Abstract
An exciting prospect in the field of magnetic fluid hyperthermia (MFH) has been the integration of noble rare earth elements with biopolymers (chitosan/dextran) that have optimum structures to tune specific effects on magnetic nanoparticles (MNPs). Remarkably, it has been demonstrated that dipole–dipole interactions have a significant influence on nanoparticle dynamics. In this article, we present an exhaustive scrutiny of dipolar interactions and how this affects the efficiency of MFH applications. In particular, we prepare chitosan and dextran-coated Tb-doped MNPs and study whether it is possible to increase the heat released by controlling the dipole–dipole interactions. It has been indicated that even moderate control of agglomeration may substantially impact the structure and magnetization dynamics of the system. Besides estimating the specific loss power value, our findings provide a deep insight into the relaxation mechanisms and bring to light how to tune the self-heating efficacy towards magnetic hyperthermia.
Similar content being viewed by others
Introduction
The comprehension of particle-intrinsic characteristics, colloidal stabilities, and spatial configurations are of utmost importance in determining the magnetic relaxation behavior of magnetic nanoparticles (MNPs), given the promising biomedical applications they offer, including bioimaging, drug delivery, and magnetic fluid hyperthermia (MFH)1,2,3,4,5. Despite numerous studies and diverse approaches to MFH, the influence of characteristic magnetic and structure parameters on the specific absorption rate (SAR) of suitably designed MNP systems remains an ongoing controversy. The principal mechanisms governing these processes are Néel (τN) and Brown (τB) relaxations6,7 which are affected by factors like controlled dipolar interactions, effective anisotropy constant (Keff) of the MNPs, particle size and shape, magnetic field strength, temperature, and the properties of the surrounding media in which the MNPs are dispersed. Nevertheless, the challenge of simultaneously optimizing all parameters under diverse experimental conditions for magnetic hyperthermia remains unresolved. In the context of in vivo applications, magnetic iron oxide nanoparticles, such as magnetite, maghemite, or a combination of both, have gained significant attention due to their promising characteristics and primarily attributed to the ease of synthesis methods and minimal toxicity levels8,9,10,11. The instability of oxidation states within Fe3O4 (FO) MNPs, poses a significant challenge, affecting magnetic tuning, structural integrity, and self-heating efficiency for MFH applications12. The presence of multiple factors, like differences in sample sizes or the impact of magnetic interactions, makes it challenging to accurately determine magnetic properties. In our previous studies, we focused on a Tb-doped FO system, primarily emphasizing the effective anisotropy to control the self-heating efficiency for MFH applications13. In the course of that investigation, we observed a notable improvement in heating efficiency. However, a further challenge arose due to the dispersion of MNPs in aqueous solutions, as their high surface energies make them prone to agglomerate13. One widely adopted strategy for controlling aggregation is successfully conjugating biomolecules, including peptides, oligonucleotides, antibodies, and natural polymers14,15,16,17,18,19. Recent studies have demonstrated that intra and inter-aggregate dipolar interaction effects are renowned for modifying the heating efficacy of the MNPs20. However, despite achieving magnetic nanoparticles with improved functionalities, dispersibility, and the challenge of obtaining MNPs with enhanced functionalities/dispersibility and high SAR still persists21,22. Notably, dextran and chitosan-coated Fe3O4 MNPs stand out as excellent options for clinical trials in hyperthermia applications due to their non-antigenic, biocompatible, bio-functional, and biodegradable nature17,23.
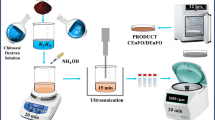
Expanding upon our previous research on the impact of Tb doping on Fe3O4 MNP, this study continues our investigation to correlate the dipolar interaction and analyze the effective magnetic anisotropy for enhancing the heating efficiency13. Herein, the study introduces an experimental approach for precise assessment of magnetic characteristics and heating efficiency, aiming to distinguish the energy contributions arising from magnetic dipolar interactions by employing a model system comprising Tb-doped magnetite MNPs with controlled polymer coatings that act as interspacing material as depicted in Fig. 1.
Materials and methods
Synthesis of the samples
The comprehensive technique for synthesizing the Tb-doped Fe3O4 (TbFO) MNPs is illustrated in our prior work13. Therefore, we enclose the concise expression of synthesizing procedure for chitosan and dextran coated Tb doped Fe3O4 MNPs only.
All designed MNPs were prepared through the simple co-precipitation technique, optimized the reactant concentration, and experimented with different temperatures for the final control over the synthesis method. The attained TbFO is further encaptulated using biopolymers, namely, chitosan and dextran, abbreviated as CTbFO and DTbFO throughout the article. In the experimental procedure, 0.26 g of chitosan was dispersed in 100 ml of 0.1 M acetic acid solution and was added dropwise to 0.5 g of TbFO, kept in 10 ml of double-deionized water under continual stirring at 180 rpm for 20 min and then ultrasonicated for 15 min. Whereas, ultrasonication is used for dispersion of the nanoparticles in the synthesis process. Then the reference MNPs had washed with ethanol/double-deionized water and centrifuged at 2100 rpm for 15 min. The precipitate attained from the experimentations was dried in a vacuum oven at 90° C for 12 h and lastly ground to powder for obtaining the engineered MNPs. Similarly, the same procedure was followed for synthesizing DTbFO MNPs. Herein, dextran was directly dissolved in double-deionized water instead of 0.1 M acetic acid solution.
