Abstract
As the global demand for food increases, aquaculture plays a key role as the fastest growing animal protein sector. However, existing aquafeeds contain protein ingredients that are not sustainable under current production systems. We evaluated the use of microbial community-based single cell protein (SCP), produced from soybean processing wastewater, as a partial fishmeal protein substitute in juvenile Asian seabass (Lates calcarifer). A 24-day feeding trial was conducted with a control fishmeal diet and a 50% fishmeal replacement with microbial community-based SCP as an experimental group, in triplicate tanks containing 20 fish each. Both diets met the protein, essential amino acids (except for lysine), and fat requirements for juvenile Asian sea bass. The microbial composition of the SCP was dominated by the genera Acidipropionibacterium and Propioniciclava, which have potential as probiotics and producers of valuable metabolites. The growth performance in terms of percent weight gain, feed conversion ratio (FCR), specific growth rate (SGR), and survival were not significantly different between groups after 24 days. The experimental group had less variability in terms of weight gain and FCR than the control group. Overall, microbial community-based protein produced from soybean processing wastewater has potential as a value-added feed ingredient for sustainable aquaculture feeds.
Similar content being viewed by others
Introduction
It is estimated that 1250 million tons of animal-derived protein would need to be produced per year to meet the global demand in 2050 at current consumption levels1. However, increasing meat production for this purpose is not sustainable because the conversion of plant to meat protein is inefficient2. Protein production by conventional agriculture-based food supply chains is also a major issue in terms of global environmental pollution. The livestock sector produces 14.5% of the global anthropogenic greenhouse gas (GHG) emissions, and about 33% of the land surface and 75% of freshwater resources are used for livestock or crop production3. Thus, new solutions for protein production are needed to ensure sustainable food security.
The supply of seafood, wild-caught and farmed, for human consumption was around 175 million tons in 2020, making it the largest protein industry worldwide4,5. Aquaculture accounted for about 50% of that sector and 21% of total animal protein production6. With the global human population predicted to increase by 30% in 2050 compared to 2018, it is estimated that aquaculture production would need to increase by 57.2% to meet the growing demand for seafood5. High trophic level aquaculture species still rely on fishmeal and fish oil derived from wild capture fisheries7,8,9, highlighting the need for alternatives to fishmeal as a protein source in aquaculture.
Protein can be produced through the cultivation of various microbes, which is generally denoted as microbial protein or single cell protein (SCP)10. A recent life cycle assessment (LCA) showed a lower environmental impact of SCP production compared to that of soybean meal manufacturing11. SCP represents a promising alternative to fishmeal as a protein source in aquaculture4, as it does not compete with the human food supply. In fact, it can transform the so-called “organic waste” from the human food industry into a valuable and sustainable animal feed ingredient. Thus, it may offer solutions for a wide variety of products and production approaches in aquaculture, but considerable research, development, and scale-up efforts are required to reach its potential12,13.
In particular, SCP grown on food processing wastewaters (FPWW) holds promise as a sustainable source of protein in animal feed14. Collectively, FPWW possess attractive features for SCP production, such as a continuous global production of process water rich in dissolved carbon (C), nitrogen (N) and phosphorus (P) compounds15. These wastewaters are also considered to be free of pathogens, heavy metals, and other harmful contaminants. FPWW can support microbial growth in bioreactors, from which cells can be recovered and dried leading to products suitable for animal feed ingredients, while concurringly avoiding the cost of treating such wastewaters, and hence represent an important step toward a circular bioeconomy16.
