Abstract
Improvement of prenatal identification of large-for-gestational-age (LGA) infants could lower the risk for adverse outcomes. Therefore, we sought to evaluate the association of a combination of maternal waist circumference (WC) and abdominal fat depths with infant birth size. A cohort study including 1240 women was performed between 2015 and 2018 at Uppsala University Hospital, Sweden. Maternal WC was measured at the first antenatal visit, and visceral (VF) and subcutaneous (SCF) fat depths by ultrasound at the second-trimester anomaly scan. Waist circumference, VF, and SCF were categorized as low or high (cut-offs WC ≥ 88 cm, VF ≥ 54 mm, SCF ≥ 21 mm). Outcomes were birth weight standard deviation score (BWSDS) and LGA (BWSDS > 90th and > 97th percentile). Secondary outcome was small-for-gestational-age (SGA, BWSDS < 10th and < 3rd percentile). Univariate analysis of variance and logistic regression analyses were performed adjusted for maternal weight, height, parity, smoking, country of birth, pregestational diabetes, and chronic hypertension. For both high and low WC, high VF was positively associated with BWSDS and LGA. There was no association with SGA. The results did not demonstrate any value of the combination of WC and fat depth measures in predicting infant birth size but suggested VF as a marker for large infants.
Similar content being viewed by others
Introduction
Being born large-for-gestational-age (LGA), often defined as a birthweight > 90th percentile after adjustments for gestational age and sex1, is associated with adverse pregnancy outcomes for both the mother and the infant. For the mother, giving birth to an LGA-infant increases the risk for emergency caesarian section, perineal injuries, and postpartum bleeding2. The LGA-infant is at increased risk of shoulder dystocia, plexus brachialis injury2, low Apgar score3, and neonatal hypoglycemia4. Being born LGA might also have long-term consequences, as it is associated with malignancies in childhood5, obesity6, diabetes mellitus5,6, breast cancer, cardiovascular disease, and psychiatric disorders5.
Several maternal factors are associated with increased fetal growth and high birth weight, such as overweight and obesity, excessive pregnancy weight gain7, and diabetes mellitus8,9. Previous studies have suggested that prenatal identification of LGA-fetuses, which enables appropriate interventions at delivery, could lower the risk for adverse outcomes3,10. Hence, early detection of LGA-infants is of high clinical importance, and improving methods for early identification of LGA-fetuses is necessary.
Maternal BMI is a recognized predictor of infant birth weight and is used in routine care for risk stratification of pregnant women. However, BMI may be insufficient as an individual risk predictor because it does not directly estimate adiposity11. In fact, BMI has high specificity but low sensitivity in identifying individuals with excess body fat; only 50% of non-pregnant individuals with adiposity-related risk are identified by BMI12. Waist circumference (WC) is suggested as an independent risk marker of cardiometabolic complications due to its ability to target individuals with central adiposity, i.e. increased visceral fat mass. Waist circumference measurement is recommended to identify non-pregnant individuals with the highest risk of obesity-related complications13.
Maternal WC correlates with infant birth size14,15, and an independent association between early mid-pregnancy visceral fat depth and birth weight has previously been reported by our group16. We hypothesize that a combination of early pregnancy WC and early mid-pregnancy fat depth measures, especially visceral fat, could be used to predict increased infant birth weight. This population-based cohort study, including 1240 women and child-dyads, sought to evaluate the value of the combination of early pregnancy WC and early mid-pregnancy ultrasound estimated abdominal fat depths in predicting infant birth size.
Methods
Study population
From January 2015 to December 2017, WC measurement was implemented as a clinical routine at the first antenatal visit in Uppsala County, Sweden. A total of 5827 pregnant women underwent WC measurement during this period. In addition, measurement of visceral fat depth (VF) and subcutaneous fat depth (SCF) was implemented as a clinical routine at the second-trimester anomaly scan at Uppsala University Hospital, Sweden. It was a coincidence whether the scan included fat depth measurement since the personnel booking the scans was not involved in the study. During the study period, 2844 women underwent a scan including fat depth measurements17. Eligible participants for this cohort study were women who had undergone both WC and fat depth measurements (n = 1366).