Characterization of the MNPs
The phase purity and crystallographic characteristics of the reference magnetic nanoparticles were analyzed using X-ray diffraction (Rigaku Ultima IV), and the XRD characteristics were directed with Cu-Kα radiation (λ = 1.5406 Å). The elemental states and functional groups were examined using Fourier transform infrared spectra (FTIR) analysis accomplished on an Agilent Technology Cary 630 instrument. The morphology and average particle size were studied via ZEISS Gemini 300 field-emission scanning electron microscope (FESEM), while the selected area electron diffraction (SAED) and d-spacing measurements were studied with JEOL, JEM 2100 high-resolution transmission electron microscope (TEM). The chemical and electronic states of the MNPs were extensively examined using X-ray photoelectron spectroscopy on a Thermo Fisher Scientific Excalab Xi + instrument. X-ray radiation from an Al Kα source was employed for the analysis. Further, a thermogravimetric study (TGA) was conducted from room temperature to 800ºC under a nitrogen (N2) atmosphere through a Hitachi STA7300 thermal analyzer. The magnetic states of the MNPs were investigated by a Lakeshore 7410 series vibrating sample magnetometer (VSM) and Electron Spin Resonance (ESR; JEOL, JES-FA200) techniques. The reference MNPs underwent a self-heating study using an induction heating setup (Easy Heat-8310, Ambrell make, U.K.).
Statistical analysis
To gain more insights into the structural parameters, Rietveld refinement (RR) was performed using Full Proof software, and Vesta software is used to visualize the Ball-and-stick model of fcc cubic spinel structure of the processed MNPs.
Results and discussion
Structural and morphological study
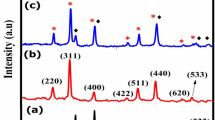
The initial analysis of powdered magnetic nanoparticles (MNPs) focused on their crystal structure, size, and shape. The XRD scans of synthesized chitosan and dextran-coated Tb doped FO MNPs, as portrayed in Fig. 2, reveal a pure crystalline phase, which exhibits distinct diffraction peaks identified as (220), (311), (400), (511), and (440). These peaks correspond to the crystallographic pattern of the cubic spinel structure of Fe3O4, as documented in the ICDD PDF card number 01–075-0033 with space group Fd \(\overline{3 }\) m24. Furthermore, dextran and chitosan have a non-crystalline structure; thus, no alternation of the crystalline structure was detected for DTbFO and CTbFO MNPs25.
In addition, to gain more insights into the structural parameters, Rietveld refinement (RR) was performed using Full Proof software, and the analysis allowed for a detailed examination of cation distribution and cell constants, as enclosed in Fig. 2, which showed fine agreement with the theoretical and experimental curves26,27. Table 1 presents the computed parameters obtained from Rietveld refinement (RR), including cation distribution, structure parameters such as crystallite size calculated by using the Scherrer formula28, U parameter, Bond Lengths (A-O and B-O), cell volume, Octahedral radius RB, and Tetrahedral radius RA. The analysis of Rietveld refinement revealed that the functionalized dextran and chitosan coatings noticeably influenced the cation distribution within the FO matrix. Consequently, the incomplete coordination of oxygen ions at the surface of nanoscale spinel FO systems can reduce coordination symmetry, impacting the magnetic parameters of the processed magnetic nanoparticles.
The slightly increased crystallite size and cell volume with the developed strain suggest the influence of dextran and chitosan in the system9. Furthermore, the presence of dextran and chitosan coatings in our ferrite matrix resulted in an enrichment of bond lengths (A-O/B-O), as indicated in Table 1. The observed increase in bond length can be attributed to various factors such as cation substitution, lattice strain, finite size effect, lattice stress29, etc. In Fig. 3, the Ball-and-stick model of the fcc cubic spinel structure of TbFO MNPs is presented, generated from VESTA software. The visualization reveals that, prior to coating, the Tb ions prefer to occupy the octahedral voids relatively than the tetrahedral voids within the FO matrix, which further uphold the cation distribution of RR analysis.
The FTIR spectra of designed nanoparticles are illustrated in Fig. 4, covering ~ 400 to 4000 cm−1 frequency range. The spectra of the processed nanoparticles displays two clear absorption peaks at around 428 and 540 cm−1, align with the characteristic features of the ferrite structure13. The absorption spectra at approximately 540 cm−1 (υ1) and 428 cm−1 (υ2) arise from the stretching vibration of the Fe–O bonds in the tetrahedral and octahedral metal complexes, respectively30. Figure 4 illustrates the observed shift of the absorption band (υ2) from approximately 428 cm−1 to higher wavenumbers, confirming the influence of Tb ions occupying the octahedral voids in the Fe3O4 system, supporting the results obtained from the computed XRD analysis. Furthermore, a broad absorption peak ranging from nearly ~ 3450 to 3550 cm−1 was observed, consistent to the stretching vibrations of O–H bonds in the absorbed H2O molecule, and the present observation is consistent with previous findings31. The presence of two additional absorption peaks at approximately 1595 cm−1 and 1371 cm−1 indicates the occurrence of N–H bending and C–O stretching, which strongly proposes the effective encapsulation of chitosan on the surface of the processed CTbFO MNPs23,31, 32. In the DTbFO spectra, an additional peak observed in the range of approximately 1000 to 1250 cm−1 can be accredited to the C–O and C–O–C stretching of the polymeric chain of dextran, suggesting the efficient encapsulation of dextran onto the TbFO system17.
