Abstract
Childhood obesity is a global health concern affecting over 150 million children worldwide, with projections of a rise to 206 million by 2025. Understanding the mechanisms underlying this epidemic is crucial for developing effective interventions. In this study, we investigated circulating levels of Growth Differentiation Factor 10 (GDF10), a novel regulator of adipogenesis. Previous studies report diminished circulating GDF10 levels contribute to obesity and hepatic steatosis in mice. To further understand the role of plasma GDF10 in childhood obesity, a prospective case–control study was conducted. Using an enzyme-linked immunosorbent assay, plasma GDF10 levels were measured in children aged 5–17 years of age with normal (n = 36) and increased (n = 56) body mass index (BMI). Subsequently, plasma GDF10 levels were compared to various cardio-metabolic parameters. Children with increased BMI exhibit significantly lower levels of plasma GDF10 compared to children with normal BMI (p < 0.05). This study not only supports previous mouse data but is the first to report that lower levels of GDF10 is associated with childhood obesity, providing an important human connection for the relevance of GDF10 in obesity. Furthermore, this study revealed a significant correlation between low plasma GDF10 levels and elevated LDL-cholesterol and total cholesterol levels dependent on BMI (95% CI, p < 0.05). This study supports the hypothesis that children with obesity display lower plasma levels of GDF10, which correlates with elevated cholesterol levels. These insights shed light on potential mechanisms contributing to childhood obesity and may lead to future therapeutic interventions targeting GDF10 to mitigate adverse effects of adipogenesis in cardiometabolic health.
Similar content being viewed by others
Introduction
The high prevalence of obesity in children presents a significant global public health concern, with numerous adverse outcomes associated with these conditions1,2,3,4,5,6,7,8,9. In Canada, nearly 30% of children aged 5–17 are classified as overweight or obese, highlighting the urgency of addressing this issue3. Childhood obesity often persists into adulthood, as evidenced by the fact that 77% of children with obesity become adults with obesity10.
Adipogenesis is the process of cell differentiation by which pre-adipocytes become mature adipocytes capable of storing lipids. Dysregulation of the adipogenic program is associated with the early onset of obesity11. A secreted ligand of the transforming growth factor beta superfamily termed growth differentiation factor 10 (GDF10), also known as bone morphogenic protein-3b, is expressed in all adipose depots, although higher expression is observed in pre-adipocytes than in mature adipocytes. Several reports indicate that GDF10 secretion is responsible for blocking adipogenesis12,13,14,15,16. GDF10 secretion inhibits adipogenesis by suppressing key transcriptional factors, peroxisome proliferative factor receptor gamma (PPARγ) and CCAAT/enhancer-binding protein alpha15. GDF10’s ability to negatively regulate PPARγ is also effective in reducing lipid accumulation in human hepatocytes17.
A recent report has shown that high-fat diet can significantly reduce fibroadipogenic progenitor-derived GDF10 transcript levels in mice, directly leading to the induction of fat infiltration in a paracrine manner18. We and others have also reported that mice deficient in GDF10 are prone to increased weight gain when fed a regular chow or high-fat diet17,19. Mice deficient in GDF10 display increased whole body abdominal adiposity and adipocyte hypertrophy, independent of changes in food consumption. Moreover, these mice develop abnormal metabolic features including impaired fasting glucose, hyperinsulinemia and dyslipidemia marked by increased circulating plasma triacylglycerol compared to wild-type controls17. Loss of GDF10 contributes to pathological cardiac remodeling and elevated cardiovascular risk19. These observations highlight the emerging potential of GDF10 as a therapeutic target due to its inverse association with obesity in vivo.
Despite this growing body of evidence, to the best of our knowledge, plasma GDF10 levels has not been correlated with BMI in children. This study not only supports previous mouse data but is the first to reinforce the notion that lower levels of GDF10 is associated with increased BMI in childhood, providing an important human connection for the relevance of GDF10 in obesity. Finally, it strongly suggests that therapies aimed at increasing plasma GDF10 levels or the pathways that are activated by GDF10 may represent a new approach for the treatment and/or management of childhood obesity.