A research database including WC and fat depth measurements was created. Information on maternal age, maternal weight at the first antenatal visit, maternal height, smoking at the first antenatal visit, maternal country of birth, chronic illness, infant birth weight, gestational age, and sex was linked from the Medical Birth Register held by the Swedish National Board of Health and Welfare. Following linkage, the study population database was pseudo-anonymized.
Exposures
Waist circumference was measured with a standardized measurement tape between the lower rib margin and the iliac crest with the woman standing18. The midwives who measured WC received verbal and written information repeatedly during the study period on how to perform measurements. We defined a low WC measure as < 88 cm and a high as ≥ 88 cm. This cut-off was selected based on the WHO-suggested WC cut-off for (non-pregnant) women, where WC ≥ 88 cm implies a substantially increased risk for metabolic complications associated with obesity18. In addition, this WC cut-off has been used by others in the same research field19,20.
Maternal VF and SCF were measured at the second-trimester anomaly scan at 18–19 weeks’ gestation, as first described by Armellini et al.21, with a minor adjustment regarding the placement of the probe. The measurements were performed with a GE Voluson E6, E8, or E10 ultrasound machine (GE Medical Systems, Zipf, Austria) with the woman in the supine position. The ultrasound probe was placed at the body’s midline 10 cm above the umbilicus. The VF was defined as the distance between the inner border of the rectus abdominis muscle and the anterior border of the aorta, and the SCF was defined as the distance between the dermis and the surface of the rectus abdominis muscle. Both fat depths were measured in millimeters. All midwives who performed the measurements were certified obstetric ultra-sonographers. Additional training sessions were held throughout the study period to maximize the scan quality. The intraclass correlation coefficient of the inter-examiner variation was 0.83 for VF and 0.85 for SCF, indicating good reliability22. Visceral fat depth and SCF were categorized in quartiles (VF quartile 1–4 and SCF quartile 1–4). Quartile 4 was defined as high (VF ≥ 54 mm and SCF ≥ 21 mm), and quartiles 1–3 as low (VF < 54 mm and SCF < 21 mm).
Main outcomes
Infant birth size was evaluated as birth weight standard deviation score (BWSDS) and LGA. The BWSDS is a population-based z-score and was calculated by the use of national reference standards for birth weight with respect to gestational age and sex23. We used two definitions of LGA: BWSDS above the 90th percentile and BWSDS above the 97th percentile (used as a proxy for + 2 standard deviations, the clinical definition of LGA used in Sweden). We also evaluated small-for-gestational-age (SGA) as a secondary outcome. Two definitions of SGA were used: BWSDS below the 10th percentile and BWSDS below the 3rd percentile (used as a proxy for − 2 standard deviations, the clinical definition of SGA used in Sweden).
Ethical approval
The study was approved by the Regional Ethical Review Board in Uppsala (Dnr: 2014/353 and 2015/366). All research was performed in accordance with relevant national and international guidelines. Informed consent was waived by the Swedish Ethical Review Authority (Dnr: 2019–00391).
Statistics
The Welch ANOVA test, followed by the Games-Howell post-hoc test, was used to evaluate WC, VF, and SCF in relation to BMI classes since the assumption of homogeneity of variances was not met. Pearson correlation coefficients were calculated to examine the associations between BMI, WC, VF, and SCF.
To evaluate the value of different combinations of central adiposity measures in predicting infant birth size, the cohort was divided into eight groups based on WC and fat depths (Table 1). The low-risk group (low WC/low VF/low SCF) was the reference. Univariate analysis of variance was performed to evaluate the difference in BWSDS between the low-risk group and the other seven groups. The analysis was adjusted for maternal weight at the first antenatal visit (kg), maternal height (cm), parity (nulliparous or parous), smoking at the first antenatal visit (yes or no), maternal country of birth (EU or outside EU), pregestational diabetes (yes or no), and chronic hypertension (yes or no). Covariates were selected based on previous prediction models for large infants24,25.
Logistic regression was used to evaluate differences in the odds of giving birth to an LGA or SGA infant between the low-risk group and the other seven groups. The same covariates as described above were included in the models. All data were analyzed using IBM SPSS Statistics version 28. The statistical significance level was set at p < 0.05.