Figure 5 displays the surface morphology micrographs of the chitosan and dextran-coated MNPs. The SEM images parade moderate agglomeration, berry-like, nearly uniform, and ~ spherical morphology. Further, it upholds the TEM analysis. Addedly, EDX analyses also displays the presence of the element Fe, O, and Tb for the characterized TbFO MNPs (Fig. 6), which is approximately equivalent to the stoichiometric one.
The TEM images depicted in Fig. 7 demonstrate that the predominance of the synthesized nanoparticles exhibit a ~ spherical morphology, and the issue of agglomeration is effectively addressed, aligning well with the findings observed through SEM analysis. The lognormal distribution estimates the particle size of FO, TbFO, DTbFO, and CTbFO MNPs. This estimation method strongly agrees with the results obtained from XRD analysis, as presented in Table 1 and Fig. 7h. Moreover, Fig. 7 reveals a noticeable increment in particle size, and it can be attributed to the influence of functionalized chitosan and dextran on the structure, resulting in modifications to both the particle size and shape. A potential explanation for the disparity in size between the nanoparticles coated with chitosan and dextran is the presence of charges originating from chitosan macromolecules, which are absent in dextran-coated MNPs. Chitosan is primarily attributed to the existence of reactive functional groups, namely hydroxyl (–OH) and amino (–NH2) groups. The presence of positively charged amino groups on the coated MNPs induces electrostatic repulsion among the chitosan-coated nanoparticles, thereby restricting their aggregation and leading to the formation of smaller, isolated particles4. Conversely, the absence of charges in dextran can contribute to partial agglomeration of the NPs, leading to an increase in particle sizes. Besides, the d-spacing consistent with the (311) plane of characterized TbFO, CTbFO, and DTbFO MNPs perfectly matches the calculated XRD data [Fig. 7b,d,f]. The SAED inset has concentric rings with spots, signifying the MNPs are polycrystalline in nature33. The visible diffraction rings correspond to the crystallographic planes (220), (311), (400), (422), (511), and (440), respectively, aligning consistently with the findings derived from the XRD analysis.
The X-ray Photoelectron Spectroscopy (XPS) analysis was performed to study the chitosan coating combined with Tb-doped magnetite nanoparticles. Figure 8a, the overall survey scan of chitosan-coated Tb-doped FO MNPs, depicted the successful encapsulation of chitosan and proper doping of Tb in the respective FO matrix. Figure 8b displays the high-resolution core Fe 2p electron spectra of the processed nanoparticles, revealing the spin-orbital parameters of 2p3/2 and 2p1/2 at approximately 711 eV and 722 eV peaks8, respectively. The scan results indicate that the Fe ions exhibit valence states of + 2 and + 3. Furthermore, the satellite Fe peaks observed in the scan correspond to the formation of bonds initiated by both octahedral and tetrahedral sites. Figure 8c and d show the observed C 1 s, N 1 s, and O 1 s spectra, with the nitrogen peak appearing at ~ 399 eV, providing strong evidence for the presence of chitosan functionalization of the characterized MNPs. The deconvolution of Tb 3d5/2 ions, revealing approximate binding energies of around ~ 124 eV and ~ 128 eV, suggests the existence of two non-equivalent bonds originating from the Tb ions inhabiting both tetrahedral and octahedral voids, as depicted in Fig. 8e. The overall survey scan of TbFO MNPs, demonstrating the optimum doping of Tb in the FO matrix, as depicted by Fig. 8f.
Thermogravimetric analysis (TGA)
To assess the thermal stability, phase-crystallinity, and weight percentage (wt%) of dextran and chitosan on the surface of the Tb-doped Fe3O4 nanoparticles, a Thermogravimetric Analysis (TGA) is conducted under an N2 atmosphere, covering the temperature range from room temperature to 800 °C. In Fig. 9, the degradation of aqueous compounds, metal hydroxides, and unreacted organic traces is depicted. Notably, there is a weight loss of ~ 10 ± 1 wt% observed at temperatures below 100 °C for all the synthesized MNPs, which can be accredited to the removal of absorbed H2O from the pure FO MNPs. Moreover, the incorporation of Tb ions in the FO matrix positively affects the thermal stability, leading to a decrease in weight loss to approximately 6 ± 1 wt%. Conversely, the MNPs functionalized with dextran and chitosan demonstrate a significant weight loss of 12.85 ± 1 wt% and 28.23 ± 2 wt%, respectively, within the temperature range of 150 to 600 °C. These results align well with previous studies reported in the literature22,25, 34, 35. Beyond 600 °C, a gradual weight loss is evident in Fig. 9, indicating that only the residual nanoparticles remain after complete decomposition of dextran and chitosan. Utilizing the TGA findings, the percentage of dextran and chitosan within TbFO MNPs was quantified at nearly 3.14 ± 1 wt% and 18.55 ± 2 wt%, correspondingly.