Traditionally, SCP production involves the use of single strains in axenic cultures, incurring high initial and operational costs. For example, a recent study used pure and enriched cultures of purple bacteria as a protein ingredient (5–11% protein replacement) in whiteleg shrimp nursery feed17. A promising alternative would be a whole-community approach for SCP production, leveraging the microorganisms already present in the wastewater14. Comparatively, microbial community-based SCP reduces the need for costly specific growth media, as well as the overall energy input required for SCP production. Microbial communities can be more robust and resilient to process fluctuations, leading to more stable SCP production. Further, the use of a microbial community may result in a more diverse and nutritionally rich SCP, as various microorganisms contribute different amino acids, vitamins, and other essential nutrients to the final product. A recent study showed that microbial community-based SCP produced directly from soybean processing wastewaters contained essential amino acids for fish as well as taxa with probiotic potential18. However, the feasibility of using such SCP as fish feed ingredient for aquaculture has yet to be established. Hence, the aim of this study was to evaluate the use of microbial community-based SCP, produced from soybean processing wastewater, as a value-added alternative ingredient to fishmeal for juvenile Asian seabass (Lates calcarifer) aquaculture.
Materials and methods
Fish source and experimental culture conditions
Unvaccinated Asian sea bass (Lates calcarifer, also known as barramundi) juveniles were obtained from a commercial hatchery (Allegro Aqua, Singapore). Fish were fed with a commercial sea bass diet (43% protein, Uni-President, Vietnam) for a period of 3 weeks to acclimate the fish to lab conditions prior to the start of the 24-day feeding trial. Juvenile sea bass of 19.7 ± 2.6 g were randomly distributed in rectangular glass aquaria (0.58 m × 0.58 m × 0.38 m, 100-L water volume) at a stocking density of 20 fish per aquaria. Subsequently, three aquaria were randomly assigned to each dietary treatment (n = 3). The glass aquaria were connected to a recirculating system with mechanical and biological filtration and aeration was supplied to each aquarium. Water temperature ranged from 27 to 29 °C. Other water quality parameters, i.e., dissolved oxygen (> 4 mg L−1), pH (7.0–7.9), salinity (15 ppt), ammonia (< 0.5 ppm) and nitrite (< 0.5 ppm) were kept within acceptable levels for the species. The study was approved by the Institutional Animal Care and Use Committee (IACUC) under number 202003-155A. All experiments were performed in accordance with relevant guidelines and regulations.
Experimental diets and feeding protocol
A control fishmeal-based (FM) diet and an experimental 50% fishmeal replacement diet with microbial community-based SCP (50SCP), were formulated to contain at least 45% protein and 12% fat (Table 1). These were prepared with 30% fishmeal (FM) and with 50% replacement of the fishmeal with SCP (50SCP), while adjusting the other ingredients to maintain the crude protein and fat levels (Table 1). The nutritional composition of both diets is detailed in Table 2. Diet preparation involved mixing the dry feed ingredients first, followed by the addition of the liquids, including 20% v/v water, in a stand mixer (Model 5 QT, KitchenAid, St Joseph, MI, USA). The resulting mash was passed through a meat grinder attached to the stand mixer and fitted with a 3-mm diameter die. The moist strings were dried overnight in a ventilated drying oven at 45 °C. The dry strings were hand-broken and stored in air-tight plastic containers at 4 °C until used. Both diets were fed twice a day (0900 h, 1600 h) to apparent satiation, and feed intake was measured daily. Fish were group-weighed at the beginning and individually at the end of the 24-day feeding trial.
Bioreactor setup and operational parameters for SCP production
To produce microbial SCP, four 4-L bioreactors were operated as sequencing batch reactors (SBRs) on continuous 12-h cycles with intermittent aeration for 136 days. Reactors received wastewater from a soybean processing company in Singapore as described in Vethathirri et al.18. Water temperature was maintained at 30 °C, and the sludge was continuously mixed at 375 rpm. The feeding phase occurred during the initial 5–10 min of a cycle, followed by 180 min anoxic/anaerobic and 540 min aerobic phases, after which the biomass was left to settle for 60 min and 1.35 L of the supernatant was discarded. Thereafter, the reactor was filled with the same volume of soybean wastewater, starting a new cycle. The pH was measured between 6.0 and 8.5, and the dissolved oxygen (DO) concentration was controlled between 0.2 and 0.5 mg L−1 during the aerobic phase. Each of the SBRs employed in this study was equipped with a magnetic stir plate to ensure mixed liquor homogeneity, a pair of EasySense pH and DO probes with their corresponding transmitters (Mettler Toledo), dedicated air and feed pumps, a solenoid valve for supernatant discharge, and a surrounding water jacket connected to a re-circulating water heater. The different portions of the cycle were controlled by computer software specifically designed for these reactors (VentureMerger, Singapore).