Results
Out of 1366 women with both WC and fat depth measurements, 126 women were excluded from further analysis due to missing information on BMI at first antenatal visit (n = 11), multiple pregnancy (n = 2), intrauterine fetal death (n = 3), missing data from the delivery (n = 30), WC measurement not from current pregnancy (n = 32), WC measured < 5 or > 16 weeks’ gestation (n = 45), and error value of BMI, VF or SCF measure (n = 3). The final study population consisted of 1240 women who gave birth to singleton infants between 8 July 2015 and 2 September 2018. The women were 15–45 years old, 559 (45.1%) were nulliparous, and 515 (41.5%) were either overweight or obese. A WC ≥ 88 cm was observed in 333 women (26.9%). The study population characteristics are presented in Table 2.
Waist circumference and fat depth measures in relation to BMI
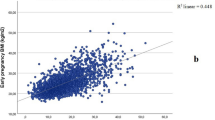
The WC measurements were obtained at a mean of 64 days’ gestation (standard deviation (SD) 15 days). The vast majority (91%) of the women had their WC measured < 12 weeks’ gestation. Overall, WC ranged 60–150 cm, VF 2–108 mm, and SCF 1–52 mm. Waist circumference, VF, and SCF measures in relation to WHO BMI classes are presented in Supplementary Table S1. All three adiposity measures increased with increasing BMI (Supplementary Fig. S1, panel A–C).
Waist circumference and BMI were highly correlated (r = 0.84)26 (Supplementary Table S2). A low correlation was seen between VF and BMI (r = 0.44), whereas SCF and BMI were moderately correlated (r = 0.67) (Supplementary Table S2). There was a low correlation between WC and VF (r = 0.41) and a moderate correlation between WC and SCF (r = 0.62) (Supplementary Table S2).
Associations of the combination of WC and fat depths with BWSDS
In comparison with the low-risk group, increased BWSDS was observed in the low WC/high VF/low SCF group (mean difference 0.23, CI 0.03 to 0.44, p = 0.025), and in the high WC/high VF/low SCF group (mean difference 0.42, CI 0.10 to 0.73, p = 0.010) (Table 3).
Associations of the combination of WC and fat depths with LGA and SGA
In comparison with the low-risk group, there was an increase in the odds of giving birth to an infant LGA (defined as BWSDS > 90th percentile) in the following groups: low WC/high VF/low SCF (odds ratio (OR) 2.20, CI 1.21 to 4.05, p = 0.010), low WC/high VF/high SCF (OR 4.04, CI 1.58 to 10.31, p = 0.004), high WC/high VF/low SCF (OR 2.47, CI 1.11 to 5.50, p = 0.027), and high WC/high VF/high SCF (OR 2.40, CI 1.06 to 5.44, p = 0.037) (Table 4). In the analyses evaluating the stricter definition of LGA (BWSDS > 97th percentile), the odds were increased in the same groups, and the estimates were higher (Table 5). In the analyses evaluating SGA (BWSDS < 10th percentile and < 3rd percentile), there were no significant results (Supplementary Table S3 and Supplementary Table S4).
Discussion
This is, to the best of our knowledge, the first study to evaluate the value of a combination of early pregnancy WC and early mid-pregnancy ultrasound estimated fat depths in predicting infant birth size. Infants of mothers with a high VF, regardless of WC and SCF measures, had higher odds of LGA compared with infants of mothers in the low-risk group. Additionally, the BWSDS was higher among infants of mothers with high VF and low SCF, independent of WC. These findings indicate that VF is a stronger predictor for large infants than WC or SCF. Interestingly, the highest odds of LGA were observed among women with low WC and high fat depth measures. This group might include women with an unhealthy metabolic profile despite a low WC. Women with a low WC are likely to have a low BMI since these measures are highly correlated. From a clinical perspective, using ultrasound to detect VF might have the greatest value in women with low BMI, as these are not otherwise identified as at risk.