Magnetic analysis
The magnetic characteristics, spin-related phenomena, of the MNPs were analyzed via Electron Spin Resonance (ESR) spectroscopy (Fig. 10). The findings from the ESR measurements are summarized in Table 2, which includes parameters such as spin–spin relaxation time (τ1), resonance field (Hr), resonance linewidth (Hpp), effective g value, and full width at half maximum (FWHM) of the absorption spectra (ΔH = √3 Hpp)36. The broadening of the ESR graph exhibits g ~ 2, indicating the superparamagnetic phase of the processed MNPs37. Notably, the broadening of the curves indicates the existence of the pre-dominating dipolar interaction38. The listed increasing Hr values for CTbFO MNPs revealed the constant drop in the magnetic anisotropy and saturation magnetization of their internal fields and can be further established through VSM analysis. Additionally, the small value of g and Hpp signifies the strong super-exchange interaction. After this, the lower g and Hpp values for dextran and chitosan-coated MNPs illustrate a stochastic arrangement of magnetic moments with enhancing particle size39,40,41. As enumerated in Table 2, the listed lower spin–spin relaxation time (τ1) for coated samples rather than the uncoated one can lead to an ability of higher power dissipation of the synthesized MNPs42.
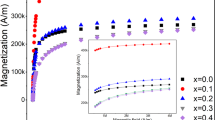
The magnetic characteristics of the processed magnetic nanoparticles (MNPs) are depicted in Fig. 11. The hysteresis graphs were analyzed using the Langevin fit method, which revealed the ~ nearly superparamagnetic nature of the nanoparticles43. To accurately determine the saturation magnetization (Ms) and effective anisotropy constant (Keff), we employed the Law of Approach to Saturation Magnetization (LAS) to fit the hysteresis graphs44. The obtained magnetic parameters, including saturation magnetization (Ms), effective anisotropy constant (Keff), retentivity (Mr), and coercivity (Hc), are summarized in Table 3 based on the S-shaped hysteresis graphs.
According to the findings presented in Table 3, it can be noted that the Ms of the pure FO MNPs exhibits a notable reduction in comparison to the TbFO MNPs. The saturation magnetization of the TbFO MNPs, as observed in the results, is significantly higher than often reported in the existing literature45. There are a few possible reasons; for instance, the concentration of oxygen vacancies present in TbFO leading to the overall enrichment in the magnetization, as it promotes an increase in the amount of Fe2+ to Fe3+ ions transitions for compensation of excessive positive charge46. Addedly, incorporating a small amount of Tb ions into the system can significantly increase the saturation magnetization due to the higher spin magnetic moment of Tb3+ ions (~ 9.72 µB) compared to Fe2+ ions (~ 4.9 µB)13. Conversely, the decrease in Ms for coated MNPs can primarily be attributed to the spin canting at the surface, magnetic dilution caused by the non-magnetic nature of chitosan and dextran coatings, as well as interfacial effects and collective oscillation19,25, 42, 47. Moreover, the coated samples hinder the aggregation resulting from the dipolar interaction among magnetic cores, enhancing colloidal stability. This property also brings the benefit of impeding the interaction between Fe2+ ions and enzymes, thereby reducing the promotion of reactive oxygen species generation via the Fenton reaction48. Fenton’s reaction begins with the oxidation of the ferrous ion (Fe2+ cation) to the ferric ion (Fe3+ cation) in the presence of hydrogen peroxide, which acts as an oxidizing agent. This results in the formation of a hydroxide ion and a hydroxyl free radical as byproducts. In addition, the Rietveld refinement also supports the rearrangement of cation distribution on CTbFO and DTbFO MNPs. The observed enhancement in the Ms value of CTbFO relative to DTbFO is attributed to the ligand properties of chitosan and dextran, which act as ligands and influence the crystal field splitting following the principles of ligand field theory49,50. Observations indicate that chitosan-coated MNPs exhibit a higher inclination towards the low spin state of Fe3+ in the octahedral site, primarily attributed to the prevalence of the amine group in chitosan49. In contrast, dextran-coated MNPs prefer a high spin state of Fe3+ in the octahedral site, attributed to a hydroxyl group acting as a weak field ligand51.
Furthermore, the analysis presented in Table 3 highlights a decrease in coercivity (Hc), and effective magnetic anisotropy (Keff) for the chitosan and dextran-coated MNPs in compared to the uncoated FO and TbFO MNPs. In this regard, the reduction in the Keff in CTbFO and DTbFO MNPs can be attributed to the weakened interaction between Tb and Fe, leading to a decrease in the ratio of orbital to spin moments of 4f electrons and a subsequent weakening of the spin–orbit coupling52. The decreasing trend of coercivity (Hc) for the designed MNPs is due to the lower value of the anisotropy constant (Keff), which can be explained via the Stoner- Wohlfarth model53.
Self-heating efficiency study
To confirm the applicability of the characterized MNPs towards magnetic hyperthermia, their magnetic heating efficiency is most likely stimulated by an AC magnetic field at a frequency of 337 kHz, an alternating current of 250 A, and the magnetic field strength of 14.91 kAm-1, under the clinical safety limits (H.f ≤ 5 × 109 Am−1 s−1)54. Figure 12 displays the time-dependent temperature variant curve at 1 mg/mL concentration, fitted with the Box-Lucas model55 to estimate specific absorption rates (SAR) and intrinsic loss power (ILP) of the reference MNPs. The presence of optimal MNPs concentrations is evidently pivotal in both sustaining the hyperthermic range and amplifying the SAR. Haase et al. theoretically introduced the concept of an optimal concentration to achieve maximal SAR56 and experimentally validated it by Lahiri et al.57. Analogously, Tan et al. reported the impact of optimum concentration considerably affects the heating power amplitude through Monte Carlo simulations58. Moreover, the graph (Fig. 12) indicates that all the reference samples parade the hyperthermic threshold phase (42 °C to 47 °C)59.
