Abstract
This study aimed to develop and validate a predictive model to determine the risk of in-hospital mortality in patients with acute paraquat poisoning. This retrospective observational cohort study included 724 patients with acute paraquat poisoning whose clinical data were collected within 24 h of admission. The primary outcome was in-hospital mortality. Patients were randomly divided into training and validation cohorts (7/3 ratio). In the training cohort, the least absolute shrinkage and selection operator regression models were used for data dimension reduction and feature selection. Multivariate logistic regression was used to generate a predictive nomogram for in-hospital mortality. The prediction model was assessed for both the training and validation cohorts. In the training cohort, decreased level of consciousness (Glasgow Coma Scale score < 15), neutrophil-to-lymphocyte ratio, alanine aminotransferase, creatinine, carbon dioxide combining power, and paraquat plasma concentrations at admission were identified as independent predictors of in-hospital mortality in patients with acute paraquat poisoning. The calibration curves, decision curve analysis, and clinical impact curves indicated that the model had a good predictive performance. It can be used on admission to the emergency department to predict mortality and facilitate early risk stratification and actionable measures in clinical practice after further external validation.
Similar content being viewed by others
Introduction
Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride [PQ]) is a non-selective contact herbicide widely used in many countries since the 1960s1. Acute PQ poisoning (APP) has been associated with suicides and accidents, with PQ causing sequential organ damage/failure through oxidative stress and systemic inflammatory responses. The most prominent manifestations include acute lung and kidney injuries2. The mortality rate of patients with APP remains tremendously high (50–90%)3,4,5,6. Consequently, PQ has been banned in most countries, including China. However, PQ is still legally used in some areas7,8.
Early assessment of the severity and prognosis of patients with APP is crucial to guide treatment (hemodilution, immunosuppressive therapy)9,10. Currently, no standardized method exists for predicting the prognosis of APP. Previous studies have demonstrated the utility of the Severity Index of PQ Poisoning (SIPP) as a specific scoring system for prognosticating APP11. However, calculating the SIPP score necessitates the measurement of serum PQ concentration, which demands costly equipment. Consequently, its usage is limited in most countries, particularly in developing nations12,13. Furthermore, research indicates that the SIPP score might overestimate the survival rate of patients with APP14, especially those admitted to the hospital more than 24 h after poisoning15. Prior research has identified complete blood cell count16, liver and kidney function indicators17, serum anion gap18,19, and chest computed tomography20 as independent prognostic indicators for APP. Nevertheless, these indicators are relatively individualistic and demonstrate limited predictive power. The currently available scoring systems for predicting patient prognosis, including the Acute Physiology and Chronic Health Evaluation II (APACHE II) score21, Sequential Organ Failure Assessment (SOFA) score22, and Poisoning Severity Score (PSS)23, are designed for critically ill patients rather than individuals with low exposure or mild symptoms.
Furthermore, due to the intricate calculation process, these scoring systems are incapable of promptly predicting the mortality rate or conducting risk assessments for PQ poisoning patients24. Accordingly, it is imperative to develop an efficient, straightforward, and universally applicable predictive model based on commonly employed laboratory indicators. A nomogram is a simple multivariate prediction model that incorporates multiple variables affecting prognosis to calculate an individual’s survival probability25. Therefore, this study aims to develop a nomogram model based on clinical data collected within 24 h of admission that can identify, at an early stage, patients who are at high risk of in-hospital mortality due to APP.
Methods
Setting
We performed a retrospective, observational cohort study in an emergency department (ED) with more than 3000 beds in an extensive tertiary care teaching hospital in Chengdu City, Sichuan Province, China, per the amended Declaration of Helsinki. The West China Hospital approved the study of the Sichuan University Biomedical Research Ethics Committee (No. 2022-1591). Due to the retrospective nature of the study, the need of informed consent was waived by the Sichuan University Biomedical Research Ethics Committee. All analyses were performed following the Transparent Reporting of a multivariate prediction model for Individual Prognosis or Diagnosis statement26.