Our results are in line with a previous study reporting a positive association of first trimester VF, but not WC, with birth weight centile27. Other previous studies have studied either WC or VF as proxies for central adiposity in relation to infant outcomes. Of seven previous studies evaluating the association between WC and infant birth size, six report a positive association with birth weight, LGA, and macrosomia (≥ 4000 g)14,15,20,28,29,30. These results are different from ours, we did not find any association between WC and birth size when a combination of central adiposity measures was evaluated. One could speculate that WC might predict large infants in a model not including VF. When both measures are available, as in our study, VF seems to be superior to WC. Our results are also supported by the findings of three previous studies reporting a positive association between maternal VF, measured by ultrasound, and infant birth size16,27,31.
We used ultrasound to measure the fat depths, but the gold standard methods for examination of intra-abdominal fat mass are CT and MRI32. However, for abdominal fat distribution assessment during pregnancy, ultrasound is a more feasible method for several reasons. First, pregnant women are already being examined by a trained ultrasonographer at the routine antenatal ultrasound scan, and fat depth measurements could easily be implemented at this time point with no need for further health care visits. Second, ultrasound is more accessible and less expensive than CT and MRI. Third, ultrasound does not involve any radiation, which could be harmful to the fetus. Abdominal ultrasound is a reliable and reproducible method for examination of intra-abdominal fat mass; a correlation coefficient of 0.81 (p < 0.001) between ultrasound and CT measures has been reported, indicating a strong association33.
Waist circumference measurement also has advantages. It is a cheap, easy, and fast method for body fat distribution assessment that could be implemented in routine care of pregnant women, especially in low-resource settings. However, we only found a weak correlation between early pregnancy WC and early mid-pregnancy VF, indicating that WC is not a good proxy for visceral fat accumulation in pregnant women.
The possible causal pathways linking maternal visceral fat accumulation to increased birth weight are not fully elucidated, but insulin resistance and hyperglycemia could be partly responsible. Early pregnancy visceral fat thickness correlates positively with diastolic blood pressure and levels of insulin, blood glucose, triglycerides, and cholesterol34. The physiology of normal pregnancy includes peripheral insulin resistance and hyperlipidemia35, and excessiveness of these normal metabolic changes among pregnant women with central obesity could possibly underpin the association between visceral fat and high birth weights.
This cohort study had strengths and limitations. Limitations included that the number of study participants was small in some of the groups, which might have lowered the power of the study. Yet another limitation was that the WC and fat depths were measured only once. A strength was that the study cohort was population-based. To ensure that the WC measure belonged to the current pregnancy and to avoid the impact of the growing uterus, we only included women with a WC measure obtained > 5 and < 16 weeks’ gestation (91% had their WC measured < 12 weeks’ gestation). This range is applicable since there is no relation between WC measured at 6‒16 weeks’ gestation and gestational length36. In addition, WC is considered to be generally unaffected by the pregnancy until 20 weeks’ gestation, when the uterus reaches the umbilical level37. Furthermore, the timing of the VF measurement in our study (18‒19 weeks’ gestation) was unlikely prone to bias, as there is no significant difference in visceral fat thickness between the first trimester (8–12 weeks’ gestation) and the second trimester (24–27 weeks’ gestation)38. Yet another strength of this study was the outcome birth size. Birth weight was measured at the hospital in a standardized way soon after delivery. It was also beneficial that we used a standardized score taking gestational length and infant sex into account, which otherwise could have biased the results.
Conclusions
This study did not show any predictive value of the combination of WC and fat depth measures on increased birth size but suggested VF as a marker for this outcome. Further studies are required to confirm our results. It could be valuable to evaluate possible cut-off points for VF, perhaps with respect to BMI classes, in relation to outcomes. The adiposity measure VF might improve the ability to identify pregnant women with the most hazardous obesity phenotype and to target health care interventions to those with the greatest risk for complications.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cartwright, R. D. et al. Neonatal morbidity and small and large size for gestation: A comparison of birthweight centiles. J. Perinatol. 40, 732–742. https://doi.org/10.1038/s41372-020-0631-3 (2020).
Beta, J. et al. Maternal and neonatal complications of fetal macrosomia: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 54, 308–318. https://doi.org/10.1002/uog.20279 (2019).