It is worth mentioning that Neel relaxations dominate over Brownian relaxation for the superparamagnetic MNPs within the size range ~ 16 ± 3 nm. Therefore the heat generation for the synthesized MNPs will be predominated by Neel relaxations60. Zubarev et al. reported that MNPs with diameters of 16–20 nm exhibit a significant increase in heat production, up to 30%, due to their intrinsic interactions57,58. Although the lower magnetization parameters (Ms and Keff) of DTbFO and CTbFO MNPs show better heating efficacy (Fig. 13) than the uncoated ones, which can be explained via dipolar interaction energy20,61, 62. According to the ESR analysis, it also reflects the dominance of spin–spin relaxation, highlighting the significant contribution of dipole–dipole interaction in enhancing the SAR within our system63. It has been reported that the dipolar interaction energy64 (ɛDipolar) per particle is ɛDipolar = αμ2/d3, where α represents the proportionality constant, μ is the magnetic moment of the MNPs, and d is the mean interparticle distance. Notably, the dipolar interaction energy is directly proportional to the demagnetizing field65. Henceforth, the existence of ɛDipolar induces a random torque, which perturbs the spin relaxation process by the competition between intraparticle interactions and anisotropy among the MNPs. As a consequence, the competition gives rise to frustrated magnetic moments or randomization of easy anisotropy axes orientation, so demagnetizing effect arises in the system, which may cause reduced heating efficacy in uncoated MNPs. Another stimulating result from Fig. 13 is that the heating response is higher for CTbFO than the DTbFO system. As we can observe from the TEM analysis, CTbFO MNPs are more dispersed than DTbFO MNPs, which resembles decreased dipolar interaction energy leading to improving SAR. Based on the magnetic findings (Fig. 14), it has been observed that the saturation magnetization is relatively high for CTbFO MNPs than DTbFO MNPs, suggesting that this likely contributes to the improved heating efficiency66. Henceforth, these results have empirical consequences for MFH optimization. In light of this, the chitosan-coated Tb-doped Fe3O4 MNPs exhibit a high heating efficacy among all the engineered samples, making them a safe and effective agent for hyperthermia applications.
Conclusion
In summary, a methodology has been explained for designing chitosan and dextran-functionalized Tb-doped FO nanoparticles; interestingly, the biopolymer encapsulation provides a promising strategy to control the agglomeration of the reference MNPs. Incorporating chitosan and dextran into the TbFO MNPs enhances the self-heating efficiency of the characterized MNPs and makes them suitable for hyperthermia applications. Moreover, these results suggest that the reported SAR value (789.21 W/g) for CTbFO MNPs, which is under safety limit, can lead towards the finite possibility for future investigations to explore the feasibility of using these MNPs in vivo and to optimize the treatment parameters for an exciting new avenue toward successful MFH applications.
Data availability
The data that support the findings of this study are available within the article.
Change history
26 March 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-024-57685-9
References
Pan, J., Xu, Y., Wu, Q., Hu, P. & Shi, J. Mild magnetic hyperthermia-activated innate immunity for liver cancer therapy. J. Am. Chem. Soc. 143(21), 8116–8128. https://doi.org/10.1021/jacs.1c02537 (2021).
Lastovina, T. A., Bugaev, A. L., Kubrin, S. P., Kudryavtsev, E. A. & Soldatov, A. V. Structural studies of magnetic nanoparticles doped with rare-earth elements. J. Struct. Chem. 57(7), 1444–1449. https://doi.org/10.1134/S0022476616070209 (2016).
Park, J. C. et al. Surface design of Eu-doped iron oxide nanoparticles for tuning the magnetic relaxivity. ACS Appl. Mater. Interfaces 10(30), 25080–25089. https://doi.org/10.1021/acsami.8b06569 (2018).
Aranaz, I. et al. Chitosan: An overview of its properties and applications. Polymers (Basel) 13(19), 3256. https://doi.org/10.3390/polym13193256 (2021).
Li, Q. et al. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 7(1), 1–4. https://doi.org/10.1038/s41598-017-09897-5 (2017).
Kerroum, M. A. A. et al. Quantitative analysis of the specific absorption rate dependence on the magnetic field strength in Znxfe3−xo4 nanoparticles. Int. J. Mol. Sci. 21(20), 1–24. https://doi.org/10.3390/ijms21207775 (2020).
Chalifour, A. R., Davidson, J. C., Anderson, N. R., Crawford, T. M. & Livesey, K. L. Magnetic relaxation time for an ensemble of nanoparticles with randomly aligned easy axes: A simple expression. Phys. Rev. B 104(9), 1–11. https://doi.org/10.1103/PhysRevB.104.094433 (2021).
Priya, K., Fopase, R., Pandey, L. M. & Borah, J. P. Influence of Gd-doping on structural, magnetic, and self-heating properties of Fe 3 O 4 nanoparticles towards magnetic hyperthermia applications. Phys. B Phys. Condens. Matter 645(July), 414237. https://doi.org/10.1016/j.physb.2022.414237 (2022).
Hazarika, K. P. & Borah, J. P. RSC advances parameter tuning, and self heating in magnetic nanoparticles. RSC Adv. 2023(13), 5045–5057. https://doi.org/10.1039/D2RA07924K (2023).
Kermanian, M. et al. Inulin-coated iron oxide nanoparticles: A theranostic platform for contrast-enhanced MR imaging of acute hepatic failure. ACS Biomater. Sci. Eng. 7(6), 2701–2715. https://doi.org/10.1021/acsbiomaterials.0c01792 (2021).
