Abstract
We evaluated modifications in the hemostatic balance of different concentrations of apixaban (APIX) in 25 healthy donors and 53 patients treated with aspirin (ASA, n = 21), ASA and clopidogrel (ASA + CLOPI, n = 11), or ASA and ticagrelor (ASA + TICA, n = 21). Blood samples from participants were spiked ex vivo with apixaban 0 (APIX0), 40 (APIX40), and 160 ng/mL (APIX160). We assessed the effects of APIX on (1) clot formation, by ROTEM thromboelastometry; (2) thrombin generation primed by platelets; and (3) platelet and fibrin interactions with a thrombogenic surface, in a microfluidic model with circulating blood. APIX caused dose-related prolongations of clotting time with minimal impact on other ROTEM parameters. Thrombin generation was significantly inhibited by APIX160, with ASA + TICA actions showing the strongest inhibition (p < 0.01 vs APIX0). Microfluidic studies showed that APIX160 was more potent at suppressing platelet and fibrin interactions (p < 0.001 vs. APIX0). APIX40 demonstrated a consistent antithrombotic action but with a favorable protective effect on the structural quality of fibrin. APIX potentiated the antithrombotic effects of current antiplatelet regimens. APIX at 40 ng/mL, enhanced the antithrombotic action of single or dual antiplatelet regimens but was more conservative for hemostasis than the 160 ng/mL concentration.
Similar content being viewed by others
Introduction
The incidence of atrial fibrillation (AF) and deep vein thrombosis (DVT) increases with age. It is estimated that 2% of the population in the EU and the USA will have a diagnosis of AF, and 80% of these patients will require anticoagulant treatment1. Twenty percent of the patients with AF will require revascularization procedures during their lives2,3. It is estimated that 5–7% of patients undergoing percutaneous coronary interventions (PCI) will have indications for chronic oral anticoagulant therapy4.
Patients undergoing PCI, peripheral artery revascularization, or post-acute coronary syndrome (ACS), require dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 receptor antagonist5. The recent availability of newer direct oral anticoagulants (DOACs) and more potent antiplatelet agents (i.e., prasugrel, ticagrelor) has further increased the complexity of managing the thrombosis-bleeding balance in these patients6. A recent meta-analysis of 4 randomized controlled trials including 42,411 patients on DOACs, of which 33.4% were also on antiplatelet drugs, reported similar efficacy in thromboembolism prevention in patients on DOACs alone compared with those on both treatments (DOACs + antiplatelet). However, higher rates of bleeding were observed in patients exposed to combination therapy7.
Thrombin formation, platelet deposition, and fibrin formation play a major role not only in physiological hemostasis but also in the formation of occlusive thrombi. These processes are modulated by local rheologic conditions8. At the doses approved for treatment or prevention of thrombotic complications, DOACs significantly inhibit thrombin generation. In addition to being responsible for the conversion of fibrinogen into fibrin, thrombin is the most powerful platelet agonist9. There is a need for studies exploring the mechanisms involved in the additive or synergistic prohemorrhagic effects of triple therapies in patients on chronic dual antiplatelet administration and undergoing revascularization procedures.
Previous in vitro studies with circulating human blood have demonstrated that apixaban (APIX), at a concentration compatible with the Cmax (160 ng/mL) reached in patients with the standard dose for AF (5 mg BID)10, reduced both fibrin and platelet depositions preventing thrombus formation11. Interestingly, lower concentrations of APIX (corresponding to 40 ng/mL) compatible with levels reached after 2.5 mg b.i.d. did not inhibit fibrin deposition but still showed some antiplatelet effects under dynamic flow conditions. These studies suggest that there is a differential inhibitory effect of DOACs on fibrin formation and platelet aggregation. This dose-dependent effect may have a significant detrimental impact on hemostasis when concomitantly used with strong antiplatelet therapies.
We aimed to evaluate the antithrombotic/prohemorrhagic actions of standard (160 ng/mL) and a lower (40 ng/mL) concentration of APIX in patients receiving antiplatelet therapies. The studies were carried out ex vivo, spiking APIX concentrations into blood samples drawn from healthy individuals and from patients receiving antiplatelet treatments for primary or secondary prevention of ischemic events.
Results
Demographics of the study population
Figure 1 summarizes the study design and the distribution of control and treated groups Characteristics of patients and control groups are summarized in Table 1. The mean age of the participants was 62 years, 65% of whom were male. The patients in the study had a significant burden of cardiovascular risk factors; 43% were smokers; 52% had a history of hypertension, 24% had diabetes and 43% had dyslipidemia. Data Among all screened patients, 103 patients were finally recruited, and 52 patients completed the full study. Participants in the study were healthy participants not subjected to antithrombotic treatment (Controls), patients on aspirin treatment (ASA), or dual antiplatelet therapy with aspirin + clopidogrel (ASA + CLOPI) or aspirin + ticagrelor (ASA + TICA).
The diagram summarizes the design of the study. APIX was spiked on blood samples from the different study groups: healthy controls, or patients under treatment with aspirin (ASA), aspirin plus clopidogrel (ASA + CLOPI), or aspirin plus ticagrelor (ASA + TICA). APIX was initially solved in dimethyl sulfoxide (DMSO)and further diluted in phosphate-buffered saline (PBS) containing a low concentration of DMSO. Volumes spiked to blood samples for Control or treatment groups were equal (100 µL per 2 mL of blood or equivalent). Concentrations of APIX were corrected for the small volume change. Concentrations of DMSO were kept identical (< 0.05%) in all blood samples.
