Abstract
Through microorganism in the rumen of ruminant, plant fiber can be converted to edible food such as meat and milk. Ruminants had a rich and complex microbial community within the rumen, and the bacteria comprised the dominant proportion of the ruminal microbes. High-throughput sequencing offered a viable solution for the study of rumen microbes. In this study, rumen fluid samples were taken from 11 cattle from Inner Mongolian, the DNA of 11 rumen fluid samples were extracted and bacterial amplicons of the V4 regions of 16S rRNA were subjected to Illumina sequencing. More than 90,000 raw reads and 60,000 effect Tags per sample were obtained. 28,122 operational taxonomic units (OTUs) were observed from 11 samples, in average 2557 ± 361 OTUs for each sample. Bacteroidetes (44.41 ± 7.31%), Firmicutes (29.07 ± 3.78%), and Proteobacteria (7.18 ± 5.63%) were the dominant phyla among the bacteria of rumen, accounting for 82%. At the genus level, the highest relative abundance was Prevotella. Their functions were predicted using the Kyoto Encyclopedia of Genes and Genomes (KEGG). The results showed that they included metabolism, genetic information processing, environmental information processing and cellular processes. It explored the bacterial community diversity and composition of the rumen of Mongolian cattle. On the whole, our research showed that there was a high diversity as well as rich bacterial flora function of rumen bacteria in Mongolian cattle. Meanwhile, these findings provided information for further studies on the relationship between the community, diversity, functions of rumen bacteria and the nutritional physiological functions of the host.
Similar content being viewed by others
Introduction
Ruminants were the source of dairy milk and meat. As an herbivore, the functionality of their digestive system could convert plant fiber into volatile fatty acids and other products. This capacity was of enormous significance to man, and its realization depended on the microorganism of ruminants. About 75% of the fiber in the feed were digested in the rumen1. Rumen was a huge and complex microbial ecosystem, which depended on the joint action of bacteria, fungi, protozoa and other microorganisms. Among them, bacteria were the main component of rumen microorganisms, representing about 95% of the total microbes2. The rumen microorganisms served for the host as nutrition, immune regulation and physiological processes3. The composition and function of rumen microorganisms were not static, they would change with host, environment, diet and other factors.
As an anaerobic environment, only about 20% of rumen microorganisms were cultured conventionally in the laboratory and observed under microscope. It was possible to study microbial diversity, abundance and function for the uncultured microorganisms through metagenomics sequencing without culture. The 16S rRNA gene was an important symbol for a unique microbial. As it included nine hypervariable regions in the gene sequence, and the sequences of the hypervariable regions in a 16S rRNA gene correspond to a unique bacterium4. Consequently, the 16S rRNA gene amplicon sequencing was a vital tool to study the communities and diversity of bacteria in rumen5,6. The 16S rRNA gene high-throughput sequencing was used for the microbial of the gastrointestinal in 2005 for the first time. It provided a new method for the identification of the uncultured microbial7. In order to study the changes of bile acid and intestinal microflora in the perinatal period of Sha ling sows. Jie Wang8 carried out 16S rRNA sequencing and bile acid targeted metabolome detection on the feces of 42 sows. The results showed that there was more much bile acid when the intestinal microflora was increasing in the perinatal period of Sha ling sows. Dong SX9 used 16S rRNA sequencing on the intestinal microorganisms of wild striped goose in Tibet Autonomous Region. A total of 513,505 original data reads were obtained. The results showed that the phylum Chlamydomonas (67.34%) was the dominant phylum, followed by Proteobacteria (29.03%) and Cyanophyta (1.97%). The findings were also very useful and valuable for the study of genetics and high altitude in Tibet Autonomous Region.
Mongolian cattle are mainly fed in the north of China, Mongolia and the east of Russia10, where the altitude here is high, and the weather was cold and dry. Mongolian cattle are hardy, disease resistant, adaptable to harsh environmental conditions and have relatively low food requirements, being able to digest roughage with a high fibre content. It is a treasure of microbial community research because of its unique living environment setting and eating habits11.
In order to study the composition and structure of microorganisms in the rumen, we analyzed the sequencing results of the bacterial amplicons of the V4 regions of 16S rRNA of 11 Mongolian cattle rumens. The findings could be helpful for the study of rumen microbial diversity of Mongolian cattle, and they could provide basis for future research on rumen bacteria of Mongolian cattle. Based on the experimental results, the functional bacteria producing cellulase or ethanol was screened from the rumen of Mongolian cattle. In addition, understanding the characteristics of Mongolian cattle rumen microorganisms could provide guidance for their feeding.
Methods
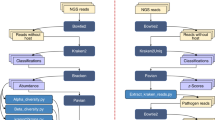
Sample collection
A total of 11 Mongolian cattle with two years old were used in this study. And these cattle were originated from Zhenglan Banner, XilingGol League, Inner Mongolia, in China (115°00′-116°42′E, 41°56′-43°11′N). All the animals were raised under the same conditions. The experimental cattle were slaughtered in the same abattoir, where 11 rumen fluid samples were collected immediately. It was necessary to wear sterile gloves when collecting rumen fluid samples. Rumen fluid (approximately 50 mL) was strained through four layers of cheesecloth and centrifuged at 13,000 × g for 20 min at 4 °C to collect the supernatant. Samples were rapidly frozen in liquid nitrogen and stored at − 80°C. They were labeled as N1 to N11.