Alotaibi, I. et al. Synthesis, characterization and heating efficiency of Gd-doped maghemite (γ-Fe2O3) nanoparticles for hyperthermia application. Phys. B Condens. Matter 2022(625), 2–10. https://doi.org/10.1016/j.physb.2021.413510 (2021).
Nazari, M., Ghasemi, N., Maddah, H. & Motlagh, M. M. Synthesis and characterization of maghemite nanopowders by chemical precipitation method. J. Nanostruct. Chem. 4(2), 2–6. https://doi.org/10.1007/s40097-014-0099-9 (2014).
Hazarika, K. P. & Borah, J. P. Biocompatible Tb doped Fe3O4 nanoparticles with enhanced heating efficiency for magnetic hyperthermia application. J. Magn. Magn. Mater. 2022(560), 251–256. https://doi.org/10.1016/j.jmmm.2022.169597 (2022).
Xiang, H. et al. One-pot synthesis of water-soluble and biocompatible superparamagnetic gadolinium-doped iron oxide nanoclusters. J. Mater. Chem. B 8(7), 1432–1444. https://doi.org/10.1039/c9tb02212k (2020).
Fu, R., Yan, Y., Roberts, C., Liu, Z. & Chen, Y. The role of dipole interactions in hyperthermia heating colloidal clusters of densely-packed superparamagnetic nanoparticles. Sci. Rep. 8(1), 1–10. https://doi.org/10.1038/s41598-018-23225-5 (2018).
Kurihara, Y., Yokota, H. & Takahashi, M. Water-dispersible carboxymethyl dextran-coated melamine nanoparticles for biosensing applications. ACS Omega https://doi.org/10.1021/acsomega.2c05653 (2022).
Naha, P. C. et al. Dextran-coated cerium oxide nanoparticles: A computed tomography contrast agent for imaging the gastrointestinal tract and inflammatory bowel disease. ACS Nano 14(8), 10187–10197. https://doi.org/10.1021/acsnano.0c03457 (2020).
Rajan, A., Sharma, M. & Sahu, N. K. Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci. Rep. 10(1), 1–15. https://doi.org/10.1038/s41598-020-71703-6 (2020).
Sugumaran, P. J., Liu, X. L., Herng, T. S., Peng, E. & Ding, J. GO-functionalized large magnetic iron oxide nanoparticles with enhanced colloidal stability and hyperthermia performance. ACS Appl. Mater. Interfaces 11, 22703–22713. https://doi.org/10.1021/acsami.9b04261 (2019).
Valdés, D. P., Lima, E., Zysler, R. D., Goya, G. F. & De Biasi, E. Role of anisotropy, frequency, and interactions in magnetic hyperthermia applications: Noninteracting nanoparticles and linear chain arrangements. Phys. Rev. Appl. 15(4), 1–18. https://doi.org/10.1103/PhysRevApplied.15.044005 (2021).
Shams, S. F. et al. Element-specific contributions to improved magnetic heating of theranostic CoFe2O4 nanoparticles decorated with Pd. Sci. Rep. 11(1), 1–15. https://doi.org/10.1038/s41598-021-95189-y (2021).
Lesiak, B. et al. Surface study of Fe3O4 nanoparticles functionalized with biocompatible adsorbed molecules. Front. Chem. https://doi.org/10.3389/fchem.2019.00642 (2019).
Lachowicz, D. et al. Enhanced hyperthermic properties of biocompatible zinc ferrite nanoparticles with a charged polysaccharide coating. J. Mater. Chem. B 7(18), 2962–2973. https://doi.org/10.1039/c9tb00029a (2019).
Shatooti, S., Mozaffari, M., Reiter, G., Zahn, D. & Dutz, S. Heat dissipation in Sm3+ and Zn2+ co-substituted magnetite (Zn0.1SmxFe2.9-XO4) nanoparticles coated with citric acid and pluronic F127 for hyperthermia application. Sci. Rep. https://doi.org/10.1038/s41598-021-96238-2 (2021).
Pourmortazavi, S. M., Sahebi, H., Zandavar, H. & Mirsadeghi, S. Fabrication of Fe3O4 nanoparticles coated by extracted shrimp peels chitosan as sustainable adsorbents for removal of chromium contaminates from wastewater: The design of experiment. Compos. B Eng. 2019(175), 107130. https://doi.org/10.1016/j.compositesb.2019.107130 (2019).
Antic, B., Kremenovic, A., Nikolic, A. S. & Stoiljkovic, M. Cation distribution and size-strain microstructure analysis in ultrafine Zn–Mn ferrites obtained from acetylacetonato complexes. J. Phys. Chem. B 108(34), 12646–12651 (2004).
Rodríquez-Carvajal, J. & Roisnel, T. Line broadening analysis using Fullprof: Determination. Mater. Sci. Forum 444, 123–126. https://doi.org/10.4028/www.scientific.net/MSF.443-444.123 (2004).
Gul, I. H., Abbasi, A. Z., Amin, F., Anis-ur-Rehman, M. & Maqsood, A. Structural, magnetic and electrical properties of Co1-XZnxFe2O4 synthesized by co-precipitation method. J. Magn. Magn. Mater. 311(2), 494–499. https://doi.org/10.1016/j.jmmm.2006.08.005 (2007).
Paswan, S. K. et al. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J. Phys. Chem. Solids 2021(151), 109928. https://doi.org/10.1016/j.jpcs.2020.109928 (2020).
