K. Data collection and analysis were performed by J.H.K., S.K., Y.R.K., C.H.L., and J.M.R. The first draft of the manuscript was written by H.G.P., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, H., Lee, J., Choi, Y. et al. Screening patients requiring secondary lumbar surgery for degenerative lumbar spine diseases: a nationwide sample cohort study. Sci Rep 14, 1295 (2024). https://doi.org/10.1038/s41598-024-51861-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51861-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.