Methods
Genome DNA extraction and PCR amplification
Total genome DNA of rumen fluid from 11 samples was extracted by Genome DNA Extraction Kit (Qiagen, Germany). The concentration and purity of the genome DNA were monitored using 1% agarose gels. For the procedure of Polymerase Chain Reaction (PCR), DNA was diluted to 1 ng µL−1 using sterile water according to the concentration.
Specific primers 515F-806R were used to amplify the 16S rRNA gene of the 16S V4 region. All PCR reactions were carried out with 15 µL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs). In each reaction forward and reverse primers were 2 µM, and template DNA was 10 ng. Thermal cycling was followed below: an initial denaturation at 98 °C for 1 min; 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s; 72 °C for 5 min. The concentration and purity of the PCR products were monitored on 2% agarose gel. The mixture of PCR products was purified with Qiagen Gel Extraction Kit (Qiagen, Germany).
16S rRNA gene amplicon library preparation
Genome DNA libraries of 11 rumen fluids were generated using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) and index codes were added. At last, 11 libraries were sequenced through an Illumina NovaSeq platform. 250 bp paired-end reads were generated after sequencing and were named raw data.
Processing of sequencing data
The raw data were spliced and controlled on quality to obtain clean tags. Chimerism were filtered to obtain effective tags. The effective tags were clustered with 97% identity and named OTUs (operational taxonomic units). Representative sequences for each OTU were screened and annotated taxonomic information according to Silva Database (http://www.arb-silva.de/)12. MUSCLE (Multiple Protein Sequence Alignment) software (Version 3.8.31, http://www.drive5.com/muscle/) was used to analyze the relationship of different OTUs and the dominant species among the 11 samples13.
Alpha diversity
For studying the species diversity of the 11 samples, alpha diversity was used on 6 indices, including Observed-species, Chao1, Shannon, Simpson, ACE, and Good-coverage. The 6 indices of the 11 samples were calculated with QIIME (Quantitative Insights into Microbial Ecology) (Version 1.7.0) and displayed with R software (Version 2.15.3). The Chao1 estimator (http://www.mothur.org/wiki/Chao) and the ACE estimator (http://www.mothur.org/wiki/Ace) were selected to identify community richness of the 11 samples. The Shannon index (http://www.mothur.org/wiki/Shannon) and the Simpson index (http://www.mothur.org/wiki/Simpson) were used to identify the community diversity of the 11 samples. The Good’s coverage (http://www.mothur.org/wiki/Coverage) was used to characterized Sequencing depth of the 11 samples.
Beta diversity
In order to analyze the species complexity among the 11 samples, beta diversity analysis was used. Beta diversity included weighted and unweighted unifrac. Weighted and unweighted unifrac were calculated using QIIME software (Version 1.9.1).
Principal Coordinate Analysis (PCoA) was a visualized form of the sequencing data. In the principal coordinate analysis, a set of orthogonal axe was obtained from a distance matrix of weighted or unweighted unifrac among the 11 samples. The maximum variation factor was demonstrated by first principal coordinate, and the second maximum one by the second principal coordinates, and so on. PCoA analysis was displayed by WGCNA (Weighted correlation network analysis) package, stat packages and ggplot2 package in R software (Version 2.15.3).
Unweighted Pair-group Method with Arithmetic Means (UPGMA) clustering was performed as a type of hierarchical clustering method to interpret the distance matrix using average linkage and was conducted by QIIME software (Version 1.9.1).
Function prediction
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was a group of pathways showing the relation networks for: 1. Metabolism 2. Gene information transduction 3. Environmental information processing 4. Cell signal transduction 5. Organism Systems 6. Human diseases 7. Drug development.
KEGG pathways were built using the Tax4Fun program. The Tax4Fun function prediction was analyzed by the nearest neighbor method based on the minimum 16S rRNA sequence similarity. The procedure of the Tax4Fun function prediction was followed below: firstly, extracting the whole genome 16S rRNA gene sequences of prokaryotes in KEGG database (www.kegg.jp/kegg/kegg1.html)14,15, to achieve an unrivaled speed of the metabolic profiling approach. Secondly, comparing the 16S rRNA gene sequence to SILVA SSU Ref NR database (SSURef 115 NR) by BLASTN algorithm (BLAST bitscore > 1500). Thirdly, establishing a correlation matrix. Fourthly, function annotating using functional information of KEGG database annotated by UProC and PAUDA methods and the SILVA database. The OTUs of samples with SILVA database were used as reference sequences in the annotation of functional prediction. For the generation of clustered heatmaps, the pheatmap package (version 1.0.12) in R (https://cloud.r-project.org/) was used16.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Sequencing data analysis
A total of 1,153,578 raw reads were obtained from 11 samples, with an average of 104,870 ± 4224 sequences for each sample (Table 1). The original reads were spliced and filtered to obtain effective data. On average, 102,823 ± 4091 raw tags, 101,953 ± 4032 clean tags, and 65,898 ± 2766 effective tags were obtained (Table 1). The average length of effective tags was 253 nucleotides (nt). The Q20 and Q30 of effect tags were 99.04 ± 0.06% and 97.44 ± 0.13% respectively (Table 1). Operational taxonomic units (OTUs) clustering and species analysis were conducted based on the effect tags. Based on 97% nucleotide sequence identity, the total number of OTUs in the 11 samples reached 28,122 with an average of 2557 ± 361 per sample.