Patange, S. M. et al. Elastic properties of nanocrystalline aluminum substituted nickel ferrites prepared by co-precipitation method. J. Mol. Struct. 1038, 40–44. https://doi.org/10.1016/j.molstruc.2012.12.053 (2013).
Nam, P. H. et al. Physical characterization and heating efficacy of chitosan-coated cobalt ferrite nanoparticles for hyperthermia application. Phys. E Low-Dimens. Syst. Nanostruct. 2021(134), 114862. https://doi.org/10.1016/j.physe.2021.114862 (2020).
Shakil, M. S. et al. In vivo toxicity studies of chitosan-coated cobalt ferrite nanocomplex for its application as MRI contrast dye. ACS Appl. Bio Mater. 3(11), 7952–7964. https://doi.org/10.1021/acsabm.0c01069 (2020).
Abbas, Y. M., Mansour, A. B., Ali, S. E. & Ibrahim, A. H. Investigation of structural and magnetic properties of multiferroic La 1–x Y x FeO 3 perovskites, prepared by citrate auto-combustion technique. J. Magn. Magn. Mater. 482(March), 66–74. https://doi.org/10.1016/j.jmmm.2019.03.056 (2019).
Kulkarni, A. B., Mathad, S. N. & Bakale, R. P. The evaluation of kinetic parameters for cadmium doped Co-Zn ferrite using thermogravimetric analysis. Ovidius Univ. Ann. Chem. 30(1), 60–64. https://doi.org/10.2478/auoc-2019-0011 (2019).
Arsalani, N., Fattahi, H. & Nazarpoor, M. Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym. Lett. 4(6), 329–338. https://doi.org/10.3144/expresspolymlett.2010.42 (2010).
Massoudi, J. et al. Magnetic and spectroscopic properties of Ni-Zn-Al ferrite spinel: From the nanoscale to microscale. RSC Adv. 10, 34556–34580. https://doi.org/10.1039/d0ra05522k (2020).
Mukherjee, S., Pal, A. K., Bhattacharya, S. & Chattopadhyay, S. Field-induced spin—Flop transitions of interacting nanosized α -Fe 2 O 3 particles dispersed in a silica glass matrix. J. Phys. Condens. Matter 20, 055204. https://doi.org/10.1088/0953-8984/20/05/055204 (2008).
Das, R., Chaudhuri, U., Chanda, A. & Mahendiran, R. Broadband electron spin resonance study in a Sr2FeMoO6Double Perovskite. ACS Omega 5(28), 17611–17616. https://doi.org/10.1021/acsomega.0c02070 (2020).
Shukla, V. K. & Mukhopadhyay, S. Transverse Spin relaxation and magnetic correlation in Pr1-XCaxMnO3: Inuence of particle size variation and chemical doping. arXiv 3, 1–8. https://doi.org/10.1063/1.4977580 (2017).
Vasilchikova, T. et al. Peculiarities of magnetic ordering in the S=5/2 Two-dimensional square-lattice antimonate NaMnSb O4. Phys. Rev. B 101(5), 1–12. https://doi.org/10.1103/PhysRevB.101.054435 (2020).
Lado, J. L., Ferrón, A. & Fernández-Rossier, J. Exchange mechanism for electron paramagnetic resonance of individual adatoms. Phys. Rev. B 96(20), 1–6. https://doi.org/10.1103/PhysRevB.96.205420 (2017).
Massoudi, J. et al. Magnetic and spectroscopic properties of Ni-Zn-Al ferrite spinel: From the nanoscale to microscale. RSC Adv. 10(57), 34556–34580. https://doi.org/10.1039/d0ra05522k (2020).
Lemine, O. M., Madkhali, N., Hjiri, M., All, N. A. & Aida, M. S. Comparative heating efficiency of hematite (α-Fe 2 O 3) and nickel ferrite nanoparticles for magnetic hyperthermia application. Ceram. Int. 46(18), 28821–28827. https://doi.org/10.1016/j.ceramint.2020.08.047 (2020).
Gholizadeh, A. A comparative study of the physical properties of Cu-Zn ferrites annealed under different atmospheres and temperatures: Magnetic enhancement of Cu 0.5 Zn 0.5 Fe2O4 nanoparticles by a reducing atmosphere. J. Magn. Magn. Mater. 452, 389–397. https://doi.org/10.1016/j.jmmm.2017.12.109 (2018).
Akhtar, P. et al. Structural and magnetic evaluations of rare-earths (Tb, Pr, Ce, Gd, Y)-doped spinel ferrites for high frequency and switching applications. J. Mater. Sci. Mater. Electron. 32(6), 7692–7703. https://doi.org/10.1007/s10854-021-05487-4 (2021).
Wrzesińska, A. et al. Structural, electrical, and magnetic study of La-, Eu-, and Er- doped bismuth ferrite nanomaterials obtained by solution combustion synthesis. Sci. Rep. 11(1), 1–12. https://doi.org/10.1038/s41598-021-01983-z (2021).
Myrovali, E. et al. Toward the separation of different heating mechanisms in magnetic particle hyperthermia. ACS Omega https://doi.org/10.1021/acsomega.2c05962 (2023).
León Félix, L. et al. Gold-decorated magnetic nanoparticles design for hyperthermia applications and as a potential platform for their surface-functionalization. Sci. Rep. 9(1), 1–11. https://doi.org/10.1038/s41598-019-40769-2 (2019).