Materials/subjects and methods
Study population
Study population (n = 92) of both sexes aged 5–17 years old were recruited from the Children’s Exercise and Nutrition Centre at McMaster Children’s Hospital in Hamilton, Canada and during well-child visits in a Pediatrician’s office20. Written and informed consent was obtained from the legal guardian with child provided assent. This study was approved by The Research Ethics Board at Hamilton Health Sciences in accordance with the Declaration of Helsinki. Demographic information (age and sex) was collected by questionnaire. All measurements were taken at a single visit the morning after a minimum fasting period of 8 h. The study visit included anthropometric measures and collection of a fasting blood sample. All study data was saved into a database as deidentified information. Considering the World Health Organization (WHO) proposed body mass index (BMI) cut-off points for adolescent populations, normal BMI is defined as BMI-for-age < + 1SD, overweight as BMI-for-age > + 1SD, and obesity as BMI-for-age > + 2SD. Thus, in this study we categorized the children as normal BMI defined as BMI-for-age < + 1SD (n = 36) and increased BMI defined as children with a BMI-for-age > + 1SD above the mean (n = 56; 12 participants with BMI-for-age > + 1SD and 44 with BMI-for-age > + 2SD)21. The study excluded children under 5 years old or over 17 years old. The study excluded those receiving pharmacological treatment for obesity or obesity related complications based on the potential impact of health conditions and treatments that may influence food uptake among participants22.
Assessments
Anthropometric parameters were measured as previously described20. Briefly, height was measured using a Harpenden Stadiometre (London, UK). Weight was measured using a Tanita electronic scale. BMI (kg/m2) and BMI-Z score (World Health Organization) were calculated using NUTSTAT, a component of the EpiInfo program. Waist circumference was measured half-way between the iliac crest and lower rib23.
Metabolic parameters were measured as previously described20. Fasting glucose (minimum 8 h), total cholesterol, high density lipoprotein (HDL)-cholesterol, and triglyceride levels were assessed using the Roche analyzer as previously reported20. Low density lipoprotein (LDL)-cholesterol was calculated according to the Friedewald formula as previously reported20.
Human GDF10 measurement
Growth Differentiation Factor 10 ELISA Kit (Elabscience, Wuhan, China) was used to measure circulating GDF10 levels from plasma samples that had been stored in a – 80 °C freezer since collection and were limited to one freeze–thaw cycle as per the manufacturer’s protocol. The optimal detection range for GDF10 is 31.25–2000 pg/mL (sensitivity as low as 18.75 pg/mL). The intra- and inter-assay variations were less than 5%.
Statistics
GraphPad software was used to perform all statistical analyses. p < 0.05 is considered significant for all analyses.
Descriptive statistics of the population
Anthropometric and metabolic data were tested for normal distribution using skewness and kurtosis. Categorical traits are expressed as percentages (%) in Table 1. Comparisons of categorical traits were made between groups using the Chi-square test for independence and effect size was measured by odd’s ratio (Supplementary Table 1). Two-way ANOVA (Tukey’s multiple comparison) was performed to compare male and female age between the normal BMI and increased BMI group (Supplementary Fig. 1A). Comparison of continuous variables between the normal and increased BMI group were performed with a student’s independent T-test (two-tailed) (Table 1). Continuous variables include age (years), BMI (kg/m2), BMI-Z, waist to hip ratio, standardized waist to hip ratio, baseline weight, low density lipoprotein (LDL) cholesterol (mmol/L), high density lipoprotein (HDL) cholesterol (mmol/L), total cholesterol (mmol/L), triglycerides (mmol/L) and fasting glucose (mmol/L). Data is expressed as mean ± standard deviation for continuous traits.
Investigating the association of plasma GDF10 levels with baseline characteristics
Two-way ANOVA (Tukey’s multiple comparison) was performed to compare male and female plasma GDF10 levels between the normal BMI and increased BMI group (Supplementary Fig. 1B).
The correlation coefficients and probability values in Table 2 were calculated with Pearson product moment. A Pearson’s correlation heat map of continuous anthropometric and metabolic variables is presented in Supplementary Fig. 2A. Correlation of plasma GDF10 levels compared to LDL- and total cholesterol are shown as scatterplots in Supplementary Fig. 2B.
Multiple linear regression (least square estimates) was used to measure the association between plasma GDF10 levels (dependent variable) and the categorical trait for BMI classification (0-normal and 1-increased BMI) adjusted for age and sex (1-male, 2-female) (Table 3). Multiple linear regression (least square estimates) was used to test the association between GDF10 (dependent variable) with LDL-cholesterol and total cholesterol adjusted for age and sex, independent of BMI (Table 3). Anthropometric markers such as BMI, BMI-Z, height, weight, and waist-to-hip ratio demonstrate multicollinearity. Similarly, metabolic markers such as triglycerides, LDL-, HDL- and total cholesterol demonstrate multicollinearity.