Alpha diversity analysis
As the number of sequences increased, the rarefaction curve flattened out (Fig. 1). It was indicated that the depth of sequencing was sufficient to accurately represent the bacterial composition of the rumen (with the Good’s coverage > 99%). Furthermore, rumen microbial diversity and richness were estimated using alpha diversity index (Table 2), including Shannon, Observed species, PD whole tree, Simpson, Chao1, and ACE.
Beta diversity analysis
Beta diversity analysis was performed to carry out a comparative analysis of the microbial community composition of 11 samples. The samples with high similarity in community structure were clustered together in the plot, and the samples with large community differences would be far apart in the polt. The first and second principal coordinates of the PCoA diagram are 45.16% and 19.13% respectively (Fig. 2A). Microbial community characteristics varied widely across N8 and other samples. Similar results to PCoA mentioned above, samples were divided into three groups: (A) N1, N2, and N4; (B) N3, N5, N6, N7, N9, N10, and N11; (C) N8 (Fig. 2B).
The bacteria composition and community of rumen in Mongolian cattle
Cumulative bar charts of relative species abundance at the taxonomic level of the phylum (Fig. 3A) and genus (Fig. 3B) were produced to view the species with high relative abundance at different taxonomic levels and their proportion in each sample. The top ten genera in terms of relative abundance in 11 samples were Prevotella (14.30 ± 4.13%), Succinimonas (2.42 ± 4.19%), Rikenellaceae_RC9_gut_group (6.66 ± 1.25%), Succinivibrionaceae_UCG-002 (1.65 ± 2.23%), Lactobacillus (4.57 ± 2.62%), Escherichia-Shigella (0.79 ± 1.96%), Saccharofermentans (1.98 ± 1.03%), Christensenellaceae_R-7_group (2.44 ± 0.60%), unidentified_F082 (0.96 ± 0.82%), and Ruminococcaceae_NK4A214_group (1.88 ± 0.48%) (Figs. 3B and 4). These genera accounted for 37.66 ± 6.16% of total bacterial sequences and belonged to the three phyla with the highest relative abundance (Fig. 3A): Bacteroidetes (44.41 ± 7.31%), Firmicutes (29.07 ± 3.78%), and Proteobacteria (7.18 ± 5.63%). Firmicutes and Bacteroides were the core phylum in the rumen accounting for 73.48 ± 5.60%. In addition, the ratio of Firmicutes to Bacteroidetes is about 1.57.
The heat maps (Fig. 3C) was plotted to visualize the top 35 genera in terms of abundance among 11 samples. The relative abundances of Micrococcus, Enterococcus, and Morganella Morganii in sample N2 were higher than those in other samples. But the relative abundances of Christensenellaceae R-7 group and Ruminococcaceae_ NK4A214_ group in N2 were lower than others. The relative abundance of Methanobrevibacter, Veilonellaceae-ucg-001, and Saccharofermentans in sample N8 were higher than these in other samples. But the relative abundance of Prevotrlla, Prevotrllaceae_Ucg-001, and Prevotellaceae UCG-003 were lower than these in other samples.
Functional annotation
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway at level 1 and 2 were performed estimated by Tax4Fun to analysis the potential function of bacteria in the 11 samples. Functional relative abundance annotation results showed that the pathways were extraordinary analogous among samples. At KEGG level 1 (Fig. 5A), the biological pathways of the samples mainly included metabolism (45.09 ± 0.39%), genetic information processing (25.13 ± 0.61%), environmental information processing (11.72 ± 0.74%), cellular processes (7.82 ± 0.15%), Unclassified (5.31 ± 0.12%), human diseases (2.86 ± 0.06%), and organism systems (2.06 ± 0.07%). The top three functional paths in terms of relative abundance accounting for more than 80%.
Hierarchical clustering heat map showing abundance information for the top 35 relative abundances of KEGG pathways in 11 samples (Fig. 5B). As the results of level 1, the functional pathways were similar among 11 samples. The KEEG pathways with high relative abundance in the 11 samples mainly included carbohydrate metabolism, replication and repair, translation, membrane transport and amino acid metabolism. The cellular community prokaryotes, membrane transport, transcription and infectious diseases were the mainly pathways in the samples N1, N4, N8, and N9. In the sample N4, the cellular community prokaryotes and membrane transport were play an important role. The folding, sorting and degradation, lipid metabolism, and metabolism of terpenoids and polyketides were the mainly metabolic pathways in the sample N3.