Trueba, A., Aramburu, J. A., Barriuso, M. T. & Moreno, M. Spectrochemical series and the dependence of Racah and 10 Dq parameters on the metal—Ligand distance: Microscopic origin. J. Phys. Chem. A 2011(115), 1423–1432. https://doi.org/10.1021/jp110586e (2011).
Chakraborty, I. et al. Magnetic field-dependent photoluminescence of tartrate-functionalized gadolinium-doped manganese ferrite nanoparticles: A potential therapeutic agent for hyperbilirubinemia treatment. ACS Appl. Nano Mater. 4(5), 4379–4387. https://doi.org/10.1021/acsanm.0c03073 (2021).
Tassa, C., Shaw, S. Y. & Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 44(10), 842–852. https://doi.org/10.1021/ar200084x (2011).
Luo, C. et al. The influence of Nd dopants on spin and orbital moments in Nd-doped permalloy thin films. Appl. Phys. Lett. 105(8), 082405. https://doi.org/10.1063/1.4894180 (2014).
Kahmann, T., Rösch, E. L., Enpuku, K., Yoshida, T. & Ludwig, F. Determination of the effective anisotropy constant of magnetic nanoparticles—Comparison between two approaches. J. Magn. Magn. Mater. 519, 167402. https://doi.org/10.1016/j.jmmm.2020.167402 (2021).
Kossatz, S. et al. High therapeutic efficiency of magnetic hyperthermia in xenograft models achieved with moderate temperature dosages in the tumor area. Pharm. Res. 31(12), 3274–3288. https://doi.org/10.1007/s11095-014-1417-0 (2014).
Lanier, O. L. et al. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 36(1), 687–701. https://doi.org/10.1080/02656736.2019.1628313 (2019).
Haase, C. & Nowak, U. Role of dipole-dipole interactions for hyperthermia heating of magnetic nanoparticle ensembles. Phys. Rev. B Condens. Matter Mater. Phys. 85(4), 2–6. https://doi.org/10.1103/PhysRevB.85.045435 (2012).
Ranoo, S., Lahiri, B. B., Nandy, M. & Philip, J. Enhanced magnetic heating efficiency at acidic PH for magnetic nanoemulsions stabilized with a weak polyelectrolyte. J. Colloid Interface Sci. 579, 582–597. https://doi.org/10.1016/j.jcis.2020.06.093 (2020).
Tan, R. P., Carrey, J. & Respaud, M. Magnetic hyperthermia properties of nanoparticles inside lysosomes using kinetic Monte Carlo simulations: Influence of key parameters and dipolar interactions, and evidence for strong spatial variation of heating power. Phys. Rev. B Condens. Matter Mater. Phys. 90(21), 1–12. https://doi.org/10.1103/PhysRevB.90.214421 (2014).
Rosensweig, R. E. Heating magnetic fluid with alternating magnetic field. J. Urol. 252(6), 370–374. https://doi.org/10.1016/S0022-5347(17)32321-2 (2002).
Torres, T. E. et al. The relevance of Brownian relaxation as power absorption mechanism in magnetic hyperthermia. Sci. Rep. 9(1), 1–11. https://doi.org/10.1038/s41598-019-40341-y (2019).
Landi, G. T. Role of dipolar interaction in magnetic hyperthermia. Phys. Rev. B Condens. Matter Mater. Phys. 89(1), 1–6. https://doi.org/10.1103/PhysRevB.89.014403 (2014).
Pelayo García-Acevedo,* Manuel A. González-Gómez, Ángela Arnosa-Prieto, Lisandra de Castro-Alves, Yolanda Piñeiro, * and José Rivas. Role of Dipolar Interactions on the Determination of the Effective Magnetic.Pdf. 2022, p 2203397.
Hergt, R., Dutz, S. & Zeisberger, M. Validity limits of the Néel relaxation model of magnetic nanoparticles for hyperthermia. Nanotechnology 21(1), 015706. https://doi.org/10.1088/0957-4484/21/1/015706 (2010).
Allia, P. et al. Granular Cu-Co alloys as interacting superparamagnets. Phys. Rev. B Condens. Matter Mater. Phys. 64(14), 1444201–14442012. https://doi.org/10.1103/PhysRevB.64.144420 (2001).
Held, G. A., Grinstein, G., Doyle, H., Sun, S. & Murray, C. B. Competing interactions in dispersions of superparamagnetic nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 64(1), 124081–124084. https://doi.org/10.1103/physrevb.64.012408 (2001).
Mondal, D. K., Jonak, S., Paul, N. & Borah, J. P. Dextran mediated MnFe2O4/ZnS magnetic fluorescence nanocomposites for controlled self-heating properties. RSC Adv. 11(21), 12507–12519. https://doi.org/10.1039/d0ra09745d (2021).
Acknowledgements
The authors would like to acknowledge the Central Instrument Facility (CIF), IIT Guwahati for providing all the necessary facilities required for the characterization of the samples.
Author information
Authors and Affiliations
Contributions
K.P.H. collected, synthesized, evaluated, and wrote the main manuscript, and J.P.B. conceptualized and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-024-57685-9
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hazarika, K.P., Borah, J.P. RETRACTED ARTICLE: A comprehensive scrutiny to controlled dipolar interactions to intensify the self-heating efficiency of biopolymer encapsulated Tb doped magnetite nanoparticles. Sci Rep 14, 427 (2024). https://doi.org/10.1038/s41598-023-50635-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50635-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.