Abstract
Avocado fruit is a climacteric fruit that has a short life after harvest. Chitosan (Ch) and Arabic gum (AG) have a pronounced effect on the storability of fruits. This investigation aimed to determine the effect of individual or combined use of Ch and AG as well as Ch/AG enriched with 2, 4, 8% Zn–NPs on physio-biochemical attributes and antioxidant capacity of Hass avocado fruit during cold storage (7 °C). The result showed that Ch or AG alone succeeded in maintaining fruit quality of Hass fruit during cold storage. Also, combined application of Ch/AG was more effective than individual application of Ch or AG in reducing fruit weight and polyphenol oxidase activity (PPO) as well as increasing total antioxidant capacity (TAC) and malondialdehyde (MDA). Moreover, Ch/AG coating enriched with 8% Zn–NPs recorded the lowest fruit weight loss, fruit decay %, TSS fruit content, fruit firmness and improved fruit skin and pulp color significantly compared to Ch/AG and control. Coating with Ch/AG/2%Zn NPs recorded the highest peroxidase (POD) activity, while Ch/AG/8% Zn–NPs recorded the highest TAC and the lowest PPO activity. Moreover, enriched Ch/GA with Zn–NPs recorded the highest CAT and POD activity compared to the control. This study shows the efficiency of Ch/AG enriched with Zn–NPs on preserving Hass avocado fruit quality during cold storage by delaying ripening process and activating enzymatic defense mechanisms.
Similar content being viewed by others
Introduction
Avocado (Persea americana Mill) is a high-calorie fruit due to its high content of monounsaturated and polyunsaturated fatty acids. Additionally, it has a high nutritional value due to its content of nutrients (K, Mg, Fe, P), vitamins (B, C, E), and antioxidants1,2,3. In addition to the greater economic importance of international trade. It is considered a productive plants4,5. Hass avocados are among the world's most popular cultivars in retail and consumption, due to their organoleptic characteristics (Taste, appearance, smell, flavor and texture) and high nutritional value1. Avocado is classified as a climacteric fruit, which is highly perishable due to its high rate of fruit respiration and increased production of ethylene which leads to accelerated fruit ripening and reduced post-harvest life6. The large amounts of post-harvest fresh fruit losses are due to significant weight loss and shrinkage7. Another negative impact on the environment is caused by postharvest losses, which emit 4.2 tons of CO2 per ton of wasted food8,9. One method that has been developed to increase the storability of fruits is the use of edible coatings.
Chitosan (Ch) is a high molecular weight polysaccharide, serving as promising semi-permeable edible coatings substance due to its safe, biodegradable, biocompatible properties, antimicrobial activities and modulating fruit atmosphere as well reducing fruit weight loss resulting from internal moisture loss10,11. Previous research has shown that Ch coating improves fruit quality and storability of many perishable fruits, such as papayas12, litchis10, loquat13 and Red Raspberries14. However, Ch still has weak mechanical strength15. Thus, to enhance the properties of chitosan film, composite nano-coatings could be a promising technology. It has recently been shown that coatings containing nano-titanium dioxide (TiO2) and Ag/TiO2 nanocomposite help in prolonging postharvest life of strawberries and melons fruits16,17,18.
Arabic Gum (AG) or Gum Arabic is a dried gummy substance from Acacia senegal and related Acacia species19. It is considered one of the oldest and most popular remedies and has a therapeutic for chronic kidney diseases since 5000 years20. It has excellent water solubility, indigestibility and lower solution viscosity than the other gums19. Recent studies indicate its cardioprotective, renal, gut-protective, anticoagulant, antimicrobial and anti-inflammatory implications. It has achieved measurable success when used in drug, and nano-technology21. In tomato fruit, combined application of 6% CaCl2 and 10% gum Arabic reduced fruit weight loss, and decay percent of fruits as well as maintained fruit firmness, TA, and TSS were preserved compared with the control22. Coating mangoes by 10% AG and 1% Ch reduced decay incidence, weight loss, ethylene production and respiration rate23. Nowadays, several studies have used AG combined with Ch as an edible coating for extending shelf life of many fruit species such as mango24.
Zinc oxide nanoparticles (Zn–NPs) have multifunctional properties in inhibiting microbial growth25,26,27,28,29,30, cosmetics, drug delivery and medical devices31. Furthermore, the U.S. Food and Drug Administration has listed Zn–NPs as generally safe (21CFR182.8991; 32. The application of 0.5% Zn–NPs reduced weight loss and microbial load as well as maintained strawberry fruit with higher firmness, antioxidant capacity and vitamin C content 33. Moreover, Zn–NPs have been used as an antibacterial agent and packaging film additive26,34. However, there is not enough information available about direct application as coating for avocado fruit.
However, the individual or combined application of Ch and AG as well as AG/Ch + Zn–NPs as composite coatings and their physio-biochemical mechanisms for preserving perishable avocado fruits has not been studied. Therefore, this study aimed to was to evaluate the efficiency of Ch, AG, AG/Ch and AG/Ch enriched with Zn–NPs as coatings on Hass avocado fruit quality and its physio-biochemical mechanisms during cold storage.
Material and methods
Materials
Chitosan (Ch, medium molecular weight, De-acetylation ≈ 75%), polyvinyl alcohol (PVA; Mwt = 30,000), and zinc acetate were purchased from Sigma Aldrich. Acetic acid (AcOH) and glycerol were obtained from Elnasr Pharmaceutical Chemicals Co. All chemicals were used in analysis grade without any purification required. Permission was obtained to use the avocado variety in this study which complies with relevant institutional, national and international guidelines and legislation.
Preparation of Arabic Gum (AG)/Chitosan (Ch)/Zn–NPs nanocomposite film
Nano Zinc oxide (Zn–NPs) was prepared by refluxing zinc acetate (3.942 g) in alkaline ethanol (1.44 g NaOH/1L ethanol) at 70°C for 2 h, after that deionized water was added. The Zn–NPs were obtained as fine white powder by centrifugation of the previous solution for 10 min at 5000 rpm, and then calcined for 2h at 500°C.
A 2% Ch solution was prepared by dissolving Ch in 1% acetic acid. AG solution (4%) was prepared by dissolving AG powder in distilled water at 40 °C for 1h using a magnetic stirrer. The composite AG/Ch was obtained by mixing Ch and AG solution by volume ratio 1:1 under a magnetic stirrer for 10 min. Finally, the nanocomposites were fabricated by the addition of various concentration of Zn–NPs to previous mixture to prepare 2, 4 and 8% AG/Ch/ZnO–NPs Nano composite. In addition, the glycerol was added by 10%/volume to all the prepared coating as plasticizers35,36. The composition of the as-prepared coatings was illustrated in Table 1.
Characterization of coating materials
At room temperature, Fourier-transform infrared (FTIR) spectra between 400 and 4000 cm−1 were captured for as-prepared samples using a Mattson 5000 FT-IR spectrophotometer (Unicam, UK).
With a magnification of 600,000, a resolution power of 0.4 nm, and an operating voltage of 120 kV, the Jem 1230 Transmission Electron Microscope (TEM) from JEOL, Japan, was used to examine transmission electron micrographs. Scanning electron images were obtained by Quanta FEG-250 Scanning Electron the surface morphology of as-prepared samples was obtained by Quanta FEG-250 Scanning Electron Microscopy (SEM) instrument, Ametek Holland.