Jolly, M. C., Sebire, N. J., Harris, J. P., Regan, L. & Robinson, S. Risk factors for macrosomia and its clinical consequences: A study of 350,311 pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 111, 9–14. https://doi.org/10.1016/s0301-2115(03)00154-4 (2003).
Groenendaal, F., Elferink-Stinkens, P. M., Netherlands Perinatal Registry. Hypoglycaemia and seizures in large-for-gestational-age (LGA) full-term neonates. Acta Paediatr. 95, 874–876. https://doi.org/10.1080/08035250500544948 (2006).
Magnusson, A. et al. The association between high birth weight and long-term outcomes-implications for assisted reproductive technologies: A systematic review and meta-analysis. Front. Pediatr. 9, 675775. https://doi.org/10.3389/fped.2021.675775 (2021).
Johnsson, I. W., Haglund, B., Ahlsson, F. & Gustafsson, J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr. Obes. 10, 77–83. https://doi.org/10.1111/ijpo.230 (2015).
Mayer, C. & Joseph, K. S. Fetal growth: A review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet. Gynecol. 41, 136–145. https://doi.org/10.1002/uog.11204 (2013).
O’Sullivan, E. P. et al. Atlantic Diabetes in Pregnancy (DIP): The prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia 54, 1670–1675. https://doi.org/10.1007/s00125-011-2150-4 (2011).
Billionnet, C. et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 60, 636–644. https://doi.org/10.1007/s00125-017-4206-6 (2017).
Boulvain, M. et al. Induction of labour versus expectant management for large-for-date fetuses: A randomised controlled trial. Lancet 385, 2600–2605. https://doi.org/10.1016/s0140-6736(14)61904-8 (2015).
National Clinical Guideline Centre. in Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults: Partial Update of CG43 (National Institute for Health and Care Excellence (NICE). Copyright © National Clinical Guideline Centre, 2014., 2014).
Okorodudu, D. O. et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. (Lond.) 34, 791–799. https://doi.org/10.1038/ijo.2010.5 (2010).
Ross, R. A.-O. X. et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. (2020).
Harville, E. W., Juonala, M., Viikari, J. S. & Raitakari, O. T. Preconception metabolic indicators predict gestational diabetes and offspring birthweight. Gynecol. Endocrinol. 30, 840–844. https://doi.org/10.3109/09513590.2014.937336 (2014).
Gao, X. et al. The mutual effect of pre-pregnancy body mass index, waist circumference and gestational weight gain on obesity-related adverse pregnancy outcomes: A birth cohort study. PLoS One 12, e0177418. https://doi.org/10.1371/journal.pone.0177418 (2017).
Lindberger, E. et al. Association of maternal central adiposity measured by ultrasound in early mid pregnancy with infant birth size. Sci. Rep. 10, 19702. https://doi.org/10.1038/s41598-020-76741-8 (2020).
Lindberger, E. et al. Associations of ultrasound estimated early mid pregnancy visceral and subcutaneous fat depths and early pregnancy BMI with adverse neonatal outcomes. Sci. Rep. 11, 4612. https://doi.org/10.1038/s41598-021-84045-8 (2021).
World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. (2008).
Pétursdóttir Maack, H. et al. Waist circumference measurement for prediction of preeclampsia: A population-based cohort study. Am. J. Hypertens. 35, 200–206. https://doi.org/10.1093/ajh/hpab156 (2022).
Mehrabi, E., Kamalifard, M., Yavarikia, P. & Ebrahimi Mameghani, M. The relation between early pregnancy anthropometric indices among primiparous women and macrosomia. J. Caring Sci. 1, 153–158. https://doi.org/10.5681/jcs.2012.022 (2012).
Armellini, F. et al. The contribution of sonography to the measurement of intra-abdominal fat. J. Clin. Ultrasound 18, 563–567. https://doi.org/10.1002/jcu.1870180707 (1990).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163. https://doi.org/10.1016/j.jcm.2016.02.012 (2016).
Marsal, K. et al. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 85, 843–848. https://doi.org/10.1111/j.1651-2227.1996.tb14164.x (1996).