Chemical analyses of fish diets and tissue
The nutritional composition, i.e., proximate composition (moisture, crude protein, crude fat, crude fibre, ash), amino acids profile, fatty acids profile, calcium, phosphorus, selenium, zinc, iron, and vitamins A, D, and B12 content of the control and experimental diets, as well as fish tissue (one sample per group) were analyzed according to standard methods19 by BV-AQ laboratories (Singapore).
Microbial characterization of microbial community-based SCP
Three samples of 0.5 g each were collected randomly from a 2-L bottle containing 222 g of dry mixed-culture SCP to be used in the aquaculture trial. These were subjected to DNA extraction and as previously described20. Amplicon preparation was done by PCR using the primer set 341f/785r targeted the V3-V4 variable regions of the 16S rRNA gene21, as described in22. Bacterial 16S rRNA amplicon sequencing was done in two steps as described in Santillan et al.23. The libraries were sequenced on an Illumina MiSeq platform (v.3) with 20% PhiX spike-in and at a read-length of 300 bp paired-end. Sequenced sample libraries were processed following the DADA2 bioinformatics pipeline24. DADA2 allows inference of exact amplicon sequence variants (ASVs) providing several benefits over traditional clustering methods25. Illumina sequencing adaptors and PCR primers were trimmed prior to quality filtering. Sequences were truncated after 280 and 255 nucleotides for forward and reverse reads, respectively, the length at which average quality dropped below a Phred score of 20. After truncation, reads with expected error rates higher than 3 and 5 for forward and reverse reads, respectively, were removed. After filtering, error rate learning, ASV inference and denoising, reads were merged with a minimum overlap of 20 bp. Chimeric sequences (0.03% on average) were identified and removed. For a total of 3 samples, on average 26,426 reads were kept per sample after processing, representing 52% of the average input reads. Taxonomy was assigned using the SILVA database (v.138)26.
Growth performance and statistical analyses
The individual growth performance of the fish was evaluated by calculating the mean weight gain (mean final weight − mean initial weight), percent weight gain (mean weight gain × mean initial weight−1 × 100), specific growth rate (SGR = (lnmean final weight − lnmean initial weight) × trial duration−1 × 100), feed conversion ratio (FCR = mean feed intake × mean weight gain−1), and survival rate. Univariate analysis of growth performance parameters were done via Welch’s t-test assuming unequal variances in R (v.3.6.3). All reported P values for statistical tests in this study were corrected for multiple comparisons using a false-discovery rate (FDR) of 5%27. Heat maps for bacterial relative abundances of the SCP were constructed using the DivComAnalyses package (v.0.9) in R28.
Preprint
A previous version of this manuscript was published as a preprint.
Results and discussion
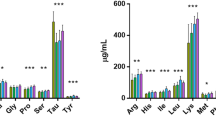
Both the control fishmeal-based diet (FM) and the microbial community-derived SCP experimental diet (SCP50) met the protein (> 45%) and fat (> 12%) requirements for juvenile Asian sea bass (Table 1). They were also formulated to provide essential amino acids to fish; however, at 18% and 14% in the FM control diet and 50SCP experimental diet, respectively, lysine was below the required level (Table 2). The remaining essential amino acid requirements were met, in agreement with a recent study by Vethathirri et al.18 where microbial community-derived SCP biomass produced from soy bean FPWW in lab-scale reactors was reported to fully or partially meet the essential amino acids requirements for fish and shrimp.