At room temperature, X-ray powder diffraction (XRD) patterns were captured using a Ni-filter and Cuk radiation source (λ = 1.54 Ǻ) operated at 40 kV and 40 mA in the 2θ range 5–80° at the scan speed of 0.05° per second on a Philips PW 1390 Diffractometer from Japan.
Under a nitrogen atmosphere, a Shimadzu TGA-50 thermo-gravimetric analyzer, Columbia, EUA, was used to carry out the thermal analysis in the range of 25–600 °C at a heating rate of 10 °C/min.
The cup method was used to measure the water vapour transmission rate (WVT, GBPI Co., China) using the GBPI W303-B Water Vapor Permeability Analyzer. WVT was determined as the volume of water vapour that permeates a given area during a given period at a given humidity level (4–10%) and temperature (38 °C). JIS Z0208, 53,122–1, TAPPI T464, ASTM D1653, ISO 2528, and ASTM E96 were followed in the assessments35,36.
Fruit sampling
This study was conducted during 2021 at the National Research Center (Horticultural Crops Technology Lab and Department of Polymers and Pigments) and Cairo University (Pomology department, Faculty of Agriculture), Giza, Egypt. During October, high quality Hass avocados fruit were harvested from Salmiya private orchard (30°41′42″ N and 30°23′16″ E, elevation 9 m) in El Behera Governorate, Egypt. Avocado fruits were harvested at 20% dry matter mainly37 with full green color, firm stage (40 kg/cm3), uniform size and weight (150 g) as well as free from mechanical defects. The harvested fruit were carefully packed into open display boxes and immediately transferred to the Lab. The fruits were distributed to seven postharvest treatments: Ch, AG, AG/Ch, AG/Ch/2%ZnO–NPs, AG/Ch/4%ZnO–NPs, AG/Ch/8%ZnO–NPs and control.
Edible coatings application
A total of seven treatments i.e., control, AG, Ch, AG/Ch, AG/Ch/2% ZnO–NPs, AG/Ch/4% ZnO–NPs, and AG/Ch/8% ZnO–NPs were used in this study. The fruits were divided into 7 groups, each one containing 60 fruits which were dipped in the previously prepared coating for 5 min, then dried (25 °C/20 min), packed in perforated boxes, and stored up to 3 months (7 ± 1 °C and 90% RH). During cold storage, the following fruit quality measurements were recorded every 15 days.
Fruit physical quality parameters
Weight loss %
A digital balance was used to determine fruit weight loss percent using the following equation:
The fruit decay %
The percentage of fruit decay was determined visually by the following equation:
Decayed fruits were classified as moldy spots visible on the fruit surface.
Fruit firmness (kg/cm3)
Fruit Firmness (kg/cm3) was measured on the two opposite sides of each unpeeled fruit sample using penetrometer (FT327, FACCHINI srl, Alfonsine, Italy) according to Abd El-Moniem et al.38.
Fruit peel and pulp color
Fruit skin and pulp color were measured every two weeks according to Mcguire39. For skin color, three fruits for each replicate were labeled on the equatorial region (four regions/fruit) and then color on the same labeled spot was recorded. While for pulp color measurement, three fruits for each replicate were divided into 4 slices and then color was measured on each slice. Fruit color was measured by the colorimetric method using a Minolta Chroma Meter CR-2000 (Chroma Meter; Konica Minolta Sensing, Inc., Osaka, Japan). Values were recorded as L* (lightness = 100, black = 0), b* (yellow–blue scale) and a* (green--red).
Fruit chemical quality parameters
Total titratable acidity % (TA)
Total titratable acidity in the extracted juice was determined and expressed as citric acid % by titration with NaOH (0.1 N) to a stable faint pink color as end point using 2 drops of phenolphthalein (1gm solved in 100 mL ethanol) as indicator. Titration was performed in 20 mL of final solution (20 g of fruit pulp mixed in a blender with 100 mL of distilled water) and repeated three times. TA determination performed from A*B equation
Vitamin C (mg g−1FW)
In the previous extracted juice vitamin C (ascorbic acid) was determined using 2,6 dichlorophenolindophenol titration method40.
Fruit TSS content
TSS was determined by a digital hand refractometer (PR32, Atago Palete ATago CO.LTD. Japan) in the previous extracted juice and expressed as Brix12.
Respiration rate
The respiration rate was measured on three avocados per replicate using the closed system method. It was measured based on the amount of CO2 produced by the fruits and per unit of fresh weight. The fruits were carefully placed in sealed 2-L glass jars for 24 h under the same experimental conditions. The respiration rate was then measured using an O2/CO2 gas analyzer (Model 1450-Servomex 1400, Japan) from the jar headspace through the rubber septum and expressed as mL of CO2 kg−1 fruit/h41.
Enzyme analysis
Extraction and determination of fruit enzymes were performed in 1.0 g of frozen fresh fruit tissue42.
Extraction
A sample of the fruit was taken each time the sample was taken and immediately kept at − 30 °C for enzymatic analysis. To extract the enzymes, the mixture was formed by potassium phosphate buffer (50 mM) at pH 7.0 containing 1% PVP, 1 mM PMSF, 0.2 mM EDTA (w/v), and 0.1% Triton X100 (v/v). Extraction solution (5mL) was added to avocado tissue (1g), grounded with liquid nitrogen and homogenized. After centrifugation (22,000 g for 10 min, 4 °C) the supernatant was ready for the enzymatic assay.
Soluble proteins
The microassay procedure from protein assay kit (Bio-Rad) was used. Preparing reaction mixture composite of 80 μL sample, 200 μL of dye reagent and 720 μL of distilled water. This mixture was vortexed (DLAB MX-S, DLAB Scientific Co., Ltd, China), incubated at room temperature (30 min) and absorbance read at 595 nm (Optizen POP UV/VIS spectrophotometer, Serial POP, KLAB, Republic of Korea) and then BSA stock solution used as standard curve for proteins calculation.
Total antioxidant capacity (TAC)
The antioxidant capacity was obtained by mixing the enzyme extract (20 µL) and 125 µL of 60 µM of 2,2-diphenyl-1-picrylhydrazyl (DPPH). This mixture was then incubated (30 min) in the dark and the absorbance was measured at 517 nm (Thermo Scientific Genesys, China). TAC was calculated using a trolox as a standard curve and was expressed as µmoles g−1 DW43.
Catalase activity (CAT)
The enzyme mixture included phosphate buffer (2.55 mL) at pH 7.0, 50 mM, H2O2 (250 μL) and enzyme extract (200 μL). The absorption reduction of H2O2 (37.5 mM) at 240 nm was used to determine CAT activity expressed in U mg−1 of protein..
Lipid peroxidation via malondialdehyde (MDA)
Lipid peroxidation via malondialdehyde (MDA) was determinded according to Bailly and Kranner44 protocol. Avocado tissue (0.5 g) was ground and homogenised with 0.5% thiobarbituric acid (5 mL) and 20% trichloroacetic acid. This mixture was heated at 95 °C (water bath) during 30 min, then rapidly cooled on ice. The mixture was then centrifuged (22000 g for 2 min) and the supernatant (2 mL) used to measure MDA absorbance (532 and 600 nm). Then MDA was calculated and expressed as U mg−1 of protein of fresh weight.
Peroxidase activity (POD)
Peroxidase activity (POD) was assessed by mixing the enzyme extract(140 μL) and 570 μL of guaiacol (20 mM). Then the mixture was incubated (5 min/30 °C) and then added 290 μL of H2O2 (50 mM) and the absorbance read (460 nm) every 30 s for 5 min and POD expressed in U mg−1 of protein.