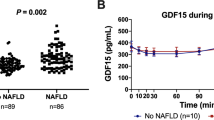
An independent student's T-test (two-tailed) was used to test the statistical significance of plasma GDF10 levels between normal BMI and increased BMI groups (Fig. 1). A simple logistic regression was used to calculate the odd’s ratio between plasma GDF10 levels and BMI groups (normal compared to increased BMI).
Results
Baseline characteristics of the study subjects
Table 1 presents the baseline characteristics of the study participants, comparing children with a normal BMI (n = 36) to those with an increased BMI (n = 56). No differences in sex distribution between the two groups were identified (Supplementary Table 1). Moreover, the ages of male and female participants did not significantly differ between normal BMI and increased BMI groups (Supplementary Fig. 1). Thus, age and sex were comparable between groups.
Consistent with group assignment, children in the increased BMI group exhibited significantly higher values for BMI, waist-to-hip ratio, weight, and BMI Z-score. However, there were no significant differences in height between the study groups. Children in the increased BMI group also had higher circulating levels of triglycerides, total cholesterol, and LDL-cholesterol and lower levels of HDL-cholesterol (Table 1). These findings are consistent with the expectation that children with obesity exhibit an at-risk lipid profile24,25,26,27. The levels of fasting glucose were within normal range and did not differ significantly between the normal and increased BMI groups.
Plasma GDF10 levels were similar in males and females in both the normal BMI and increased BMI groups suggesting limited effect of sex (Supp Fig. 1B). Table 2 provides correlations between plasma GDF10 levels and various anthropometric and metabolic parameters. Although there was a trend towards a negative correlation between plasma GDF10 levels and several anthropometric measures including BMI, BMI Z-score (WHO), waist circumference, hip circumference, and body weight, these correlations were not statistically significant (Table 2).
Plasma GDF10 levels are significantly reduced in children with increased BMI and inversely associated with LDL- and total cholesterol in a BMI-dependent manner
Plasma GDF10 levels were inversely correlated with total cholesterol (correlation coefficient: − 0.232, p = 0.026) and LDL-cholesterol (correlation coefficient: − 0.227, p = 0.03) (Table 2) in univariate analysis (Supp. Fig. 2). Multiple linear regression analyses indicate that GDF10 is negatively associated with LDL- and total cholesterol when adjusted for age and sex, however, this significant association is dependent on BMI (Table 3).
Plasma GDF10 levels are significantly reduced in children with increased BMI compared to normal BMI (p < 0.012) (Fig. 1). Multiple linear regression tested the relationship between plasma GDF10 levels as a continuous trait associated with BMI classification (Table 3). BMI classification is a significant predictor of plasma GDF10 levels when adjusted for age and sex (unadjusted analyses also demonstrate a significant association), the higher classification assigned to increased BMI negatively correlates with plasma GDF10 levels (p < 0.01) (Table 3). Cumulatively, this data suggests a relationship between lower plasma GDF10 levels and adiposity regulation.
Discussion
Lower plasma GDF10 levels in youth with increased BMI
Accumulating evidence demonstrates the ability of GDF10 to negatively regulate adiposity by suppressing key adipogenic transcription factors in rodent models15,16,17,28. Understanding the mechanistic role of GDF10 secretion in obesity is of paramount importance as it offers valuable insights into the underlying processes involved in adiposity regulation. This study reports significantly reduced plasma GDF10 levels in children with increased BMI compared to those with normal BMI. These findings suggest that lower GDF10 secretion may play a role in the development and progression of obesity or that plasma levels of GDF10 are reduced in the context of obesity.
This study is the first to report the inverse correlation between plasma GDF10 levels and LDL-cholesterol and total cholesterol levels in children. The relationship between plasma GDF10 levels and cholesterol metabolism is dependent on BMI. These findings are consistent with the reported loss of GDF10 resulting in dyslipidemia and hypercholesterolemia in mouse models of cardiovascular disease and obesity19. Following a high-fat meal, single-nucleotide polymorphisms of GDF10 was linked to blood pressure loci and shown to impact lipid metabolism29,30. GDF10’s protective role against dyslipidemia and hypercholesterolemia may be attributed to its inhibitory effect on PPARγ as previously shown15,16,17. Further research is warranted to elucidate the causal relationship between GDF10-mediated inhibition of PPARγ and cholesterol metabolism independent of obesity.