Patients
We conducted a study involving patients diagnosed with APP who were admitted to the ED of West China Hospital, Sichuan University, between September 1, 2010, and January 31, 2022. Inclusion criteria comprised of patients aged ≥ 14 years, those with poisoning via gastrointestinal intake, and individuals with PQ plasma concentrations ≥ 0.01 mg/L by high-performance liquid chromatography. Exclusion criteria encompassed patients whose PQ concentrations were not detected in the plasma, those with incomplete clinical data, those with other concomitant poisoning (such as alcohol poisoning), and pregnant women.
For patients who developed symptoms within 6 h of onset, gastric lavage was recommended. Those who developed symtoms within 12 h of onset were administered activated charcoal via oral or nasogastric route. Patients with positive concentrations of PQ in blood tests were advised to undergo blood purification treatment, including blood perfusion, hemodialysis, or continuous venovenous hemofiltration. After excluding contraindications such as gastrointestinal bleeding, all patients should be given methylprednisolone sodium succinate at a dosage of 80 mg/day for anti-inflammatory treatment through intravenous infusion27.
Predictors
We collected anonymous clinical data within 24 h of admission to the Emergency Department (ED) from electronic medical records. The collected data comprised the following variables: time from poisoning to treatment at the study site, sex, age, level of consciousness (LOC), heart rate (HR), respiration rate (RR), systolic blood pressure (SBP), diastolic blood pressure (DBP), white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet count (PLT), total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine, cystatin C, carbon dioxide combining power (CO2CP), blood potassium, plasma PQ concentrations, and SIPP. Carbon dioxide combining power (CO2CP) measures a substance or solution's ability to react with and bind carbon dioxide (CO2) molecules. It quantifies the substance's capacity to combine with CO2 chemically. The normal range of CO2CP typically falls between 22 and 30 milliequivalents per liter (mEq/L)28. Decreased LOC is defined as a Glasgow Coma Scale (GCS) score below 1529. The severity of PQ poisoning was estimated quantitatively by using the SIPP, which was calculated by multiplying the elapsed time (hours) from ingestion to arrival by the serum PQ level(μg/ml)11. All patient data were anonymized and de-identified. Variables were treated as continuous variables, except for sex and disturbance of consciousness, which were binary variables. Finally, the continuous variables used to construct the nomogram were transformed into categorical variables based on the actual data. This was achieved by categorizing them based on the normal upper and lower bounds, median, tertiles, and quartiles.
Clinical outcome
The patients were divided into two groups: survivor and non-survivor group, according to the occurrence of in-hospital death. The in-hospital mortality discussed in this study refers to all-cause mortality of APP patients, including deaths in the ED.
Statistical analysis
Data were analyzed using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value < 0.05 indicated statistical significance. Data are presented as medians (interquartile ranges) for continuous variables and as numbers (percentages) for categorical variables, as appropriate. The non-parametric Mann–Whitney U test, chi-square analysis, and Fisher’s exact test were used to test for differences between groups, as appropriate. In this study, grouping was achieved through simple random non-relaxation sampling using the simple_ra() function in the R software. All the baseline characteristics were comparable between the training and validation cohorts.
In the training cohort, the least absolute shrinkage and selection operator (LASSO) regression was used to decrease the potential collinearity of variables assessed from the same patient and the overfitting of variables. The latent variables in the LASSO regression analysis were included in the multivariate analysis of stepwise forward selection to determine independent risk factors affecting in-hospital mortality. The results are reported as odds ratios (ORs) and 95% confidence intervals (95% CI). A simple nomogram based on the independent risk factors was developed to predict the probability of mortality. The prediction model was assessed using the concordance index (C-index), area under the receiver operating characteristic (ROC) curve (AUC), calibration curves, decision curve analysis (DCA), and clinical impact curves for both the training and validation cohorts30,31,32.