Polyphenol oxidase activity (PPO)
Polyphenol oxidase activity (PPO) was assessed by mixing the enzyme extract (0.1 mL), grade water (0.9 mL), 0.001 M catechol (1 mL), 0.5 M K-phosphate buffer (1 mL) at pH 6.5. Then PPO activity was determined within 3 min by monitoring the increase of absorbance (280 nm) according to Worthington and Worthington45 and PPO activity was expressed in U mg−1 of protein.
Statistical analysis
The design of this experiment was in a two way factorial. The treatments layout in a randomized complete block design (RCBD) with two factors (storage periods and postharvest materials) for employ treatments with three replicates for each treatment. Analysis of variance (ANOVA) was calculated via MSTAT-C software package46. Treatments means were compared by the least significant difference test at level of p < 0.0547. The data presented in the figures are means ± standard error (SE).
Ethics approval
The authors confirmed that the institutional committee and licensing committee approved the experiments, including all relevant details and that all experiments have been performed in accordance with specified named guidelines and regulations.
Ethics approval and consent to participate
All methods were in accordance with relevant institutional, national, and international guidelines and legislation
Results and discussion
Characteristics of ZnO–NPs
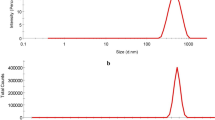
The morphological characters of as-prepared ZnO–NPs were illustrated in Fig. 1a, b using TEM and SEM, respectively, which illustrated that ZnO–NPs have particles size less than 50 nm. This results were confirmed by using particles size distribution (Fig. 1c) which demonstrated that the particles size of ZnO–NPs has narrow size and less than 50 nm.
The crystal structure of the ZnO–NPs was represented in Fig. 1d, where the XRD partum showed the peaks at main peaks 2θ = 31.9, 34.5, 36.4, 47.7°, and 57° that are corresponding to ZnO–NPs which are related to plans (100), (002), (101), (102) and (110), respectively.
Characterizations of coating films
FT-IR spectrum of as-prepared coatings was obtained in Fig. 2A. All the prepared samples exhibited the absorption band at bands 3300, 1650, 1410, and 1030 cm−1 corresponding to –OH and –NH stretching vibration, C=O and C–O stretching of the amide group, and C–O stretching, respectively. These results were in consistent with Mahmoud et al.48. on green snap bean using of chitosan and chitosan nanoparticles. Also, Salama et al.49. on common bean (Phaseolus vulgaris L.) using nanofertilizer.
The coating GA/Ch/8% ZnO–NPs exhibited an additional band in the range of 500–1000 corresponding to inorganic materials at 493 cm−1 in response of Zn–O stretching vibration.
Thermogravimetric analysis (TGA) was carried out in nitrogen atmospheres to look into the thermal stability of the as-prepared coatings. According to Fig. 2B, the coating Ch/AG and its nanocomposites exhibited two steps of decomposition. The first step is evaporating of adhering and adsorbed water while the main degradation process during TGA analysis is due to the processes of polymer chain degradation through end group-initiated mechanism and the thermal degradation of the products formed during polymer chain degradation. while the as-prepared nanocomposite coatings showed relative stability over Ch/AG coating due to the presence of ZnO–NPs, and this stability grew when ZnO–NPs loading was increased.
Transmission of water vapour (WVT), which measures how easily moisture can be absorbed and distributed through a material, has a significant impact on how packing materials are prepared to affect food shelf life. The WVT data of as-prepared coated films were illustrated in the Table 1. The data showed that the inclusion of Ch improved the blend film’s (AG/Ch) moisture barrier over the pure GA, reducing WVTR for the created blend AG/Ch. In addition, increasing the loading of ZnO–NPs in nanocomposite films creates a significant decrease in water transmissibility, since the existence of ZnO–NPs promotes the hydrophobicity of nanocomposite films. These results were in agreement with Youssef et al.36. in preserving fresh chicken breast fillets by using TiO2-NPs and cinnamon Nanoemulsion.
Fruit physical quality parameters
Fruit weight loss
As shown in Fig. 3A, the percentage of weight loss for avocado samples increased with increasing storage periods, but the rate of weight loss for the control (Cont) treatment was significantly higher than of all coated treatments. At 75 days of cold storage, application of AG/Ch as composite coating delayed avocado fruit weight loss (15.62%) compared to Ch (21.27%), AG (17.46%) and control (26.07%). Moreover, at 75 days of cold storage, AG/Ch + 8% Zn–NPs composite treatment was the most effective treatment in reducing weight loss (12.5%) compared to control (26.07%) and all other treatments. These results were in line with Tahir et al.50 who found the reduction of strawberry weight loss by Arabic Gum as coating. In tomato fruit, combined application of 6% CaCl2 and 10% gum Arabic reduced fruit weight loss compared with the control fruit22. Also, Coating mangoes with mixture of AG (10%) and Ch (1%) reduced weight loss23. Also, AG 15% + Moringa and CMC (carboxy methyl cellulose)1% + M retained fruit firmness and lowered weight loss of Maluma avocado fruit51. Moreover, AG 15% + Moringa decreased weight loss of Maluma avocado fruit51. weight loss percent of Hass avocado increased significantly during cold storage, while beetroot and turmeric extract extract significantly decreased it.
The weight loss in fruit results from water from water evaporation due to the processes of respiration and transpiration33. Tahir et al.50. reported that 15% AG-coating for strawberry significantly reduced weight loss. Chitosan coating forms a semi-permeable layer that creates a modified atmosphere that protects against O2, CO2, H2O and water loss52,53. Also, AG is a polysaccharide with functional hydrophilic properties, which had mechanical strength and slows gas exchange and moisture loss54,55.
Fruit firmness
The firmness of the fruit was significantly affected during storage so that the firmness of the control treatment from the 15th day to the 75th day went from 19.20 to 2.53 kg/cm3 and the firmness of the chitosan treatment Enriched decreased from 35.67 to 23.17 kg/cm3 (Fig. 3B). AG had significantly higher fruit firmness than Ch at the different storage periods except for 15 days. Also, AG/Ch recorded the highest significant fruit firmness followed by Ch then control at 45, 60 and 75 days of storage. Moreover, AG/Ch + 8% Zn–NPs composite coating recorded the highest significant fruit firmness during 15, 30, 60 and 75 days of storage compared to all treatments. These results were in agreement with Kubheka et al.51 who found that AG(15%) + Moringa maintained high fruit firmness of Maluma avocado fruit51. Also, Coyotl-Pérez et al.56 reported high firmness of avocado fruit treated with Ch + essential Oil. The efficiency of AG on maintaining high fruit firmness during cold storage was recorded in many fruit species such as apple57 and mango23. In tomato fruit, combined application of 6% CaCl2 and 10% gum Arabic maintained fruit firmness compared with the control fruit22.
Tahir et al.50 reported that, 15% AG-coating for strawberry significantly improved fruit firmness. Furthermore, 0.5% Zn–NPs maintained higher firmness of strawberry fruit33. Softening of fruit resulting from cell structure deterioration in both intracellular materials and the cell wall58. Coating the fruit with AG/Ch maintains high fruit firmness by covering the lenticels and cuticle which reduces respiration and delays fruit ripening59. Arabic gum(10%) + 1000 ppm Moringa oleifera seed oil treatment maintain fruit firmness of Hass avocado fruit38.