While the study provides initial insights into the association between plasma GDF10 levels, childhood obesity, and circulating lipids, it is important to acknowledge study limitations. These limitations include small sample size, limited age range, and a higher proportion of males than females. These factors restrict generalizability and increase the likelihood of random variations and reduced statistical power, which may limit the ability to draw definitive conclusions. The study focused on a specific age range, which only include children and youth, without participants from other age groups. Consequently, the findings may not fully represent the broader population, and the observed associations may differ in different age groups, such as adults or older individuals. Future studies should aim to include a wider age range in a larger and more diverse cohort, to validate and expand upon the association between plasma GDF10 levels and obesity, as measured by BMI.
Conclusion
To our knowledge, this is the first study providing compelling evidence that children with high BMI exhibit lower plasma levels of GDF10, compared to those with normal BMI. Specifically, the observed BMI-dependent inverse association between plasma GDF10 levels and LDL-cholesterol and total cholesterol levels highlights the potential role of GDF10 in regulating lipid metabolism within the context of childhood obesity. Further research should examine the intracellular processing and secretion of GDF10 specifically in children, recognizing that these mechanisms may differ from those observed in adults. A comprehensive understanding of the mechanisms underlying GDF10 secretion and its impact on obesity will potentially identify novel targets for interventions aimed at mitigating the adverse effects of childhood obesity. Developing interventions that specifically modulate GDF10 expression or activity could improve BMI reduction, cholesterol management, and overall metabolic health in affected children.
However, further research is necessary to elucidate the mechanisms by which GDF10 regulates metabolism and determine the most effective strategies for targeting this protein. Clinical trials and preclinical studies are warranted to evaluate the safety, efficacy, and feasibility of GDF10-based therapies in children with high BMI and high cholesterol.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- BMI:
-
Body mass index
- GDF10:
-
Growth differentiation factor 10
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- SMAD:
-
Mothers against decapentaplegic
References
(NCD-RisC), N. R. F. C. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387, 1377–1396 (2016).
Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311, 806 (2014).
Roberts, K. C., Shields, M., de Groh, M., Aziz, A. & Gilbert, J.-A. Overweight and obesity in children and adolescents: Results from the 2009 to 2011 Canadian Health Measures Survey. Heal. Rep. 23, 37–41 (2012).
Skinner, A. C. & Skelton, J. A. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 168, 561 (2014).
Twells, L. K., Gregory, D. M., Reddigan, J. & Midodzi, W. K. Current and predicted prevalence of obesity in Canada: A trend analysis. C. Open 2, E18–E26 (2014).
Lipnowski, S. & LeBlanc, C. Healthy active living: Physical activity guidelines for children and adolescents. Paediatr. Child Health 17, 209–210 (2012).
Zahn, K. et al. Variability in how Canadian pediatric weight management clinics deliver care: Evidence from the CANadian pediatric weight management registry. Child. Obes. 17, 420–426 (2021).
Small, L. & Aplasca, A. Child obesity and mental health. Child Adolesc. Psychiatr. Clin. N. Am. 25, 269–282 (2016).
Tevie, J. & Shaya, F. T. Association between mental health and comorbid obesity and hypertension among children and adolescents in the US. Eur. Child Adolesc. Psychiatry 24, 497–502 (2015).
Freedman, D. S., Khan, L. K., Dietz, W. H., Srinivasan, S. R. & Berenson, G. S. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: The Bogalusa Heart Study. Pediatrics 108, 712–718 (2001).
Desai, M., Beall, M. & Ross, M. G. Developmental origins of obesity: Programmed adipogenesis. Curr. Diab. Rep. 13, 27–33 (2013).
Zachara, M. et al. Mammalian adipogenesis regulator (Areg) cells use retinoic acid signalling to be non- and anti-adipogenic in age-dependent manner. EMBO J. https://doi.org/10.15252/embj.2021108206 (2022).
Schwalie, P. C. et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 (2018).
Camps, J. et al. Interstitial cell remodeling promotes aberrant adipogenesis in dystrophic muscles. Cell Rep. 31, 107597 (2020).