Results
Patients
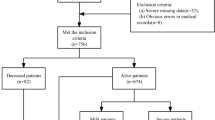
A total of 1708 patients with APP presented to our ED, of whom 984 were excluded. Finally, 724 patients who met the inclusion criteria in this study were randomly divided into two groups: training and validation cohorts group using a 7:3 ratio (Fig. 1); among them, 360/724 patients (49.7%) died. In terms of demographics, there were 325/724 (44.9%) males, with a median age of 33 (23–42) years. Among 506/724 patients in the training group, 44.1% were male, with a median age of 29 (22–42) years, and 253/506 (50%) patients died. Of the 218/724 patients in the validation group, 46.8% were male, with a median age of 31 (23–42) years, and 107/218 (49.1%) patients died. No statistical significant differences were observed between the two groups (P > 0.05) (Table 1).
Model development
In the demographic and clinical characteristics analysis of 506 patients, 22 features were reduced to nine potential predictive features. These features had non-zero coefficients in the LASSO regression model, which included age, LOC, WBC count, NLR, MLR, ALT, creatinine, CO2CP, and plasma PQ concentrations (Fig. 2a,b).
Demographic and clinical feature selection using the LASSO binary logistic regression model. (a) Optimal parameter (λ) selection in the LASSO model used 10-fold cross-validation via the minimum criteria. A partial likelihood deviance (binomial deviance) curve was plotted against log(λ). Dotted vertical lines are drawn at the optimal values using the minimum criteria and 1SE of the minimum criteria (1-SE criteria). (b) LASSO coefficient profiles of nine features. A coefficient profile plot was constructed against log(λ) sequence. A vertical line was drawn at the value selected using 10-fold cross-validation, where the optimal lambda resulted in eight features with non-zero coefficients. LASSO least absolute shrinkage and selection operator, SE standard error.
Construction of nomogram
Multivariate analysis revealed several independent risk factors for in-hospital mortality upon admission, including decreased LOC, NLR, ALT, creatinine, CO2CP, and plasma PQ concentrations. The detailed ORs and 95% CI of the multivariate analysis are summarized in Table 2.
Therefore, these six factors were used to construct the prediction model, as shown in Table 2. A simple nomogram was developed to predict the probability of death based on the independent risk factors (Fig. 3a). The NLR values were categorized into quartiles as follows: category 1 for NLR < 6.2, category 2 for NLR 6.2–10.94, category 3 for NLR 10.94–18.02, and category 4 for NLR > 18.02. ALT values were categorized based on the upper limit of normal as category 1 for ALT 0–39 IU/L and category 2 for ALT ≥ 40 IU/L. Additionally, categorical variables were created based on the median value of creatinine, resulting in category 1 for creatinine ≤ 110 μmol/L and category 2 for creatinine > 110 μmol/L. Finally, PQ values were categorized into quartiles: category 1 for PQ ≤ 0.33 g/mL, category 2 for PQ 0.33–1.66 g/mL, category 3 for PQ 1.66–11.15 g/mL, and category 4 for PQ > 11.15 g/mL. As shown in the nomogram, each factor was assigned a point, and the total nomogram points were calculated by summing the individual points of all predictors. The relationship between the total points and the probability of death is presented at the bottom of the nomogram. As an example, CO2CP was considered as a continuous variable. Where a decrease of 5 units corresponded to an approximate 35 points increase in the risk score. The nomogram depicts the predicted probability of in-hospital death resulting from APP, measured on a scale of 0 to 300. Draw a vertical line upward and assign labels to signify the respective points for each covariate. Repeated this procedure for each covariate to ascertain the cumulative.
(a) Nomogram for in-hospital mortality in patients with acute paraquat poisoning. Decreased LOC (GCS score < 15) (0, no; 1, yes), NLR (1, < 6.2; 2, 6.2–10.94; 3, 10.94–18.02; 4, > 18.02), ALT (1, 0–39; 2, ≥ 40 IU/L), CREA (1, ≤ 110; 2, > 110 µmol/L), PQ (1, ≤ 0.33; 2, 0.33–1.66; 3, 1.66–11.15; 4, ≥ 11.15 g/mL). (b) Calibration curve of the nomogram in the training set. (c) Calibration curve in the validation set. LOC level of consciousness, GCS glasgow coma scale, NLR neutrophil-to-lymphocyte ratio, ALT alanine aminotransferase, CREA creatinine, CO2CP carbon dioxide combining power, PQ paraquat.