Fruit decay%
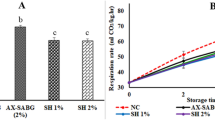
The results indicated that the rate of decay in the fruits increased with long storage periods. Up to 45 days of cold storage, all coating treatments successfully prevented fruit decay (Fig. 3C). Moreover, both AG and AG/Ch-coated avocadoes significantly reduced fruit decay by 11% and 12% compared to Ch alone (16%), while control had the highest decay rate (20%) at 75 days of storage. The minimum percentage of rotting of avocados coated with gum arabic/chitosan was observed in concentrations of 2 and 8%, which showed a significant difference with other treatments, including the concentration of 4%. Moreover, at the end of the storage period, the treatment AG/Ch + 8% Zn–NPs recorded the lowest percentage of decay compared to other treatments. Therefore, AG/Chcoating treatment with Zn–NPs could be more effective in preventing fruit decay.
In this regard, Khaliq et al.24 reported that chitosan coatings on the fruit surface acts as a barrier that protects the fruits from infection by pathogen, thereby reducing fruit decay. Also, a combined coating of Arabic gum (10%) and chitosan (1%) reduced mango fruit decay23. Also, Ch reduced fruit decay of Red Raspberries 14. Moreover, GA-coating (15%) for strawberry significantly inhibited fungal infections50. Rai-Kalal et al.60 found a reduction in strawberry decay due to treatment of 0.5% Zn–NPs. Ch inhibits the development of decay-causing pathogens, and stimulates host tissue resistance response61. Also, Ch inhibits the development of decay-causing pathogens and stimulates host tissue resistance response61. Recently, in a study, the use of a combination of chitosan and titanium oxide nanoparticles reduced the percentage of decay in mango fruit. Weight loss percent of Hass avocado fruit increased gradually during cold storage62.
Respiration
The respiration rate decreased suddenly from day 15–30, but from day 30 onwards we saw an increase in the respiration rate of fruits (Fig. 3D). At the end of storage period, AG (18.28 CO2kg−1fruit h−1), Ch (15.53 CO2kg−1fruit h−1) and AG/Ch (15.63 CO2kg−1fruit h−1) significantly reduced respiration rate compared to control (42.97 CO2kg−1fruit h−1). Also, AG/Ch + 4% Zn–NPs recorded the lowest respiration rate (8.70 CO2kg−1fruit h−1) compared to all treatments followed by AG/Ch + 8% Zn–NPs (12.27 CO2kg−1fruit h−1). In this regard, chitosan layer formed on the fruit surface modifies the gas and atmosphere exchange, directly affecting the respiration and ripening of the fruit12. Also, Handojo et al.23 reported that Arabic gum (10%) and chitosan (1%) reduced respiration rate and ethylene production of mango fruit23. Also, Khaliq et al.24 reported that coated mangoes using Arabic gum (10%) and chitosan (1%) reduced respiration rate and ethylene production. Moreover, chitosan/Nano-TiO2 composite decreased decay of mango fruits and the respiration rate peak appeared 5 days later63.
Respiration rate in avocado fruit gradually decreased during cold storage period up to 30 days of cold storage and then gradually increased in all treatments. Climacteric peak for all treatments cleared after 45 days of cold storage, with the value of each treatment varying, which led to a delay in fruit ripening. At the end of storage period (75 days) Ch/Ag + 4%Zn–NPs followed by Ch/Ag + 8%Zn–NPs and Ch/Ag + 2%Zn–NPs recorded the lowest respiration rate. In addition, individual coating of avocado fruit with Ch, GA and Ch/GA alone or enriched with Zn–NPs has the ability to form a mechanical barrier that helps control respiration rate control, prevent water loss, maintain high fruit firmness and extend storage period.
Fruit chemical quality parameters:
Vitamin C content
Vitamin C content decreased gradually with increasing storage periods from 15 till 75 days (Fig. 4A). At the end of storage period, treatments of AG/Ch (3.47 mg−1FW) followed by AG (2.07 mg−1FW) preserved higher significant vitamin C content compared to control (0.7 mg−1FW) and Ch (1.4 mg−1FW) alone. Moreover, AG/Ch + 8% Zn–NPs (2.86 mg−1FW) recorded higher vitamin C content compared to all treatments except for AG/Ch treatment. In this regard, vitamin C content significantly decreased during cold storage of “Hass” avocado fruit62. Also, Hong et al.64 reported that a 2% chitosan coating was more effective in delaying the reduction of vitamin C of guava fruit than 0.5% and 1%. Moreover, strawberry fruit treated with 0.5% Zn–NPs maintained a higher concentration of vitamin C than untreated fruit33. The decrease in Vitamin C with increasing storage periods may be due to auto-oxidation resulted from combination of ascorbic acid with oxygen in the air33. A decrease in vitamin C content has been reported in “Hass” avocado fruit during cold storage38,62. While AG enriched with moringa or algae extracts succeeded in preserving a high percentage of vitamin C in Hass avocado fruit 38.
Fruit titratable acidity
Avocado fruits recorded high titratable acidity during 15 days of cold storage, and then decreased gradually until the end of storage periods (Fig. 4B). Up to 60 days of storage, AG (1.2%) recorded a significant higher fruit acidity content than AG/Ch (0.97%), Ch (0.54%) and control (0.40%). Also, AG/Ch + 8% Zn–NPs (1.24%) and AG (1.24%) treatments recorded the highest significant titratable acidity in ‘Hass’ fruit at the end of storage. Citric acid is the main organic acid in avocado fruit. A decrease in titratable acidity during cold storage were recorded in many fruit species such as avocado38,62, strawberry33, and this may be due to the use of organic acids in the respiration process. Also, Hong et al.64 reported that a 2% chitosan coating was more effective in delaying the reduction of titratable acidity in guava fruit than 0.5% and 1% Ch which results from the ability of chitosan to modify the fruit internal atmosphere which preventing the reduction of titatable acidity.
Fruit TSS content
Figure 4C shows the changes in the TSS content of avocado during the cold storage periods. Fruit TSS content increased during storage period. Treatments of AG and Ch recorded lower TSS significantly compared to control but compared to Zn–NPs treatments, they showed more soluble solids on day 75. Moreover, composite treatments of AG/Ch + Zn–NPs at 4% and 8% recorded the lowest significant TSS at 75 days of storage compared to AG/Ch. In tomato fruit, combined application of 6% CaCl2 and 10% gum Arabic maintained fruit TSS compared with the control fruit22.
The reduction in fruit TSS content has been previously reported in chitosan-treated mangoes12. Also, Arabic Gum delayed the increase in TSS of Apples57 and strawberries50. Moreover, a composite of chitosan (1%) and Arabic gum (10%) change in TSS of mangoes23. Increasing TSS content during cold storage may due to the solubility of hemicelluloses and polyuronides which presented in cell wall64. Also, water loss due to transpiration might cause an increase in TSS65.