Discussion
Microbial diversity of samples
The sequencing platform and the sample numbers usually affected the quality of the 16S rRNA data. Meanwhile, if more than 10,000 reads obtained for one sample was sufficient for accurate and reliable results17,18,19. In this study the 16S rRNA gene of 11 samples were sequenced by Illumina NovaSeq platform. And every sample had obtained more than 90,000 reads. Consequently the results of the 16S rRNA data was accurate and reliable.
Alpha diversity indices included four main indices including Chao1, ACE, Shannon and PD whole tree. The Chao1 and ACE indices reflected the richness of the microbial communities. The Shannon indices reflected the diversity, and the PD whole tree reflected the genetic relationship of species in the community. The larger index values of Chao1, ACE, and Shannon indicated that the more abundant and diverse species were observed in one sample20. The higher alpha diversity indicated a more diverse and complex microbial composition in one rumen. It could enhance the resistance to environment and the adaptability of the host21. The rumen microbiota could regulate physiological processes that provided protection to the host, such as the production of antimicrobial substances and inhibition of the growth of digestive pathogens22,23. A good rumen microbial ecosystem can improve the growth performance of the host24. Dietary variation might be a major source of new microbial diversity, with alpha diversity often changing dynamically with diet21. In the present study, the 11 Mongolian cattle rumen samples showed similarity in terms of overall microbial diversity. The Alpha diversity indices of sample N8 was higher than those of other samples. The result indicated that sample N8 had a higher richness of species diversity.
Microbial community structure of the Mongolian cattle
In our study, the Bacteroidetes, Firmicutes, and Proteobacteria were found in all 11 samples. Firmicutes and Bacteroides were the dominant flora in the rumen of mammals, they help animals digest plant-derived feed25,26,27. When the diet of adult cows was changed to a high fibre diet, the abundance of the phylum Bacteroides increased considerably and the composition of the rumen microbiota changes28. Consistently, our findings also show Firmicutes and Bacteroides account for 70% of rumen bacteria of Mongolian cattle, and the ratio of Firmicutes to Bacteroidetes was about 1.57. In the study of Elie Jam28, they found the abundance of Bacteroidetes were inversely proportional to the fat. The results found that when the fat in the blood and tissue was increased, the abundance of Bacteroidetes was decreased. The change of Bacteroidetes ratio affected the metabolic potential of the mouse gut microbiota1. Similarly, Turnbaugh29 found that the ability of the obesity microbiome to obtain energy from the diet was increased. More importantly, that this feature was transmissible: the mice had an increase in whole body fat following the introduction of microbiota positively associated with obesity in germ-free mice. The results suggested that these floras were a cause of obesity. This was of great importance for the breeding of Mongolian cattle, as the ratio of the two floras could be adjusted to regulate the fat content and thus the health and cold tolerance of the animal30. These results suggested that the ratio of the relative abundance of Firmicutes and Bacteroides was probably related to obesity. Proteobacteria was the phylum with the third highest relative abundance in the Mongolian cattle. Proteobacteria comprised a large number of bacteria that can catabolize feedstuff components31, such as fiber plant32. The results also demonstrated that the rumen microorganisms contained Fibrobacteres, accounting for nearly 1%. It was an important phylum of cellulose-degrading bacteria33. This phylum included one cellulase producing bacteria genus named Fibrobacter. And the genus named Fibrobacter consisted of two cellulose-degrading species named Fibrobacter succinogenes and Fibrobacter enterica.
In our study, the top ten species with relative abundance at the genus level included Prevotella, Rikenellaceae_RC9_gut_group, Succinivibrionaceae_UCG_002, Lactobacillus, Escherichia Shigella, Saccharofermentans, Christensenellaceae_R-7_group、unidentified_F082、NK4A214_group. These genera belong to three phyla——Bacteroidetes, Firmicutes, and Proteobacteria.
Prevotella was the genus with the highest relative abundance among rumen bacteria34. Although Prevotella couled not degrade cellulose, it could degrade other polysaccharides such as xylan. As the main proteolytic bacteria in the rumen, Prevotella could use peptides and ammonia as nitrogen source. Additionally, it could also produce a lot of complex enzymes to degrades starch35. Meanwhile, Prevotella were associated with the production of propionate in the rumen24. Propionate was negatively correlated with methane production26,36. This showed that an increase in the relative abundance of Prevotella favored a reduction in methane emissions. Furthermore, it had an important role to play in reducing greenhouse gas emission from ruminants.
In the present study, the relative abundance of Succinimonas and Succinivibrionaceae_UCG_002 was accounting for 4%. Succinimonas were anaerobic organisms that break down starch, ferment glucose, maltose and dextrin. The metabolites were a large amount of succinic acid and a small amount of acetic acid37. The relative abundance of Succinivibrionaceae_UCG_002 was higher than others in the Succinivibrionaceae family. The abundances of all the Succinivibrionaceae members were positively correlated with the emission of methane. Because its members mainly produced succinate, that competed with methane production, thus they reduced the release of hydrogen38,39. Some studies have found that the presence of Succinivibrionaceae_UCG_002 and Succinivibrio was inversely proportional to feed efficiency40,41. Some species of the Succinivibrionaceae family from the tammar wallaby (Macropus eugenii) manipulated methane and therefore it drew attention to methane emissions in ruminant livestock42.