Frick, A. P., Syngelaki, A., Zheng, M., Poon, L. C. & Nicolaides, K. H. Prediction of large-for-gestational-age neonates: Screening by maternal factors and biomarkers in the three trimesters of pregnancy. Ultrasound Obstet. Gynecol. 47, 332–339. https://doi.org/10.1002/uog.15780 (2016).
Poon, L. C., Karagiannis, G., Stratieva, V., Syngelaki, A. & Nicolaides, K. H. First-trimester prediction of macrosomia. Fetal Diagn. Ther. 29, 139–147. https://doi.org/10.1159/000318565 (2011).
Mukaka, M. M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 24, 69–71 (2012).
Jarvie, E. M. et al. Maternal adipose tissue expansion, a missing link in the prediction of birth weight centile. J. Clin. Endocrinol. Metab https://doi.org/10.1210/clinem/dgz248 (2020).
Madhavan, A., Beena Kumari, R. & Sanal, M. G. A pilot study on the usefulness of body mass index and waist hip ratio as a predictive tool for gestational diabetes in Asian Indians. Gynecol. Endocrinol. 24, 701–707. https://doi.org/10.1080/09513590802444134 (2008).
Li, S. et al. Central adiposity and other anthropometric factors in relation to risk of macrosomia in an African American population. Obesity (Silver Spring Md.) 21, 178–184. https://doi.org/10.1002/oby.20238 (2013).
Hancerliogullari, N. et al. Correlation of maternal neck/waist circumferences and fetal macrosomia in low-risk Turkish pregnant women, a preliminary study. Fetal Pediatr. Pathol. 40, 181–188. https://doi.org/10.1080/15513815.2019.1675831 (2021).
Yusuf Ibrahim, A., Park, A. L., Berger, H. & Ray, J. G. Maternal visceral adipose tissue and risk of having a small or large for gestational age infant. J. Obstet. Gynaecol. Can. 43, 973–977. https://doi.org/10.1016/j.jogc.2020.11.019 (2021).
Shuster, A., Patlas, M., Pinthus, J. H. & Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 85, 1–10. https://doi.org/10.1259/bjr/38447238 (2012).
Stolk, R. P. et al. Validity and reproducibility of ultrasonography for the measurement of intra-abdominal adipose tissue. Int. J. Obes. Relat. Metab. Disord. 25, 1346–1351. https://doi.org/10.1038/sj.ijo.0801734 (2001).
Bartha, J. L. et al. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring, Md.) 15, 2233–2239. https://doi.org/10.1038/oby.2007.265 (2007).
Lain, K. Y. & Catalano, P. M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 50, 938–948. https://doi.org/10.1097/GRF.0b013e31815a5494 (2007).
Sattar, N. et al. Antenatal waist circumference and hypertension risk. Obstet. Gynecol. 97, 268–271. https://doi.org/10.1016/s0029-7844(00)01136-4 (2001).
Salem, W., Adler, A. I., Lee, C. & Smith, G. C. Maternal waist to hip ratio is a risk factor for macrosomia. BJOG 119, 291–297. https://doi.org/10.1111/j.1471-0528.2011.03167.x (2012).
Kinoshita, T. & Itoh, M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: The ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol. Obstet. Invest. 61, 115–118. https://doi.org/10.1159/000089456 (2006).
Acknowledgements
We would like to express our gratitude and appreciation to the midwives who performed the waist circumference measurements at the first antenatal visit and the fat depth measurements at Uppsala University Hospital.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
E.L., F.A., A.-K.W., and I.S.P. came up with the idea. E.L., F.A., A.-K.W., and I.S.P. planned the study. E.L., F.A., K.J., A.-K.W., and I.S.P. analyzed the results. E.L. was the primary author of the manuscript. F.A., K.J., A.-K.W., and I.S.P. critically revised the manuscript and contributed with important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lindberger, E., Ahlsson, F., Junus, K. et al. Combined maternal central adiposity measures in relation to infant birth size. Sci Rep 14, 725 (2024). https://doi.org/10.1038/s41598-024-51274-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51274-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.