Fruit skin color
Fruit color is the primary and most widely used perception parameter in determining fresh horticultural produce quality. Changes in L*, a*, and b* values of avocado skin color are shown in Fig. 5. For fruit skin color L* gradually decreased with increasing storage periods (Fig. 5A). Combined (19.27) or individual application of AG (20.02) and Ch (22.50) maintained high significant fruit lightness compared to control (14.10) at 75 days of storage. Also, Ch alone recorded a significantly higher L value for fruit skin (30.40, 23.44, 22.50) than AG (25.62, 21.21, 20.02) and AG/Ch (24.75, 20.07, 19.27) through 30, 60 and 75 days of storage. Moreover, Zn–NPs treatments recorded a significant higher L-value compared to Cont. While, AG/Ch + 8% Zn–NPs succeeded in maintaining a significantly higher L-value (25.98, 24.52, 23.83) during 45, 60 and 75 days of storage period. With regard to fruit skin color a* value it was increased with increasing storage periods (Fig. 5C). AG maintained lower a* value (0.32, 0.28) during 60 and 75 days after cold storage than control (1.31, 1.41). Also, AG/Ch + 4% Zn–NPs significantly decreased a* value (− 0.56) in fruit skin compared to control (1.41) at 75 days of storage. The highest values were recorded at 75 days of cold storage with AG/Ch (1.38) and control (1.41). Moreover, AG/Ch + 8% Zn–NPs gave the least significant values during all storage periods. Regarding fruit skin color (b value), the b-value of avocado skin color decreased gradually during storage periods (Fig. 5E). After 75 days of cold storage, Ch alone (6.91) significantly increased b-value during the storage periods compared to AG (5.04) and control (3.91) as did AG/Ch (6.80). Also, all Zn–NPs treatments increased color (b-value) significantly compared to control at 45, 60 and 75 days of storage. Moreover, AG/Ch + 8% Zn–NPs recorded the highest significant b-value during all storage periods except with Ch at 30 days. At 75 days of cold storage, AG/Ch + 8% Zn–NPs recorded the highest b-value (8.83).
The increase in purple (black) skin (lower b* value) was predictable in dark-skinned avocados, as this refers to the anthocyanin synthesis and chlorophyll degradation, which coincided with avocado ripening66. This result confirms the findings of Adjouman et al.67 who reported delaying in a* value increase in tomatoes due to polysaccharide coatings. The ability of these coatings to retain the green color of the avocado indicates their ability to delay the ripening of the fruits. Also, Jeong et al.68 and Cox et al.66 who reported delaying color change (green to dark purple) could extend dark-skinned cultivars shelf life. Conversely, the results showed there were sharp declines in b* values in control than coated fruit during storage time. Maftoonazad et al.69 reported a decrease in b* values in avocado indicating an increase towards darker chroma and decrease in yellowness. The color change was enhanced in the uncoated fruit and attained purple to black color during storage compared to the coated one. Although the fruit coated with AG 15% + Moringa and AG 10% + Moringa remained green up to 28 days of storage, this may result from coating materials (AG) providing a thick barrier against gas exchange and ethylene production between outer and inner environments, which delayed fruit ripening during storage.
Fruit pulp color L value
The L-value of fruit pulp (internal) color decreased from 60 at 15 days of cold storage to 33 at the end of cold storage period (Fig. 5B). Individual or combined use of Ch and AG resulted in a significant higher L-value in fruit pulp compared to Cont. Also, AG recorded a significant higher L-value (54.55, 50.88, 46.95) than Ch (51.10, 47.63, 45.33) and AG/Ch (51.10, 47.63, 45.33) at 30, 45 and 60 days of storage. During 15 days of cold storage AG/Ch had the highest significant L-values, while after 30, 45, 60 and 75 days of cold storage AG/Ch + 8% Zn–NPs had the highest significant L-values (57.06, 56.46, 55.81, 50.57) compared to all treatments. For fruit pulp color a* value it was decreased from -5 at 15 days of cold storage to 0.2 at 75 days of cold storage (Fig. 5D). Generally, GA (− 2.32, 1.57, 3.03), Ch (− 0.67, 1.52, 2.03) and AG/Ch (0.04, 0.6, 2.16) maintained a less significant pulp a* value compared to control (0.82, 3, 3.52) at 45, 60 and 75 days of storage. After 75 days of storage Ch (2.03), AG/Ch (2.16) or 2% (2.8) or 8% (2.07) AG/Ch + Zn–NPs recorded a significantly lower a* value compared to all other treatments. Regarding fruit pulp color, b-value decreased gradually from 44 at 15 days of cold storage to 22 at 75 days of cold storage (Fig. 5F). Generally AG and Ch maintain a higher significant pulp b-value compared to Cont. Meanwhile, AG/Ch recorded the highest values (44.37, 38.67, 25.62) at 15, 30, 75 days of storage compared to Ch (37.74, 35.30, 21.54), GA (38.53, 36.37, 23.64) and control (26.44, 24, 10.24). A individual application of GA maintained a significantly higher pulp b-value (35.93, 35.44) higher than Ch (28.99, 24.15) during 45 and 60 days of cold storage. During 45, 60 and 75 days of cold storage Ch/AG enriched with 4% (32.96, 30.85, 28.20) and 8% (33.77, 32.72, 28.64) Zn–NPs recorded the highest significant fruit pulp b-value compared to control (17.69, 12.85, 10.24) and Ch (28.99, 24.15, 21.54).
After 28 days of storage, Maluma avocado fruit coated with Arabic Gum at 10% or 15% + Moringa recorded the highest pulp color scores. These results were consistent with Aguiló-Aguayo et al.70 who reported that, L* values of fresh cut avocado showed a significant decrease in both untreated and pulsed light -treated samples after 15 days of storage, which indicated oxidative browning evidence during storage. Also, Gómez-López71 reported that Lightness (L*) is considered to be the most indicator for enzymatic browning in avocado fruit. Moreover, Light microscopy of apple fruit confirmed that color modifications were associated with cellular membranes breakage, which reduces the function of cell compartmentalization, and increase enzyme–substrate involved in tissue browning70,72. Severity of grey pulp in Avocado fruit significantly by MAP(modified atmosphere packaging) + Thyme oil treatment73. Gray pulp disorder occurs in cold-stored fruits during ripening74 which is associated with fruit senescence. Polyphenol oxidase (PPO) enzyme is responsible for this disorder in avocado75.
Color was recorded using the CIE L*a*b* uniform color space (CIE Laboratories), where a* indicates chromaticity on a green (-) to red ( +) axis, L* indicates lightness and b* chromaticity on a blue (-) to yellow ( +) axis76. Higher color L (more lightness) and color b (more green) plus lower color a (more blue) means that the fruit was in more freshness and quality which was recorded in 8% Zn–NPs treatment compared to lower lightness (L) and red color (a) as well as a higher yellow color (b), which means that the fruit are more ripening, which is what was recorded in the control treatments.
Enzyme analysis
TAC gradually increased with increasing storage period up to 45 days of storage and then gradually decreased (Fig. 6A). At 75 days of cold storage, AG/Ch + 4% Zn–NPs (49.07) recorded the highest TAC activity followed by AG/Ch + 2% Zn–NPs (46.82) then AG/Ch (46.42) treatment, while control (33.33) recorded the lowest significant value. All Zn–NPs treatments increased TAC significantly compared to Cont. Also, mixing AG/Ch (46.42) gave a significantly higher value than individual Ch (44.97) or AG (44.67) at 75 days of cold storage.
Effect of chitosan (Ch), Arabic gum (AG), AG/Ch, and AG/Ch enriched with 2, 4, 8% Zn–NPs on fruit Total antioxidant capacity TAC (A), catalase activity (CAT) (B), malondealdehyde (MDA) (C), peroxidase activity (POD (D), and polyphenoloxidase (PPO) (E) of Hass avocado fruit during cold storage (7C). Data were presented as means (n = 3 ± SE).