Assessment of nomogram
The calibration curve in Fig. 3b,c suggests high consistency, demonstrating that the model’s predicted probabilities are close to the observed actual probabilities. The bias-corrected C-indices for the training and validation cohorts were 0.933 and 0.947, respectively.
In the training and validation cohorts, the C-indices were 0.953 (95% CI 0.936–0.970) and 0.947 (95% CI 0.920–0.974), respectively, indicating excellent accuracy. The ROC curves and AUC for the training and validation cohorts are shown in Fig. 4a,b. The DCA demonstrated that the nomogram had a superior overall net benefit across a wide range of practical threshold probabilities (Fig. 5a,b). In addition, we plotted clinical impact curves to predict improved probability stratification for a population size of 1000. The predicted probability coincided with the actual probability in the training and validation cohorts (Fig. 5c,d).
Construction and comparison of other predictive models
Since plasma PQ concentrations accounted for much of the model and some primary hospitals could not detect them, we used LASSO and multivariate logistic regression to construct another model without PQ plasma concentrations. Finally, we determined the following independent risk factors: LOC, age, WBC count, MLR, ALT Plasma PQ concentrations, and CO2CP. Concurrently, we included the SIPP to compare the effectiveness of the three prediction models and construct the ROC curve (Fig. 6).
Comparison of other predictive models. m1, nomogram of predictive models, AUC: 0.953 (95% CI 0.936–0.970); m2, predictive model excluding plasma PQ concentrations, AUC: 0.882 (95% CI 0.853–0.911); m3, SIPP, AUC: 0.909 (95% CI 0.883–0.934); AUC area under the receiver operating characteristic curve, SIPP severity index of paraquat.
Discussion
Timely identification of the severity of patients with PQ poisoning is of utmost importance in clinical practice. It assists in formulating targeted treatment plans, allocating medical resources efficiently, and potentially enhancing patient prognosis. Nevertheless, currently, no universally accepted method exists to comprehensively evaluate the correlation between clinical indicators and the survival outcomes of patients with APP. This study bridges this gap by developing a prediction model that evaluates the risk of in-hospital mortality using clinical data collected during admission. The created nomogram exhibited exceptional performance in both the training and validation cohorts, with an AUC exceeding 0.9. The included indicators can be readily acquired upon admission, and the nomogram score can be calculated with ease. These characteristics render the model appropriate for swift and uncomplicated clinical implementation, facilitating early-stage evaluation and prognosis prediction. Its convenience further encourages widespread adoption. Additionally, based on our comprehension, the sample size employed in this study is one of the largest worldwide, and the constructed model has achieved near-perfect prediction performance. Moreover, after comparing the nomogram to SIPP, we have ascertained the superiority of our prediction model.
Prior nomogram models developed to predict the prognosis of APP patients primarily originated from the research conducted by Lu Shan. They retrospectively included a total of 80 cases of patients with APP, utilizing serological markers and imaging examinations to construct their nomogram. The AUC in their training set and validation set were 0.953 (95% CI 0.936–0.970) and 0.947 (95% CI 0.920–0.974), respectively. In comparison to their study, our current study encompasses an expanded sample size, a wider array of accessible indicators and demonstrates exceptional predictive capacity.
Our study revealed a high mortality rate in patients with decreased LOC due to APP, possibly related to toxic encephalopathy or hyperemia. Previous research has reported that PQ can stimulate glutamate efflux, leading to excitotoxicity33. Animal studies have demonstrated PQ’s ability to induce α-synuclein upregulation, promote aggregate formation, and activate microglial34.