The genus with the second highest relative abundance in 11 samples was Rikenellaceae_RC9_gut_group. And the lower pH would reduce the amount of this genus. Meanwhile the relative abundance varies by sex, with lower abundance of the genus in female cows43. A recent study36 found the abundance was also associated with rumen epithelial morphology. The rumen epithelium was important for the absorption of volatile fatty acids from feed digestion, with approximately 50–80% of volatile fatty acids (VFAs). The VFAs were absorbed directly by the epithelium44. Overall, Rikenellaceae_RC9_gut_group in the rumen has an important role in the degradation of crude fibre and in the morphological structure of the rumen epithelium35.
Lactobacillus was composed of over 170 species and 17 subspecies45. As a typical bacterium of Lactobacillus, Lactobacilli had long been used to make dairy products such as cheese and yogurt. They also had a high tolerance for very low pH conditions, especially those used to ferment foodstuffs such as mustard, cabbage, and olives. In the gut, Lactobacillus adhesion to the mucus layer of the gut wall was mediated by a protein surface layer called the S-layer. In addition, some strains of lactobacilli produced antioxidants. Ljungh and Wadström46 also reviewed that probiotic potential of lactobacilli, was the ability for immunomodulate human cells to achieve an anti-inflammatory response. In addition, Lactobacilli could produce strain-specific bacteriocins and bacteriocin-like products that could inhibit the growth of other organisms47. Lactobacillus was a partly anaerobic or strictly anaerobic bacterium, and it was involved in the hydrolysis of proteins and lipids. In particular, it could continue to multiply after participating in the formation of cheese and thus produced contamination48. As Lactobacillus fermenting glucose could release lactic or acetic acid, which produced an acidic environment that inhibited the growth of some harmful bacteria. It was helpful for the health of the host. In addition to that, Lactobacillus could also alleviate respiratory diseases and regulate respiratory immunity49 and provide some relief from the symptoms of cardiovascular disease50. But the genus Lactobacillus was not only a beneficial organism, but can also act as a pathogen for serious infections. If Lactobacillus entered the bloodstream, it might be associated with sepsis in immunocompromised patients46.
Escherichia Shigella is a facultative anaerobic microorganism as well as a pathogenic bacterium. The rapid increase of Escherichia Shigella abundance in the intestine and stomach is one of the signs of IgAN (IgA nephropathy) production, which leads to the imbalance of intestinal ecology of patients and the decline of immunity51. The study of Lee52 showed that diarrhoea caused by Escherichia-Shigella is not associated with changes in children's weight, but can slow height growth in children. The presence of large amounts of Escherichia-Shigella is detrimental to the health of the host, there is a small relative abundance of Escherichia-Shigella among samples, accounting for less than 0.00002%. It shows that the 11 samples in this study are all from healthy Mongolian cattle.
Many studies had shown that the genus Christensenellaceae R7 group was found in the intestinal tract and played an important role in amino acid and lipid metabolisms53. It was recently found Christensenellaceae was associated with the health of digestive system for human and mice54. The Christensenellaceae_R-7_group was an important member of the Christensenellaceae, and Christensenellaceae was importantly associated with mammal health. The relative abundance of Christensenellaceae was inversely proportional to fat content, with higher abundance being beneficial to health. Thus the relative abundance of Christensenellaceae was higher in centenarians53. The fermented complete feed significantly affected the microbial diversity in pig manure, with the most affected genera including Christensenellaceae_R-7_group, whose relative abundance was significantly reduced compared to the control group55. Yang’s studies indicated that the abundance of the Christensenellaceae_R-7_group was increased in the rumen as the content of n3-poly unsaturated fatty acids in longissimus dorsi muscle was increased56. And it might play an important role in the low-density fatty acids metabolism for mammals health. In present study, the relative abundances of Christensenellaceae_R-7_group were very similar among 11 samples, averagely accounting for 4.5%.
Functional pathways of rumen bacteria in the Mongolian cattle
According to the function prediction analysis of KEGG, it had found that the rumen bacteria in Mongolian cattle play roles in the metabolism of gene, cell, carbohydrate, amino acid, energy and vitamin. All findings indicated that bacteria played an important role in nutrient digestion and absorption of ruminants. The fiber was digested through the bacteria in the rumen into volatile fatty acids, such as acetic acid, propionic acid, and butyric acid57,58. Meanwhile, the bacteria could convert the nitrogen-containing substances into amino acids and provided the nitrogen source for ruminants. Consequently, the abundance and composition of the rumen bacterial population was closely correlated with the health of the ruminants59,60. Research had shown that the diversity and abundance of rumen bacteria was closely related to the composition and type of feed60. Highly nutritious feeds could reduce the variety of rumen microorganisms and even caused subacute ruminal acidosis (SARA), which was detrimental to the health of cattle and could even cause death61. Therefore, ruminal microbial composition could be controlled through scientific feed ratios to improve the stability of the rumen microbial community and increase ruminant production62. For example, when the ratio of concentrate to roughage in feeds was changed, it could maximize productivity, reduce energy losses and decrease the methane emission63.