CAT activity increased progressively with increasing periods of storage (Fig. 6B). At the end of storage period (75 days) Ch (12.50), AG(12.50), AG/Ch enriched Zn–NPs gave the highest significant CAT activity (12.50) compared to control (8.27). MDA decreased progressively with increasing storage periods (Fig. 6C). After 75 days of cold storage, AG/Ch (13.87) recorded the highest significant MDA activity followed by AG/Ch + 2%Zn–NPs (12.90) compared to the other treatments, while control (6.67) recorded the lowest significant value. All Zn–NPs treatments significantly increased MDA compared to Cont. Also, mixing AG/Ch (13.87) gave a higher significant value than individual Ch (10) or AG (11.91) during all cold storage periods.
POD activity increased gradually with increasing storage period (Fig. 6D). After 75 days of cold storage Ch (16.45) followed by both AG (15.61) and AG/Ch + 2 Zn–NPs (15.54) recorded the highest significant values while control (9.37) recorded the lowest one. All Zn–NPs treatments increased POD significantly compared to Cont.
PPO activity gradually increased with increasing storage periods (Fig. 6E). At 75 days of cold storage, the lowest PPO value (12.04) was recorded by AG/Ch + 4% Zn–NPs followed by AG/Ch (12.78), while control (21.93) recorded the highest significant value. Mixing of AG/Ch (11.63, 12.77) significantly reduced PPO at 60 and 75 days of cold storage compared to individual use of Ch (12.63, 16.78) and GA (13.13, 16.28). Also, Zn–NPs treatments significantly decreased PPO activity compared to Cont.
These results were consistent with Tesfay et al.77 as they found activation of physio-biochemical processes during storage of avocado fruit results in changes in peroxidase and catalase activity. Switala and Loewen78 found that CAT is more effective than POD in the degradation of H2O2. Coating strawberries with 15% GA significantly reduced the activity of polyphenol oxidase (PPO) activity, while it increased antioxidant capacity50. Also, Arabic gum (10%) + 1000 ppm Moringa oleifera seed oil treatment decreased the activity of polyphenol oxidase33. Moreover, Rashedy et al.62 found that turmeric acid recorded the lowest significant PPO activity while the control treatment recorded the highest PPO activity after the end of cold storage.
Mahmoudi et al.79 found that plum fruit coated with chitosan/Arg NPs (0.5%) enhanced activity of antioxidant enzymes during. Furthermore, Li et al.80 reported that nano-ZnO-enriched packaging film for preserving apple fresh-cut reduced MDA and inhibited POD and PPO. Moreover, chitosan/Nano-Titanium coating increased the activity of PPO, POD, while reducing MDA content63. Gray pulp disorder occurs in cold-stored fruits during ripening is associated with fruit senescence and Polyphenol oxidase (PPO) enzyme activity75. Also, Ch applications promoted the highest total antioxidant activity of Red Raspberries Piccolo14.
The results indicated that during the storage period there was a significant increase in CAT, POD, PPO activities as well as a significant decrease in MAD. Conversely, TAC increased till 45 days of storage and then decreased. Regarding the effect of treatments, it can be concluded that, Zn–NPs, Ch and AG treatments recorded high activity of TAC, CAT, MDA and POD with lower activity of PPO at the end of storage period (75 days) compared to the control. Individual application of Ch and AG slightly higher significant effect in CAT, MDA and POD followed by AG/Ch + 2% Zn–NPs. While AG/Ch + Zn–NPs at 8% recorded the highest TAC and the lowest PPO than individual application of Ch, AG as well as control treatments. These results were consistent with Pasquariello et al.13 who recorded enhancement of catalase activity and inhibition of polyphenol oxidase activity, which extends the shelf life of loquat fruit treated with Ch.
Our results indicate that AG/Ch + Zn–NPs treatments significantly reduced fruit decay percentage and maintained higher fruit firmness and good color during cold storage (Fig. 1a, b), which may be attributed to the antifungal properties of Zn–NPs25,26,27,28,29,30 as well as Ch10,11 attributed to lower reactive oxygen species accumulation and higher TAC as well as the higher activities of POD and CAT enzymes with lower activity of PPO activity. Our results were in agreement with81 who reported that, melatonin-treated fruit exhibited higher TAC due to lower H2O2 radicals and higher activities of antioxidant enzymes (CAT, POD, and MDA)in blueberries. The reduction in fruit decay may be contributed to higher CAT and POD activities in Zn–NPs, Ch and AG-treated avocado fruit.
Conclusion
Individual application of Ch and AG succeed in maintaining fruit quality of Hass fruit during cold storage. Also, the combined application of Ch/AG was more effective than individual application of Ch or GA in decreasing fruit weight and PPO as well as increasing TAC. Moreover, enriched Ch/GA with 8% Zn–NPs recorded the lowest fruit weight loss, decay percentage, TSS content, and improved fruit skin and pulp color significantly compared to combined application of Ch/GA as well as the control treatment. Furthermore, Ch/AG + 8% Zn–NPs was the most recommended treatment which recorded the highest TAC and the lowest PPO than the combined application of Ch/GA as well as the control treatment. Moreover, enriched Ch/GA with Zn–NPs recorded the highest CAT and POD compared to the control.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AG:
-
Arabic gum
- ANOVA:
-
Analysis of variance
- CAT:
-
Catalase
- Ch:
-
Chitosan
- DSC:
-
Differential scanning calorimetry analysis
- FTIR:
-
Fourier-transform infrared
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- CRBD:
-
Complete randomized block design
- SE:
-
Standard error
- SEM:
-
Scanning electron microscopy
- TAC:
-
Total antioxidant capacity
- TEM:
-
Transmission electron microscope
- TGA:
-
Thermogravimetric analysis
- WVT:
-
Water vapour transmission rate
- Zn–NPs:
-
ZnO-nano particles
References
Dreher, M. L. & Davenport, A. J. ‘Hass’ avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 53, 738–750 (2013).
Bill, M., Sivakumar, D., Thompson, K. & Korsten, L. Avocado fruit quality management during the postharvest supply chain. Food Rev. Int. 30, 169–202 (2014).
Alkhalaf, M., Alansari, W., Ibrahim, E. & ELhalwagy, M. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. Sci. 31, 1358–1362 (2019).
Tango JS, Turatti JM. Óleo de abacate. In: Abacate: cultura, matéria-prima, processamento e aspectos econômicos. Campinas: ITAL, 156–192 (1992).
Medina JC. Abacate: da cultura ao processamento e comercialização. Campinas: ITAL, (1998).
Blakey, R., Tesfay, S., Bertling, I. & Bower, J. Changes in sugars, total protein and oil in Hass avocado (Persea americana Mill) fruit during ripening. J. Hortic. Sci. Biotechnol. 87(4), 381–387 (2012).
Tesfay, S. Z. & Magwaza, L. S. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf Life 11, 40–48 (2017).
Quested, T. E., Parry, A., Easteal, S. & Swannell, R. Food and drink waste from households in the UK. Nutr. Bull. 36, 460–467 (2011).
Opara, U. L. & Mditshwa, A. A review on the role of packaging in securing food system: Adding value to food products and reducing losses and waste. Afr. J. Agric. Res. 8(22), 2621–2630 (2013).
Zhang, D. & Quantick, P. C. Effects of chitosan coating on enzymatic browning and decay during postharvest storage of Litchi (Litchi chinensis Sonn) fruit. Postharvest. Biol. Technol. 12(2), 195–202 (1997).