The NLR is a readily detectable inflammation marker using routine blood tests. It has been used to assess inflammatory-related lesions such as tumors, ischemic stroke, and coronary artery disease35,36,37. Neutrophils are important members of the human immune system that have gradually gained attention for their role in APP. Studies have shown that PQ can induce rapid expression of neutrophil chemoattractant proteins in bone marrow mesenchymal stem cells. Consequently, neutrophils rapidly increase in the blood and accumulate in the lungs, promoting the production of chemokines and pro-inflammatory factors (such as tumor necrosis factor α and interleukin 8) and activating the inflammatory response. Simultaneously, alveolar macrophages generated by neutrophils initiate immune responses and generate reactive oxygen species, leading to cellular nicotinamide adenine dinucleotide phosphate (reduced coenzyme II) depletion and cell membrane lipid peroxidation, thereby promoting the expression of pro-fibrotic genes in fibroblasts and resulting in pulmonary fibrosis38,39. Lymphocytes play a central role in regulating inflammatory responses in the human body. However, the specific mechanism through which PQ induces lymphocyte degradation remains unclear. Whether PQ inhibits the cellular immune function requires further investigation.
Our study showed that liver injury was an independent risk factor for death in patients with APP, which contradicts the findings of previous studies showing that toxic hepatitis is common after PQ exposure. Toxic hepatitis appears mild and transient in scope but is associated with higher complication rates, including respiratory and renal failure40.
PQ penetration into tissues and organs can cause a series of oxidative stress reactions, generating a large amount of reactive oxygen species and leading to organ damage or failure41. The kidney is the main excretory organ for PQ, eliminating 90% of PQ within 12–24 h. Consequently, patients with APP often present with acute kidney injury characterized by elevated creatinine42. In addition, renal dysfunction can significantly reduce the PQ excretion rate and increase its accumulation in the body, forming a vicious circle43,44. Further studies are needed to confirm whether correcting kidney injury improves prognosis.
CO2CP refers to the plasma CO2 content measured after isolating plasma from venous blood samples at room temperature and balancing it with the alveolar air of healthy people45. Two major acid–base disturbances, respiratory acidosis and metabolic alkalosis, both of which can result in increased CO2CP, are common in patients with respiratory diseases. If respiratory diseases such as chronic obstructive pulmonary diseases are excluded, the impact of the respiratory acid–base balance on CO2CP can be minimized46. In contrast, reduced CO2CP concentrations suggest metabolic acidosis47 or respiratory alkalosis48, both of which are indicators of poor outcomes. Most often, decreased CO2CP indicates the presence of metabolic acidosis; however, it could also reflect a decline in bicarbonate concentration as compensation for respiratory alkalosis. Therefore, it is considered a broad indicator of lung and kidney damage. In this study, the CO2CP of the mortality group was significantly lower than that of the survival group (P < 0.05). As the lungs and kidneys are important target organs of APP, this index can be regarded as a comprehensive evaluation index.
Considering that some primary hospitals cannot detect plasma PQ concentrations, we constructed a predictive model for plasma concentrations without PQ and compared it with SIPP. The second model showed the AUC of 0.882 (95% CI 0.853–0.911). Although the performance of the second model is not as good as that of the first, it is still worth promoting.
The current study has some limitations. First, it was a single-center retrospective study with selection bias; furthermore, only 724 patients were included because of missing data, which may limit the scalability of the model. Second, this study only included the clinical data of patients with APP at the time of enrollment and did not combine the clinical data after treatment to evaluate patient survival after discharge, which may have affected the results. Owing to limitations in clinical data, the GCS classification in this study was restricted to < 15/15. However, in future studies, we aim to collect more comprehensive data to improve nomogram precision. Moreover, because of the severe lack of clinical data and absence of indicators of lung injury, this study was performed retrospectively. The model must be updated when more multicenter data become available.
Conclusion
We developed a predictive model for in-hospital mortality in patients with APP. The nomogram, which includes six risk factors with favorable predictive accuracy, discrimination, and clinical utility, allows for simple and rapid individual patient risk estimates. It can be used on admission to the ED to predict mortality and facilitate early risk stratification and actionable measures in clinical practice after further external validation.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to data confidentiality. However, they can be obtained from the corresponding author upon reasonable request.
References
Wilks, M. F. et al. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med. 5, e49 (2008).
Gawarammana, I. B. & Buckley, N. A. Medical management of paraquat ingestion. Br. J. Clin. Pharmacol. 72, 745–757 (2011).