Conclusion
Our findings showed that there was higher diversity, richness, and evenness of microbial communities in the rumen. The predominant phylum were Bacteroidetes and Firmicutes in the rumen, Prevotella was the most predominant genus in the rumen. And the biological pathways in microbial communities of rumen mainly included metabolism, environmental information processing, and genetic information processing. Nevertheless, the functional profiles of 11 samples were merely a prediction; detailed analyses are still needed to elucidate this aspect. Further studies are warranted to determine the contributions of the bacterial taxa and metabolic pathways to the health, development, and physiology of cattle.
Inner Mongolia is the region with the larg number of cattle in China. Meanwhile, understanding the rumen microbial composition of Mongolian cattle is an important guide to the health of cattle. In our study, we innovatively collected rumen samples from Mongolian cattle in Xilingol League, which was rarely seen in previous studies. The experimental results showed that the rumen microbial diversity in the samples was high, including some rare and uncultured microbes. These microbes play important ecological functions in the rumen of Mongolian cattle, and were of great significance for understanding the integrity and stability of rumen microbial communities. Moreover, exploring the rumen microbial diversity was important for enriching the microbial resource. It could provide guidance for feeding cattle. For example, feed formulation could be adjusted according to the species and number of rumen microorganisms to improve feed utilization. And the feeding environment could be improved according to the ecological characteristics of rumen microorganisms to enhance the health status of Mongolian cattle. Functional gene could also be characterized, which laid the foundation for elucidating the metabolic pathways for degrading cellulose and fat, and so on. Consequently, cellulose-degrading bacterial would been collected and cellulose genes would been characterized in our laboratory from Mongolian cattle according to the results of this study.
Data availability
The raw sequencing data have been deposited on the Sequencing Reads Archive (SRA), and SRA accession number is PRJNA955685 (https://www.ncbi.nlm.nih.gov/sra/PRJNA955685).
References
Yang, Y. et al. Evaluating different extraction solvents for GC-MS based metabolomic analysis of the fecal metabolome of adult and baby giant pandas. Sci. Rep. 9(1), 12073. https://doi.org/10.1038/s41598-019-48453-1 (2019).
Zhou, M., Chen, Y. & Guan, L. L. Rumen bacteria. In Rumen Microbiology: From Evolution to Revolution (eds Puniya, A. et al.) (Springer, 2015). https://doi.org/10.1007/978-81-322-2401-3_6.
Liu, H. J. et al. Tibetan sheep adapt to plant phenology in alpine meadows by changing rumen microbial community structure and function. Front. Microbiol. 11, 587558. https://doi.org/10.3389/fmicb.2020.587558 (2020).
D’Amore, R. et al. A comprehensive benchmarking study of protocols and sequencing platforms for 16S rRNA community profiling. BMC Genom. 17(1), 1–20. https://doi.org/10.1186/s12864-015-2194-9 (2016).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl 1), 4516–4522. https://doi.org/10.1073/pnas.1000080107 (2011).
Yarza, P. et al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 12(9), 635–645. https://doi.org/10.1038/nrmicro3330 (2014).
Paul, B. E. et al. Diversity of the human intestinal microbial flora. Science 308(5728), 1635–1638. https://doi.org/10.1126/science.1110591 (2005).
Wang, J. et al. The periparturient gut microbiota’s modifications in Shaziling Sows concerning bile acids. Metabolites 13(1), 68. https://doi.org/10.3390/metabo13010068 (2023).
Dong, S. et al. First report of fecal microflora of wild bar-headed goose in Tibet Plateau. Front. Vet. Sci. 10(8), 791461. https://doi.org/10.3389/fvets.2021.791461 (2022).
Chen, Q. M. et al. Whole genome analyses revealed genomic difference between European taurine and East Asian taurine. J. Anim. Breed. Genet. https://doi.org/10.1111/jbg.12501 (2020).
Ahmad, A. A. et al. Age-dependent variations in rumen bacterial community of Mongolian cattle from weaning to adulthood. BMC Microbiol. 22(1), 1–15. https://doi.org/10.1186/s12866-022-02627-6 (2022).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590-596 (2013).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32(5), 1792–1797 (2004).
Ogata, H. et al. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 27(1), 29–34. https://doi.org/10.1093/nar/27.1.29 (1999).
Minoru, K. et al. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. https://doi.org/10.1093/nar/gkt1076 (2013).
Kolde, R. Pretty heatmaps. R package version 61 (2012). Available online at: http://CRAN.R-project.org/package=pheatmap
Myer, P. R., Wells, J. E., Smith, T. P. L., Kuehn, L. A. & Freetly, H. C. Cecum microbial communities from steers differing in feed efficiency. J. Anim. Sci. 93(11), 5327–5340. https://doi.org/10.2527/jas.2015-9415 (2015).
Paz, H. A., Anderson, C. L., Muller, M. J., Kononoff, P. J. & Fernando, S. C. Rumen bacterial community composition in Holstein and Jersey cows is different under same dietary condition and is not affected by sampling method. Front. Microbiol. 7, 1206. https://doi.org/10.3389/fmicb.2016.01206 (2016).