Liu, J. et al. Reaction mechanisms, structural and physicochemical properties of caffeic acid grafted chitosan synthesized in ascorbic acid and hydroxyl peroxide redox system. J. Agric. Food Chem. 66(1), 279–289 (2017).
Ali, A., Muhammad, M. T. M., Sijam, K. & Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 124(2), 620–626 (2011).
Adiletta, G. et al. Chitosan coating: A postharvest treatment to delay oxidative stress in loquat fruits during cold storage. Agronomy 8, 54 (2018).
Lo Piccolo, E. et al. Can chitosan applications in preand post-harvest affect the quality and antioxidant contents of red raspberries?. Horticulturae 9, 1135 (2023).
Gooneh-Farahani, S., Naimi-Jamal, M. R. & Naghib, S. M. Stimuli-responsive graphene-incorporated multifunctional chitosan for drug delivery applications: A review. Expert Opin. Drug Deliv. 16, 79–99 (2019).
Lin, B. et al. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT Food Sci. Technol. 63, 1206–1213 (2015).
Arnon-Rips, H., Porat, R. & Poverenov, E. Enhancement of agricultural produce quality and storability using citral-based edible coatings; The valuable effect of nano-emulsification in a solid-state delivery on fresh-cut melons model. Food Chem. 277, 205–212 (2019).
Tang, Y. et al. Chitosan/titanium dioxide nanocomposite coatings: Rheological behavior and surface application to cellulosic paper. Carbohydr. Polym. 151, 752–759 (2016).
Ali, A., Maqbool, M., Ramachandran, S. & Alderson, P. G. Gum Arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest. Biol. Technol. 58, 42–47 (2010).
Nasir, O. et al. Effects of gum Arabic (Acacia senegal) on renal function in diabetic mice. Kidney Blood Press Res. 35, 365–372 (2012).
Patel, S. & Goyal, A. Applications of natural polymer gum Arabic: A review. Int. J. Food Prop. 18, 986–998 (2015).
Daraghmah, F. S. & Qubbaj, T. Impact of gum arabic and cactus mucilage as potential coating substances combined with calcium chloride treatment on tomato (Solanum lycopersicum L.) fruit quality attributes under ambient storage conditions. Can. J. Plant Sci 102(2), 375–384 (2021).
Handojo, L.A., Shofinita, D., Evelina, G., Nasution, A.N. Edible coating development to extend shelf life of mangoes (Mangivera indica L.). Proc. The 4th International Conference on Food and Agriculture IOP Conf. Series: Earth and Environmental Science. 980: 012046. (IOP Publishing, 2022).
Khaliq, G., Mohamed, M. T. M., Ding, P., Ghazali, H. M. & Ali, A. Storage behaviour and quality responses of mango (Mangifera indica L.) fruit treated with chitosan and gum arabic coatings during cold storage conditions. Int. Food Res. J. 23, S141–S148 (2016).
Jones, N., Ray, B., Ranjit, K. T. & Manna, A. C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 279(1), 71–76 (2008).
Jin, T., Sun, D., Su, J. Y., Zhang, H. & Sue, H. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157:H7. J. Food Sci. 74(1), 46–52 (2009).
Aydin Sevinc, B. & Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 94(1), 22–31 (2010).
Oprea, O. et al. ZnO applications and challenges. Curr. Org. Chem. 18(2), 192–203 (2014).
Sirelkhatim, A. et al. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 7(3), 219–242 (2015).
Zhang, Z. & Xiong, H. Photoluminescent ZnO nanoparticles and their biological applications. Mater. 8, 3101–31027 (2015).
Yan, S., Salley, S. O. & Ng, K. Y. S. Simultaneous transesterification and esterification of unrefined or waste oils over ZnO–La2O3 catalysts. Appl. Catal. A Gen. 353(2), 203–212 (2009).
Xie, Y., He, Y., Irwin, P. L., Jin, T. & Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 77(7), 2325–2331 (2011).
Sogvar, O. B., Saba, M. K., Emamifar, A. & Hallaj, R. Influence of nano-ZnO on microbial growth, bioactive content and postharvest quality of strawberries during storage. Innov. Food Sci. Emerg. Technol. 35, 168–176 (2016).
Tam, K. H. et al. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films. 516(18), 6167–6174 (2008).
Youssef, A. M. et al. Development of bionanocomposite materials and its use in coating of Ras cheese. Food Chem. 1(270), 467–475 (2019).
Youssef, A. M., El-Sayed, H. S., El-Sayed, S. M., Fouly, M. & Abd El-Aziz, M. E. Novel Bionanocomposites based on cinnamon nanoemulsion and TiO2-NPs for preserving fresh chicken breast fillets. Food Bioprocess Technol. 16, 356–367 (2023).
Magwaza, L. S. & Tesfay, S. Z. A review of destructive and non-destructive methods for determining avocado fruit maturity. Food Bioprocess Technol. 8, 1995–2011 (2015).
Abd El-Moniem, E. A. A., Abdel-Hak, R. S., Yousef, A. R. M., Saleh, M. M. S. & El-lethy, S. R. Storability and fruit properties of hass avocado as affected by moringa seed oil, algae extract and Arabic gum postharvest treatments. Egypt J. Chem. 66(1), 155–168 (2023).
Mcguire, R. G. Reporting of objective color measurements. HortScience. 27, 1254–1255 (1992).
Rekha, C. et al. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans. 19(2), 303–310 (2012).
Oluwafemi, J., Caleb, V., Mahajan, U. & Corli, W. Modelling the respiration rates of pomegranate fruit and arils. Postharvest Biol. Technol. 64(1), 49–54 (2012).
Uarrota, V. G. et al. Metabolic profiling and biochemical analysis of stored Hass avocado fruit by GC-MS and UHPLC-UV-VIS revealed oxidative stress as the main driver of ‘blackspot’ physiological disorder. Food Sci. Technol. 57, 7896–7916 (2022).
Saavedra, J. et al. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 198, 81–90 (2017).
Bailly, C. & Kranner, I. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. Seed Dormancy. 773, 343–367 (2011).
Worthington Enzyme Manual. Worthington, K. and Worthington, V. (2011). Worthington Biochemical Corporation. (Date of access October 20, 2020)
Freed R, Eisensmith SP, Goetz S, Reicosky D, Smail VM, Wollberg P. MSTAT-C A Microcomputer Program for the Design, Management and Analysis of Agronomic Research Experiments. https://www.msu.edu/~freed/disks.htm., (1990).
Snedecor, G. W. & Cochran, W. G. Statistical Methods 7th edn, 507 (Iowa State University Press, 1989).
Mahmoud, S. H., Salama, D. M. & Abd El-Aziz, M. E. Effect of chitosan and chitosan nanoparticles on growth, productivity and chemical quality of green snap bean. Biosci Res. 15(4), 4307–4321 (2018).
Salama, D. M., Abd El-Aziz, M. E., Shaaban, E. A., Osman, S. A. & Abd El-Wahed, M. S. The impact of nanofertilizer on agro-morphological criteria, yield, and genomic stability of common bean (Phaseolus vulgaris L.). Sci. Rep. 12, 18552 (2022).
Tahir, H. E. et al. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J Food Biochem. 42, e12527 (2018).