No significant differences in growth performance were observed between fish fed with the FM control and those that received the experimental microbial community-based 50SCP diet (Table 3). The initial weight of the fish in the group fed with the 50SCP experimental diet was significantly lower (Welch’s t-test Padj < 0.001) than the control, despite the initial random assignment, but no significant difference was observed in the weight gain at the end of the feeding trial (Welch’s t-test Padj = 0.30) (Table 3, Fig. 1). While a significant difference in initial fish weight between groups was possible due to the random assignment of individual fish and dietary treatments between tanks, this should not be a concern. The main variables analysed here were weight gain, feed conversion ratio (FCR), specific growth rate (SGR), and survival, all of which are independent of any differences in initial weights between the groups -which, again, were due to chance from random assignment-, but rather depend on weight gain. The feed intake of the group fed with the FM control diet was not significantly different from that of the group fed with the 50SCP mix experimental diet. Hence, no significant differences were observed for FCR and SGR. No mortality was observed during the feeding trial in both groups (Table 3). Furthermore, the group fed with the FM control diet presented a 4.4- and 10.3-fold greater variability in percent weight gain and FCR than the group fed with the 50SCP experimental diet, respectively, suggesting that an SCP replacement diet could also lead to more homogeneous growth of farmed Asian sea bass. Exploring the specific molecular and physiological mechanisms responsible for the observed homogeneous fish growth is a promising avenue for future research.
Weight gain of juvenile Asian sea bass (Lates calcarifer) fed with a fishmeal-based (FM) control diet or an experimental diet with 50% fishmeal replacement with microbial community-based SCP (50SCP) over 24 d. Panels represent (A) initial and final fish weight, and (B) percent weight gain per group. The box bounds the interquartile range (IQR) divided by the median, and Tukey-style whiskers extend to a maximum of 1.5 times the IQR beyond the box. Red diamonds display mean values. Grey dots represent values for each individual fish (n = 20) in each of three replicate tanks per group on d24. Welch’s t-test P values adjusted at 5% FDR shown within panels. Initial values (d1) are averages for each tank, calculated by simultaneously weighing all the fish in each tank and dividing by 20.
To our knowledge, ours is among the first fish feeding trials with microbial community-derived SCP in the feed at a relatively high fishmeal replacement level (50%) with microbial protein. Other studies have investigated the use of pure or enriched cultures of purple non-sulfur bacteria (PNSB) as aquafeed additives for the whiteleg shrimp (Litopenaeus vannamei) at protein replacement levels of 1.2–5.8%29 or 5–11%17, and for barramundi (Lates calcarifer) at 33–100% protein replacement levels30. These studies reported growth performances for different protein replacement levels that were comparable to that of commercial fishmeal17,30, as well as probiotic properties against Vibrio pathogens17,29. In our study, the nutritional composition of the fish tissue at the end of the trial was similar for the groups fed either FM control or 50SCP experimental diets in terms of crude protein, fat, most amino acids and fatty acids, as well as some vitamins and minerals (Table 4). Nevertheless, lower concentrations were observed in the fish tissue of the 50SCP experimental group for lysine (− 17.6%), vitamin A (− 68.8%), DHA (− 20.7%), EPA (− 16.4%), and total n-3 fatty acids (− 16.5%) than in the FM control group. Future studies should focus on refining SCP production processes (refer to Vethathirri et al.14 for design considerations), exploring dietary modifications, and assessing the long-term fish health implications. Evaluating the impact of SCP in feed on immune parameters and stress tolerance, alongside optimizing SCP production to increase essential amino acid content, can collectively enhance nutritional levels in aquafeeds.
A microbial community-based approach to build SCP has several potential advantages over axenic culturing14, such as a higher protein content due to synergistic interactions between different SCP-producing microorganisms and the utilization of various carbon and nitrogen sources available in the substrate18,31, the accumulation of intracellular components32, and process stability in terms of resistance and resilience to disturbances33,34,35. The microbial composition of the microbial community-based SCP employed in the experimental diet was dominated by the genera Acidipropionibacterium and Propioniciclava (Fig. 2), which have potential as probiotics and producers of valuable metabolites. The stability and dynamics of Acidipropionibacterium and Propioniciclava in microbial cultures can be influenced by factors such as pH, temperature, and nutrient availability36. The presence of these genera and others from the family Propionibacteriaceae in the composition of microbial community-based SCP used for aquafeeds provides several potential advantages. Members of Propionibacteriaceae exhibit versatile metabolic capabilities and can thus ferment a wide range of substrates, contributing to the efficient utilization of diverse organic compounds during SCP production37,38. Furthermore, many Propionibacteriaceae are known for their ability to produce propionate through anaerobic fermentation36. Propionic acid is commonly used as a preservative in food and feed due to its antimicrobial properties. Other potentially probiotic properties39 include a positive influence on the gut microbiota of aquatic species, contributing to improved health, disease resistance, and overall well-being in aquaculture.