Zubiria, I. et al. Effect of feeding cold-pressed sunflower cake on ruminal fermentation, lipid metabolism and bacterial community in dairy cows. Animals 9(10), 755. https://doi.org/10.3390/ani9100755 (2019).
Qian, H. F. et al. Analyzing Arabidopsis thaliana root proteome provides insights into the molecular bases of enantioselective imazethapyr toxicity. J. Biotechnol. 5(1), 11975. https://doi.org/10.1038/srep11975 (2019).
Stoffel, M. A. et al. Early sexual dimorphism in the developing gut microbiome of northern elephant seals. Mol. Ecol. 29(11), 2109–2122. https://doi.org/10.1111/mec.15385 (2020).
Baumler, A. J. et al. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535(7610), 85–93 (2016).
Lettat, A. et al. Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol. 12(1), 1–12. https://doi.org/10.1186/1471-2180-12-142 (2012).
Shin, D. et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. J. Biotechnol. 14(8), e0220843. https://doi.org/10.1371/journal.pone.0220843 (2019).
de Oliveira, M. N. V. et al. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol. 164(3–4), 307–314. https://doi.org/10.1016/j.vetmic.2013.02.013 (2013).
Spence, C., Wells, W. G. & Smith, C. J. Characterization of the primary starch utilization operon in the obligate anaerobe Bacteroides fragilis: Regulation by carbon source and oxygen. J. Bacteriol. 188(13), 4663–4672. https://doi.org/10.1128/JB.00125-06 (2006).
Thoetkiattikul, H. et al. Comparative analysis of microbial profiles in cow rumen fed with different dietary fiber by tagged 16S rRNA gene pyrosequencing. Curr. Microbiol 67(2), 130–137. https://doi.org/10.1007/s00284-013-0336-3 (2013).
Jami, E., White, B. A. & Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. J. Biotechnol. 9(1), e85423. https://doi.org/10.1371/journal.pone.0085423 (2014).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122), 1027–1031. https://doi.org/10.1038/nature05414 (2006).
Evans, N. J. et al. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microb. 77(1), 138–147. https://doi.org/10.1128/AEM.00993-10 (2011).
Callaway, T. R. et al. Evaluation of bacterial diversity in the rumen and feces of cattle fed different levels of dried distillers grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 88(12), 3977–3983. https://doi.org/10.2527/jas.2010-2900 (2010).
Singh, K. M. et al. High potential source for biomass degradation enzyme discovery and environmental aspects revealed through metagenomics of Indian buffalo rumen. Biomed. Res. Int. 2014, 267189. https://doi.org/10.1155/2014/267189 (2014).
Bekele, A. Z., Koike, S. & Kobayashi, Y. Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiol. Lett. 305(1), 49–57. https://doi.org/10.1111/j.1574-6968.2010.01911.x (2010).
Purushe, J. et al. Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: Insights into their environmental niche. Microb. Ecol. 60(4), 721–729. https://doi.org/10.1007/s00248-010-9692-8 (2010).
Huang, C. et al. Microbiome and metabolomics reveal the effects of different feeding systems on the growth and ruminal development of yaks. Front. Microbiol. 12, 682989. https://doi.org/10.3389/fmicb.2021.682989 (2021).
Bryant, M. P., Small, N., Bouma, C. & Chu, H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J. Bacteriol. 76(1), 15–23. https://doi.org/10.1128/jb.76.1.15-23.1958 (1958).
Danielsson, R. et al. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front. Microbiol. 8, 226. https://doi.org/10.3389/fmicb.2017.00226 (2017).
Joch, M., Mrazek, J., Skrivanova, E., Cermak, L. & Marounek, M. Effects of pure plant secondary metabolites on methane production, rumen fermentation and rumen bacteria populations in vitro. J. Anim. Physiol. Anim. Nutr. 102(4), 869–881. https://doi.org/10.1111/jpn.12910 (2018).
Auffret, M. D. et al. Identification of microbial genetic capacities and potential mechanisms within the rumen microbiome explaining differences in beef cattle feed efficiency. Front. Microbiol. 11, 1229. https://doi.org/10.3389/fmicb.2020.01229 (2020).
Zhang, Y. K. et al. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 15(3), 100161. https://doi.org/10.1016/j.animal.2020.100161 (2021).
Pope, P. B. et al. Isolation of Succinivibrionaceae implicated in low methane emissions from Tammar wallabies. Science 333(6042), 646–648. https://doi.org/10.1126/science.1205760 (2011).
Qiu, X. et al. Serum biochemical parameters, rumen fermentation, and rumen bacterial communities are partly driven by the breed and sex of cattle when fed high-grain diet. Microorganisms 10(2), 323. https://doi.org/10.3390/microorganisms10020323 (2022).
Melo, L. Q. et al. Rumen morphometrics and the effect of digesta pH and volume on volatile fatty acid absorption. J. Anim. Sci. 91(4), 1775–1783. https://doi.org/10.2527/jas.2011-4999 (2013).
Goldstein, E. J. C., Tyrrell, K. L. & Citron, D. M. Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 60(Suppl 2), S98–S107. https://doi.org/10.1093/cid/civ072 (2015).