Kubheka, S. F., Tesfay, S. Z., Mditshwa, A. & Magwaza, L. S. Evaluating the efficacy of edible coatings incorporated with moringa leaf extract on postharvest of “Maluma” avocado fruit quality and its bio-fungicidal effect. HortScience 55(4), 410–415 (2020).
Gol, N. B., Patel, P. R. & Rao, T. V. R. Improvement of qualityand shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 85, 185–195 (2013).
Romanazzi, G., Feliziani, E., Baños, S. B. & Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 57, 579–601 (2017).
Maftoonazad, N. & Ramaswamy, H. S. Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT Food Sci. Technol. 38(6), 617–624 (2005).
Magwaza, L. S. et al. Non-chilling physiological rind disorders in citrus fruit. Hortic. Rev. 41, 131–176 (2013).
Coyotl-Pérez, W. A. et al. Improving the shelf life of avocado fruit against Clonostachys rosea with chitosan hybrid films containing thyme essential oil. Polymers 14, 2050 (2022).
El-Anany, A. M., Hassan, G. F. A. & Ali, F. M. Effects of edible coatings on the shelf-life and quality of Anna apple (Malus domestica Borkh) during cold storage. J. Food Technol. 7(1), 5–11 (2009).
Seymour, G. B., Qstergaard, L., Chapman, N. H., Knapp, S. & Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 64(1), 219–241 (2013).
Martínez-Romero, D. et al. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 39(1), 93–100 (2006).
Rai-Kalal, P., Tomar, R. S. & Jajoo, A. H2O2 signaling regulates seed germination in ZnO nanoprimed wheat (Triticum aestivum L.) seeds for improving plant performance under drought stress. Environ. Exp. Bot. 189, 104561 (2021).
Romanazzi, G. Chitosan treatment for the control of postharvest decay of table grapes, strawberries and sweet cherries. Fresh Produce 4, 111–115 (2010).
Rashedy, A., Shaban, A., El-Wakil, A., Abd El-Hamid, A. & Abdel Moniem, E. Efficiency of turmeric, hibiscus and beet root extracts as an eco-friendly edible coating to extend storage life of hass avocado fruit. Egypt. J. Chem. 66(6), 41–48 (2023).
Xing, Y. et al. Effect of chitosan/Nano-TiO2 composite coatings on the postharvest quality and physicochemical characteristics of mango fruits. Sci. Hortic. 263, 109135 (2020).
Hong, K., Xie, J., Zhang, L., Sun, D. & Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 144, 172–178 (2012).
Hernández-Muñoz, P., Almenar, E., Del Valle, V., Velez, D. & Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria ananassa) quality during refrigerated storage. Food Chem. 110, 428–435 (2008).
Cox, K. M., McGhie, T. K., White, A. & Woolf, A. B. Skin color and pigment changes during ripening of avocado fruits. Postharvest Biol. Technol. 31(3), 287–294 (2004).
Adjouman, Y. D. et al. Effect of edible coating based on improved cassava starch on post-harvest quality of fresh tomatoes (Solanum lycopersicum L.) Iran. J. Nutr. Sci. Food Technol. 4, 1–10 (2018).
Jeong, J., Huber, D. J. & Sargent, S. A. Delay of avocado (Persea americana) fruit ripening by 1-methylcyclopropene and wax treatments. Postharvest Biol. Technol. 28, 247–257 (2003).
Maftoonazad, N., Ramaswamy, H., Moalemiyan, M. & Kushalappa, A. Effect of pectinbased edible emulsion coating on changes in quality of avocado exposed to Lasiodiplodia theobromae infection. Carbohydr. Polym. 68, 341–349 (2007).
Aguiló-Aguayo, I., Oms-Oliu, G., Martín-Belloso, O. & Soliva-Fortuny, R. Impact of pulsed light treatments on quality characteristics and oxidative stability of fresh-cut avocado. LWT Food Sci. Technol. 59, 320–326 (2014).
Gómez-López, V. M. Some biochemical properties of polyphenol oxidase from two varieties of avocado. Food Chem. 77(2), 163–169 (2002).
Gómez, P. L., Salvatori, D. M., García-Loredo, A. & Alzamora, S. Pulsed light treatment of cut apple: Dose effect on color, structure, and microbiological stability. Food Bioprocess. Technol. 5(6), 2311–2322 (2012).
Sellamuthu, P. S., Mafune, M., Sivakumar, D. & Soundy, P. Thyme oil vapour and modified atmosphere packaging reduce anthracnose incidence and maintain fruit quality in avocado. J. Sci. Food Agric. 93, 3024–3031 (2013).
Lemmer, D., Kruger, F.J. Laboratory based evaluation of 1-methyl cyclopropene (1-MCP): with five South African commercial export cultivars (2003), in Vth World Avocado Congress (Actas V Congreso Mundial del Aguacate), 611–616 (2003).
Pesis, E. et al. Ethylene involvement in chilling injury symptoms of avocado during cold storage. Postharvest Biol. Technol. 24, 171–181 (2002).
Hofman, P. J. & Jobin-Décor, M. Effect of fruit sampling and handling procedures on the percentage dry matter, fruit mass, ripening and skin color of ‘Hass’ avocado. J. Hort. Sci. Biotech. 74, 277–282 (1999).
Tesfay, S. Z., Bertling, I. & Bower, J. P. Anti-oxidant levels in various tissues during the maturation of ‘Hass’ avocado (Persea americana Mill.). J. Hortic. Sci. Biotechnol. 85, 106–112 (2010).
Switala, J. & Loewen, P. C. Diversity of properties among catalases. Arch. Biochem. Biophys. 15401(2), 145–154 (2002).
Mahmoudi, R., Razavi, F. & Rabiei, P. L. Postharvest chitosan-arginine nanoparticles application ameliorates chilling injury in plum fruit during cold storage by enhancing ROS scavenging system activity. BMC Plant Biol. 22, 555 (2022).
Li, X. et al. Effect of nano ZnO coated active packaging on quality of fresh cut “Fuji” apple. Int. J. Food Sci. Technol. 46(9), 1947–1955 (2011).
Shah, H. M. S. et al. Melatonin application suppresses oxidative stress and maintains fruit quality of cold stored ‘Esperanza’ raspberries by regulating antioxidant system. Postharvest Biol. Technol. 207, 112597 (2024).
Acknowledgements
The authors thank Cairo University and National Research center for the facilities to carry out this work. Also, the authors thank Dr. M.M. Abdel Wahab (Plant Physiology Department) and Dr. H.M. Abdel-Lattif (Crops Department) for some advices in plant analysis and statistical analysis.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Author contributions statement M.E.A. and E.A.A.A. has designed the experiment and created the main idea of research. A.A.R. has controlled the conducting experiment, data collection and analyzing and preparation the manuscript. M.E.A. prepared and wrote NPs parts and M.E.A. along with A.S.E.A. analysis NPs. A.A.R., H.H.H., A.S.E.A. and H.E.E. has conducted the experiment and done the laboratory analysis. M.E.A., E.A.A.A. and A.A.R. collaborated on various parts of the manuscript preparation, analysis, and discussion. All authors revised and approved of its content. A.A.R. and M.E.A. performed most of this experiment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashedy, A.A., Abd El-Aziz, M.E., Abd-Allah, A.S.E. et al. Arabic gum/chitosan/Zn–NPs composite film maintains the quality of Hass avocado fruit by delaying ripening and activating enzymatic defense mechanisms. Sci Rep 14, 401 (2024). https://doi.org/10.1038/s41598-023-50642-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50642-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.