Characterization of the microbial community-based SCP produced from soybean processing wastewaters, assessed through 16S rRNA gene amplicon sequencing. Heat map displaying the relative abundances of the (A) top 5 phyla and (B) top 20 genera in three separate samples of the SCP mixture used in the study.
Taxa within the Acidipropionibacterium genus (previously named Propionibacterium) have a broad range of applications in the pharmaceutical and food industries due to their ability to produce vitamin B12, bacteriocins, and trehalose40. Indeed, there was 12 times more vitamin B12 in the experimental diet compared to the control (Table 2). Further, the species Acidipropionibacterium acidipropionici, which was detected in the 50SCP experimental diet, is used in the food industry as either a bio-preservative or probiotic due to its generally-recognized-as-safe (GRAS) status41. Some of these potential properties associated with a subset of the microbial community-based SCP could have contributed to the observed more homogeneous growth performance, in terms of FCR and weight gain, of the fish fed with the experimental diet compared to those fed with the control diet (Table 3). For example, Alloul et al.17 hypothesized that PNSB would be able to colonize the gastrointestinal tract and assist the digestion of white leg shrimp given that, similar to our study, the microbial protein used was freeze-dried, which allows for bacteria to be revived. However, how the microbial communities present in the experimental SCP diet interact with the indigenous gut microbiota of juvenile Asian sea bass, and to what extent these interactions contribute to the observed growth performance and probiotic potential, remains unknown. As the current knowledge of microbial community-based SCP as protein replacement in fish feed is limited, more research is needed to understand the underlying mechanisms behind our observations.
Based on the results of this preliminary trial, 50% of the fishmeal protein might be replaced with microbial community-based SCP meal without negatively affecting Asian seabass growth or survival in the short term. Additional research is required to address potential long-term effects on fish health, growth, and overall sustainability of aquaculture production, and whether these effects vary as a function of fishmeal protein replacement. Examining growth trajectories and weight gain over extended periods would help in understanding how fish respond to microbial community-based SCP as an alternative dietary component. Long-term studies could assess the efficacy of different dosages of the SCP on the immune parameters of the fish in addition to their growth performance. Moreover, other fish species and sources of FPWW could also be explored. It has been shown that microbial community-based SCP grown on wastewaters from potato and starch, brewery or dairy industries meets several of the essential amino acid requirements of trout and shrimp as reviewed by Vethathirri et al.14 and recently demonstrated for soybean processing wastewater18. However, FPWW of variable chemical composition may also lead to different mixed SCP communities18, thus more research is needed for a broader validation and implementation of this approach.
Conclusions
This preliminary trial study showed that 50% of fishmeal protein might be replaced with microbial community-based SCP produced from soybean processing wastewater without affecting Asian seabass growth or survival, and that an SCP replacement diet may also lead to less variable fish growth than a traditional diet containing only fishmeal as protein source. Future studies should consider longer growth periods and higher fishmeal replacement levels, as well as additional aquaculture species and types of FPWW. Overall, we demonstrated that microbial community-based SCP has potential as an alternative value-added ingredient for aquaculture feed, which can help in the transition to a circular bioeconomy.
Data availability
DNA sequencing data are available at NCBI BioProjects PRJNA890376.
References
Ritala, A., Häkkinen, S. T., Toivari, M. & Wiebe, M. G. Single cell protein—State-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 8, 25 (2017).
Linder, T. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Secur. 11, 265–278 (2019).
EC,. Food 2030 Pathways for Action: Alternative Proteins and Dietary Shift (European Commission: Directorate-General for Research and Innovation, 2020).