Ljungh, A. & Wadström, T. Lactic acid bacteria as probiotics. Curr. Issues Intest. Microbiol. 7(2), 73–89. https://doi.org/10.1111/j.1365-2672.1964.tb05056.x (2006).
Lievin-Le Moal, V. & Servin, A. L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 27(2), 167–199. https://doi.org/10.1128/CMR.00080-13 (2014).
Dicks, L. M. T., Fraser, T., ten Doeschate, K. & van Reenen, C. A. Lactic acid bacteria population in children diagnosed with human immunodeficiency virus. J. Paediatr. Child Health 45(10), 567–572. https://doi.org/10.1111/j.1440-1754.2009.01566.x (2009).
Du, T., Lei, A., Zhang, N. & Zhu, C. The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases. Front Immunol. 13, 908010. https://doi.org/10.3389/fimmu.2022.908010 (2022).
Nageh, H., Mansour, S. & Moustafa, A. A. Gut Microbiome and Human Health, 1, 57–59 (2018)
Zhao, J. et al. Expansion of Escherichia-Shigella in gut is associated with the onset and response to immunosuppressive therapy of IgA nephropathy. J. Am. Soc. Nephrol. 33(12), 2276–2292. https://doi.org/10.1681/ASN.2022020189 (2022).
Lee, G. et al. Effects of Shigella-, Campylobacter- and ETEC- associated diarrhea on childhood growth. Pediatr. Infect. Dis. J. 33(10), 1004–1009. https://doi.org/10.1097/INF.0000000000000351 (2014).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159(4), 789–799. https://doi.org/10.1016/j.cell.2014.09.053 (2014).
Tang, X., Zhang, K. & Xiong, K. Fecal microbial changes in response to finishing pigs directly fed with fermented feed. Front. Vet. Sci. 9, 894909. https://doi.org/10.3389/fvets.2022.894909 (2022).
Waters, J. L. & Ley, R. E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 17(1), 1–11. https://doi.org/10.1186/s12915-019-0699-4 (2019).
Xiong, Y. et al. Effects of paper mulberry silage on the growth performance, rumen microbiota and muscle fatty acid composition in hu lambs. Fermentation-Basel 7(4), 286. https://doi.org/10.3390/fermentation7040286 (2021).
Jiang, F. et al. Comparative analysis of gut microbial composition and potential functions in captive forest and alpine musk deer. Appl. Microbiol. Biotechnol. 106(3), 1325–1339. https://doi.org/10.1007/s00253-022-11775-8 (2022).
Li, C., Geng, Y., Wang, P., Shi, H. & Luo, J. Comparison of microbial diversity in rumen and small intestine of Xinong Saanen dairy goats using 16S rRNA gene high-throughput sequencing. Anim. Prod. Sci. https://doi.org/10.1071/AN20459 (2021).
Moschen, I., Broeer, A., Galic, S., Lang, F. & Broeer, S. Significance of short chain fatty acid transport by members of the monocarboxylate transporter family (MCT). Neurochem. Res. 37(11), 2562–2568. https://doi.org/10.1007/s11064-012-0857-3 (2012).
Guan, L. L., Nkrumah, J. D., Basarab, J. A. & Moore, S. S. Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 288(1), 85–91. https://doi.org/10.1111/j.1574-6968.2008.01343.x (2008).
Carberry, C. A., Kenny, D. A., Han, S., McCabe, M. S. & Waters, S. M. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl. Environ. Microbiol. 78(14), 4949–4958. https://doi.org/10.1128/AEM.07759-11 (2012).
Bevans, D. W., Beauchemin, K. A., Schwartzkopf-Genswein, K. S., McKinnon, J. J. & McAllister, T. A. Effect of rapid or gradual grain adaptation on subacute acidosis and feed intake by feedlot cattle. J. Anim. Sci. 83(5), 1116–1132. https://doi.org/10.2527/2005.8351116x (2005).
Russell, J. B. & Rychlik, J. L. Factors that alter rumen microbial ecology. Science 292(5519), 1119–1122. https://doi.org/10.1126/science (2001).
Makkar, H. P. S. & Beever, D. Optimization of feed use efficiency in ruminant production systems (2013).
Acknowledgements
Financial support by the Natural Science Foundation of Inner Mongolia Autonomous Region is gratefully acknowledged. The first author thanks Dr. YLS for her guidance.
Funding
This work was supported by Inner Mongolia Autonomous Region Natural Science Foundation Project (No. 2020MS03030), Inner Mongolia Autonomous Region Directly Affiliated Universities Basic Research Business Fee Project (No. ZTY2023032), and Inner Mongolia Autonomous Region Graduate Research Innovation Project (No. S20231131Z).
Author information
Authors and Affiliations
Contributions
Y.J.J. collected the samples,performed the data analysis and wrote the manuscript. Y.L.S. and H.Y.Q. contributed to critical discussions. Y.L.S. revised and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, Y., Shi, Y. & Qiao, H. Bacterial community and diversity in the rumen of 11 Mongolian cattle as revealed by 16S rRNA amplicon sequencing. Sci Rep 14, 1546 (2024). https://doi.org/10.1038/s41598-024-51828-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51828-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.