Hino, J., Miyazawa, T., Miyazato, M. & Kangawa, K. Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex. Int. J. Obes. 36, 725–734 (2012).
Hino, J. et al. Overexpression of bone morphogenetic protein-3b (BMP-3b) in adipose tissues protects against high-fat diet-induced obesity. Int. J. Obes. 41, 483–488 (2017).
Platko, K. et al. GDF10 blocks hepatic PPARγ activation to protect against diet-induced liver injury. Mol. Metab. 27, 62–74 (2019).
Choo, H., Kim, S.-H. & Du, D. Fibroadipogenic progenitor-derived Gdf10 regulates neuromuscular junction and fat content in tongue muscles. Physiology https://doi.org/10.1152/physiol.2023.38.S1.5730069 (2023).
Martí-Pàmies, Í. et al. Deficiency of bone morphogenetic protein-3b induces metabolic syndrome and increases adipogenesis. Am. J. Physiol. Metab. 319, E363–E375 (2020).
Morrison, K. M. et al. Cardiovascular risk factors and non-invasive assessment of subclinical atherosclerosis in youth. Atherosclerosis 208, 501–505 (2010).
Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 95, 76–85 (2007).
Cuda, S. et al. Medication-induced weight gain and advanced therapies for the child with overweight and obesity: An Obesity Medicine Association (OMA) clinical practice statement 2022. Obes. Pillars 4, 100048 (2022).
Fernández, J. R., Redden, D. T., Pietrobelli, A. & Allison, D. B. Waist circumference percentiles in nationally representative samples of African–American, European–American, and Mexican–American children and adolescents. J. Pediatr. 145, 439–444 (2004).
Friedland, O., Nemet, D., Gorodnitsky, N., Wolach, B. & Eliakim, A. Obesity and lipid profiles in children and adolescents. J. Pediatr. Endocrinol. Metab. https://doi.org/10.1515/JPEM.2002.15.7.1011 (2002).
Leopold, S. & Zachariah, J. P. Pediatric obesity, hypertension. Lipids. Curr. Treat. Options Pediatr. 6, 62–77 (2020).
Brzeziński, M., Metelska, P., Myśliwiec, M. & Szlagatys-Sidorkiewicz, A. Lipid disorders in children living with overweight and obesity—large cohort study from Poland. Lipids Health Dis. 19, 47 (2020).
Riaño-Galán, I. et al. Proatherogenic lipid profile in early childhood: Association with weight status at 4 years and parental obesity. J. Pediatr. 187, 153-157.e2 (2017).
Ahn, J., Wu, H. & Lee, K. Integrative analysis revealing human adipose-specific genes and consolidating obesity loci. Sci. Rep. 9, 3087 (2019).
Takeuchi, F. et al. Interethnic analyses of blood pressure loci in populations of East Asian and European descent. Nat. Commun. 9, 5052 (2018).
Wojczynski, M. K. et al. Genome-wide association study of triglyceride response to a high-fat meal among participants of the NHLBI Genetics of Lipid Lowering Drugs and Diet Network (GOLDN). Metabolism 64, 1359–1371 (2015).
Acknowledgements
This study was supported in part by a research grant from the Canadian Institutes of Health Research (CIHR) to RCA (FRN#148539). Financial support from The Institute of St. Joe’s Hamilton and Amgen Canada is acknowledged. RCA is a Career Investigator of the Heart and Stroke Foundation of Ontario and holds the Amgen Canada Research Chair in the Division of Nephrology at St Joseph’s Healthcare and McMaster University. Funding of Dr. Morrison’s cohort was provided by the Canadian Institutes of Health Research (FRN 123606), McMaster Children’s Hospital Foundation, Population Health Research Institute, Faculty of Health Science McMaster University.
Author information
Authors and Affiliations
Contributions
T.R.Y. and A.M.B. contributed to the study design and researched data. T.R.Y. wrote the manuscript. T.R.Y., A.M.B., K.M.M. and R.C.A. edited the manuscript. T.R.Y., A.M.B., K.M.M. and R.C.A. contributed to data interpretation and discussion and reviewed the manuscript. R.C.A. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yousof, T.R., Mejia-Benitez, A., Morrison, K.M. et al. Reduced plasma GDF10 levels are positively associated with cholesterol impairment and childhood obesity. Sci Rep 14, 1805 (2024). https://doi.org/10.1038/s41598-024-51635-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51635-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.