Jones, S. W., Karpol, A., Friedman, S., Maru, B. T. & Tracy, B. P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 61, 189–197 (2020).
Boyd, C. E., McNevin, A. A. & Davis, R. P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 20, 20 (2022).
FAO-UN. The State of World Fisheries and Aquaculture 2020: Sustainability in Action (Food and Agriculture Organization of the United Nations, 2020).
Naylor, R. L. et al. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 106, 15103–15110 (2009).
Tacon, A. G. J., Metian, M. & McNevin, A. A. Future feeds: Suggested guidelines for sustainable development. Rev. Fish. Sci. Aquac. 30, 271–279 (2022).
Slater, M., D’Abramo, L. & Engle, C. R. Aquaculture research priorities for the next decade: A global perspective. J. World Aquac. Soc. 49, 3–6 (2018).
Anupama, X. & Ravindra, P. Value-added food: Single cell protein. Biotechnol. Adv. 18, 459–479 (2000).
Spiller, M. et al. Environmental impact of microbial protein from potato wastewater as feed ingredient: Comparative consequential life cycle assessment of three production systems and soybean meal. Water Res. 171, 115406 (2020).
Verstraete, W., Yanuka-Golub, K., Driesen, N. & De Vrieze, J. Engineering microbial technologies for environmental sustainability: Choices to make. Microb. Biotechnol. 15, 215–227 (2022).
Carter, C. G. & Codabaccus, M. B. Assessing the value of single-cell ingredients in aquafeeds. Curr. Opin. Biotechnol. 76, 102734 (2022).
Vethathirri, R. S., Santillan, E. & Wuertz, S. Microbial community-based protein production from wastewater for animal feed applications. Bioresour. Technol. 341, 125723 (2021).
Muys, M. et al. Dried aerobic heterotrophic bacteria from treatment of food and beverage effluents: Screening of correlations between operation parameters and microbial protein quality. Bioresour. Technol. 307, 123242 (2020).
Durkin, A., Guo, M., Wuertz, S. & Stuckey, D. C. Resource recovery from food-processing wastewaters in a circular economy: A methodology for the future. Curr. Opin. Biotechnol. 76, 102735 (2022).
Alloul, A. et al. Purple bacteria as added-value protein ingredient in shrimp feed: Penaeus vannamei growth performance, and tolerance against Vibrio and ammonia stress. Aquaculture 530, 735788 (2021).
Vethathirri, R. S., Santillan, E., Thi, S. S., Hoon, H. Y. & Wuertz, S. Microbial community-based production of single cell protein from soybean-processing wastewater of variable chemical composition. Sci. Total Environ. 873, 162241 (2023).
AOAC. Official Methods of Analysis 16th edn. (Association of Official Analytical Chemists, 1995).
Santillan, E., Seshan, H., Constancias, F. & Wuertz, S. Trait-based life-history strategies explain succession scenario for complex bacterial communities under varying disturbance. Environ. Microbiol. 21, 3751–3764 (2019).
Thijs, S. et al. Comparative evaluation of four bacteria-specific primer pairs for 16S rRNA gene surveys. Front. Microbiol. 8, 1–15 (2017).
Santillan, E. & Wuertz, S. Microbiome assembly predictably shapes diversity across a range of disturbance frequencies in experimental microcosms. NPJ Biofilms Microbiomes 8, 41 (2022).
Santillan, E., Phua, W. X., Constancias, F. & Wuertz, S. Sustained organic loading disturbance favors nitrite accumulation in bioreactors with variable resistance, recovery and resilience of nitrification and nitrifiers. Sci. Rep. 10, 1–11 (2020).
Callahan, B. J. et al. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Callahan, B. J., McMurdie, P. J. & Holmes, S. P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11, 2639–2643 (2017).
Glöckner, F. O. et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 261, 169–176 (2017).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. (Method.) 57, 289–300 (1995).
Constancias, F. & Sneha-Sundar. (Zenodo, 2022).
Chumpol, S. et al. Optimization of culture conditions for production of antivibrio compounds from probiotic purple nonsulfur bacteria against acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus and Vibrio spp.. Aquaculture 505, 72–83 (2019).