Wu, W.-P. et al. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: A nationwide study. PLoS One 9, e87568 (2014).
Gao, J. et al. Prolonged methylprednisolone therapy after the pulse treatment for patients with moderate-to-severe paraquat poisoning: A retrospective analysis. Medicine (Baltimore) 96, e7244 (2017).
Tan, J. T. et al. Paraquat poisoning: Experience in hospital taiping (year 2008–October 2011). Med. J. Malaysia 68, 384–388 (2013).
Wang, W. J. et al. Sequential organ failure assessment in predicting mortality after paraquat poisoning: A meta-analysis. PLoS One 13, e0207725 (2018).
Gheshlaghi, F. et al. Prediction of mortality and morbidity following paraquat poisoning based on trend of liver and kidney injury. BMC Pharmacol. Toxicol. 23, 67 (2022).
Li, C. et al. Treatment outcome of combined continuous venovenous hemofiltration and hemoperfusion in acute paraquat poisoning: A prospective controlled trial. Crit. Care Med. 46, 100–107 (2018).
Wang, Y. et al. Clinical effect of haemoperfusion combined with continuous veno-veno haemofiltration in treatment of paraquat poisoning: A meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 31, 214–220 (2019).
Hsu, C.-W. et al. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS One 7, e48397 (2012).
Sawada, Y. et al. Severity index of paraquat poisoning. Lancet (London, England) 1(8598), 1333 (1988).
Proudfoot, A. T. et al. Paraquat poisoning: Significance of plasma-paraquat concentrations. Lancet (London, England) 2(8138), 330–332 (1979).
Senarathna, L. et al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM Mon. J. Assoc. Phys. 102(4), 251–259 (2009).
Xu, S. et al. APACHE score, severity index of paraquat poisoning, and serum lactic acid concentration in the prognosis of paraquat poisoning of Chinese patients. Pediatr Emerg Care 31(2), 117–121 (2015).
Suzuki, K. et al. Evaluation of severity indexes of patients with paraquat poisoning. Hum. Exp. Toxicol. 10(1), 21–23 (1991).
Chen, H. et al. An effective machine learning approach for prognosis of paraquat poisoning patients using blood routine indexes. Basic Clin. Pharmacol. Toxicol. 120(1), 86–96 (2017).
Gheshlaghi, F. et al. Prediction of mortality and morbidity following paraquat poisoning based on trend of liver and kidney injury. BMC Pharmacol. Toxicol. 23(1), 67 (2022).
Hu, L. et al. A new machine-learning method to prognosticate paraquat poisoned patients by combining coagulation, liver, and kidney indices. PloS One 12(10), e0186427 (2017).
Zhao, Y. et al. Serum anion gap at admission as a predictor of the survival of patients with paraquat poisoning: A retrospective analysis. Medicine 99(31), e21351 (2020).
Lu, S. et al. Development and validation of a radiomics nomogram for prognosis prediction of patients with acute paraquat poisoning: A retrospective cohort study. BioMed Res. Int. 2021, 6621894 (2021).
Huang, J. et al. The value of APACHE II in predicting mortality after paraquat poisoning in Chinese and Korean population: A systematic review and meta-analysis. Medicine 96(30), e6838 (2017).
Wang, W. J. et al. Sequential organ failure assessment in predicting mortality after paraquat poisoning: A meta-analysis. PloS One 13(11), e0207725 (2018).
Persson, H. E. et al. Poisoning severity score grading of acute poisoning. J. Toxicol. Clin. Toxicol. 36(3), 205–213 (1998).
Wang, J. et al. Identify the early predictor of mortality in patients with acute paraquat poisoning. BioMed Res. Int. 2020, 8894180 (2020).
Iasonos, A. et al. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 26, 1364–1370 (2008).
Lu, S. et al. Development and validation of a radiomics nomogram for prognosis prediction of patients with acute paraquat poisoning: A retrospective cohort study. Biomed. Res. Int. 2021, 6621894 (2021).