Delamare-Deboutteville, J. et al. Mixed culture purple phototrophic bacteria is an effective fishmeal replacement in aquaculture. Water Res. X 4, 100031 (2019).
Alloul, A., Muys, M., Hertoghs, N., Kerckhof, F.-M. & Vlaeminck, S. E. Cocultivating aerobic heterotrophs and purple bacteria for microbial protein in sequential photo- and chemotrophic reactors. Bioresour. Technol. 319, 124192 (2021).
Li, D., Yan, X., Li, Y., Ma, X. & Li, J. Achieving polyhydroxyalkanoate production from rubber wood waste using mixed microbial cultures and anaerobic–aerobic feeding regime. Int. J. Biol. Macromol. 199, 162–171 (2022).
Santillan, E., Constancias, F. & Wuertz, S. Press disturbance alters community structure and assembly mechanisms of bacterial taxa and functional genes in mesocosm-scale bioreactors. mSystems 5, e00471-e1420 (2020).
Santillan, E., Phua, W. X., Constancias, F. & Wuertz, S. Sustained organic loading disturbance favors nitrite accumulation in bioreactors with variable resistance, recovery and resilience of nitrification and nitrifiers. Sci. Rep. 10, 21388 (2020).
Santillan, E., Seshan, H. & Wuertz, S. Press xenobiotic 3-chloroaniline disturbance favors deterministic assembly with a shift in function and structure of bacterial communities in sludge bioreactors. ACS ES&T Water 1, 1429–1437 (2021).
Turgay, M. et al. In Reference Module in Food Science, Propionibacterium spp. and Acidipropionibacterium spp. (ed. Sad, D.) 1–12 (Elsevier, 2020).
Stackebrandt, E., Cummins, C. S. & Johnson, J. L. The Prokaryotes: Volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes. In Family Propionibacteriaceae: The Genus Propionibacterium (eds Martin, D. et al.) 400–418 (Springer, 2006).
Stackebrandt, E. In The Prokaryotes: Actinobacteria. The Family Propionibacteriaceae: Genera other than Propionibacterium (eds Eugene, R. et al.) 725–741 (Springer, 2014).
Rabah, H., Rosado Carmo, F. L. & Jan, G. Dairy propionibacteria: Versatile Probiotics. Microorganisms 5, 25 (2017).
Piwowarek, K., Lipińska, E., Hać-Szymańczuk, E., Kieliszek, M. & Ścibisz, I. Propionibacterium spp.—source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 102, 515–538 (2018).
Deptula, P. et al. Red-brown pigmentation of Acidipropionibacterium jensenii is tied to haemolytic activity and cyl-like gene cluster. Microorganisms 7, 512 (2019).
Tacon, A. G. J. The Nutrition and Feeding of Farmed Fish and Shrimp; A Training Manual 1: The Essential Nutrients 1–13 (FAO, 1987).
Funding
This research was supported by the Singapore National Research Foundation (NRF) and Ministry of Education under the Research Centre of Excellence Program, and the NRF Competitive Research Programme [NRF-CRP21-2018-0006] "Recovery and microbial synthesis of high-value aquaculture feed additives from food-processing wastewater".
Author information
Authors and Affiliations
Contributions
E.S., F.Y., D.C.P.S. and S.W. conceived the study and designed the experiment. S.W. and D.C.P.S. obtained the funding for the study. R.S.V. and E.S. produced the microbial protein. F.Y. performed the aquaculture trial. R.S.V. and S.S.T. did the laboratory chemical analyses during SCP production. H.Y.H. and E.S. performed the molecular work. E.S. did the bioinformatics analyses. E.S. and F.Y. interpreted the data, generated the results, and wrote the first draft of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santillan, E., Yasumaru, F., Vethathirri, R.S. et al. Microbial community-based protein from soybean-processing wastewater as a sustainable alternative fish feed ingredient. Sci Rep 14, 2620 (2024). https://doi.org/10.1038/s41598-024-51737-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51737-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.