Li, C. et al. Treatment outcome of combined continuous venovenous hemofiltration and hemoperfusion in acute paraquat poisoning: A prospective controlled trial. Crit. Care Med. 46(1), 100–107 (2018).
Huang, P.-F. et al. Predictive value of admission CO2 combining power combined with serum sodium for the prognosis in acute Stanford type A aortic dissection patients. Sci. Rep. 13(1), 1048 (2023).
Savonius, O. et al. PCR-confirmed malaria among children presenting with a decreased level of consciousness in Angola: A prospective, observational study. Malar. J. 22(1), 130 (2023).
Caetano, S. J., Sonpavde, G. & Pond, G. R. C-statistic: A brief explanation of its construction, interpretation and limitations. Eur. J. Cancer 90, 130–132 (2018).
Alba, A. C. et al. Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA 318, 1377–1384 (2017).
Vickers, A. J. & Elkin, E. B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 26, 565–574 (2006).
Shi, C. et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 34, 590–601 (2011).
Yang, C.-J. et al. Spectrum of toxic hepatitis following intentional paraquat ingestion: Analysis of 187 cases. Liver Int. 32, 1400–1406 (2012).
Nouri, A., Heibati, F. & Heidarian, E. Gallic acid exerts anti-inflammatory, anti-oxidative stress, and nephroprotective effects against paraquat-induced renal injury in male rats. Naunyn Schmiedebergs Arch. Pharmacol. 394, 1–9 (2021).
Sun, B. & He, Y. Paraquat poisoning mechanism and its clinical treatment progress. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 29, 1043–1046 (2017).
Mohamed, F. et al. Mechanisms underlying early rapid increases in creatinine in paraquat poisoning. PLoS One 10, e0122357 (2015).
Kim, S.-J. et al. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol. Dial. Transpl. 24, 1226–1232 (2009).
Proudfoot, A. T. et al. Paraquat poisoning: Significance of plasma-paraquat concentrations. Lancet 2, 330–332 (1979).
Hart, T. B., Nevitt, A. & Whitehead, A. A new statistical approach to the prognostic significance of plasma paraquat concentrations. Lancet 2, 1222–1223 (1984).
Scherrmann, J. M. et al. Prognostic value of plasma and urine paraquat concentration. Hum. Toxicol. 6, 91–93 (1987).
Jones, A. L., Elton, R. & Flanagan, R. Multiple logistic regression analysis of plasma paraquat concentrations as a predictor of outcome in 375 cases of paraquat poisoning. QJM 92, 573–578 (1999).
Yamamoto, I. et al. Correlating the severity of paraquat poisoning with specific hemodynamic and oxygen metabolism variables. Crit. Care Med. 28, 1877–1883 (2000).
Li, Y., Zhang, H. & Zhang, G. Early white blood cell count in predicting mortality after acute paraquat poisoning: A meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 31, 1013–1017 (2019).
Li, S. et al. The significance of CO2 combining power in predicting prognosis of patients with stage II and III colorectal cancer. Biomark. Med. 13, 1071–1080 (2019).
Hu, J. et al. Metabolic acidosis as a risk factor for the development of acute kidney injury and hospital mortality. Exp. Ther. Med. 13, 2362–2374 (2017).
Gunnerson, K. J. et al. Lactate versus non-lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. Crit. Care 10, R22 (2006).
Shirakabe, A. et al. Clinical significance of acid-base balance in an emergency setting in patients with acute heart failure. J. Cardiol. 60, 288–294 (2012).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82072156) and the Science and Technology Department of Sichuan Province (Grant No. 2022YFS0273).
Author information
Authors and Affiliations
Contributions
G.T. performed all data analysis. G.T. and J.Z. wrote the main manuscript text. J.X., Y.Y. and S.Y. prepared all figures and table. RY conducted the design and review of the article. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, G., Jiang, Z., Xu, L. et al. Development and validation of a prognostic nomogram for predicting in-hospital mortality of patients with acute paraquat poisoning. Sci Rep 14, 1622 (2024). https://doi.org/10.1038/s41598-023-50722-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50722-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.