Functional studies on platelet responses in the PFA-200 confirmed the inhibitory effects on platelet functions expected for those patients subjected to antiplatelet treatments. No obvious evidence of high on-treatment platelet reactivity (HPR) was detected in our patient population (Supplementary Material_Table 1).
Effects of apixaban on thrombus dynamics
As summarized in Fig. 2, viscoelastic parameters triggered by EXTEM, were within the normal ranges in baseline samples (APIX0) from all participants No statistically significant differences were observed for the ROTEM parameters among the different antiplatelet treatment regimes. APIX spiking was associated with dose-related prolongations of CT, with APIX160 showing statistically significant differences for ASA + CLOPI (p < 0.01) and ASA or ASA + TICA treated patients (p < 0.001) versus APIX0. APIX40 also prolonged the CT, but it was statistically significant only for the ASA group (p < 0.05 vs APIX0). Minimal reductions in MCF were observed for APIX40 or r APIX160 in the different groups of patients (refer to Supplementary Material_Table 2 for more detailed information).
Modifications in clotting times in ROTEM studies. Bars summarize the results of clotting times in seconds (mean ± SEM) for Control and treatment groups. Apixaban caused a dose-related prolongation of clotting times in all study groups. Prolongations in clotting times were more evident with APIX160 and less pronounced in the group exposed to APIX40. *p < 0.05; **p < 0.01; ***p < 0.001; vs. respective APIX0. Control (n = 25); ASA (n = 21) ASA + CLOPI (n = 11), ASA + TICA (n = 21).
Evaluations of thrombin generation assay
Figure 3A and B summarize variations in lag times and maximum thrombin peaks measured in our studies. The addition of APIX at 40 or 160 ng/mL to the healthy-control group resulted in dose-related prolongations of the lag time (p < 0.01 and p < 0.001 respectively vs. APIX0) with a concomitant significant reduction in the thrombin peak. Prolongations in lag times after the in vitro addition of APIX40 or APIX160 to blood from patients treated with ASA, ASA + CLOPI, or ASA + TICA followed similar tendencies to those observed in the Control group. A progressive reduction in maximum thrombin peak was observed when APIX40 or APIX160 were added to samples of blood from patients treated with all the antiplatelet regimens (ASA, ASA + CLOPI, or ASA + TICA). APIX160 caused a marked reduction in thrombin peaks that were more evident in blood from patients treated with ASA and ASA + TICA (p < 0.001 vs APIX0. Times to reach the thrombin peak (time to peak) were consistently associated with prolongations on lag time and reductions in thrombin generation previously commented, but differences did not reach levels of statistical significance (refer to Supplementary Material_Table 3).
Modifications in lag times (A) and thrombin peaks (B) in thrombin generation assays. Bars summarize the results expressed as mean ± SEM of lag times (minutes) and thrombin peaks (nM) for Control and treatment groups. Apixaban caused a dose-related prolongation of lag times with a subsequent reduction in thrombin peaks. Prolongations in lag times were superior with APIX160 and less pronounced in the group exposed to APIX40. Reductions in thrombin peaks were more evident with APIX160. *p < 0.05; **p < 0.01; ***p < 0.001; vs. respective APIX0. Control (n = 25); ASA (n = 21) ASA + CLOPI (n = 11), ASA + TICA (n = 21).
Figure 4 summarizes the results of the total thrombin generation expressed as area under the curve (AUC in arbitrary units) for the different study groups. Exposure to APIX 40 or 160 ng/mL resulted in progressive reductions in the AUC in healthy subjects and also in the treated groups with the inhibitory effect being always more significant for APIX160 (p < 0.001 vs APIX0). The AUC in control studies (APIX0) was reduced in the presence of APIX 40 or 160 ng/mL (p < 0.05 and p < 0.001 respectively vs. APIX0). AUC for the ASA group was significantly reduced with APIX40 or APIX160 (p < 0.01 and p < 0.001 respectively vs. APIX0). Reductions in AUC were also observed in patients exposed to ASA + CLOPI or ASA + TICA with differences reaching statistical significance at p < 0.05 for APIX40 and p < 0.001 for APIX160 vs. APIX0.
Areas under the curve (AUC) calculated during the thrombin generation assay for the different study groups. Bars summarize the results of total thrombin generation in the different groups. The solid lines provide a visual estimate of the progressive effects calculated as a percentage of the AUC in the absence of apixaban (APIX0). Reductions in total thrombin generation appear more intense in the group of patients exposed to APIX + TICA, less pronounced in the group of patients treated with ASA, and somewhere intermediate in the group of patients treated with ASA + CLOPI. AUC expressed as arbitrary units *p < 0.05; **p < 0.01; ***p < 0.001; vs. respective APIX0. Control (n = 25); ASA (n = 21) ASA + CLOPI (n = 11), ASA + TICA (n = 21).
Effect on platelet and fibrin interactions
Figure 5 illustrates the inhibitory activity of APIX on platelet and fibrin deposition on the thrombogenic surface of our microfluidic chamber. The inhibition effect was dependent on the apixaban concentrations and more evident with APIX160. The control group showed a platelet surface coverage of 9.7 ± 0.8, 6.3 ± 0.7, and 2.6 ± 0.3% for APIX 0, 40, and 160 ng/mL respectively (p < 0.01 and p < 0.001 respectively vs. APIX0). Of interest, the platelet coverage for the ASA-treated patients was 13.2 ± 1.5, 7.7 ± 1.4, and 4.2 ± 0.6% for APIX 0, 40, and 160 ng/mL (p < 0.05 and p < 0.001 respectively vs. APIX0), respectively. In patients treated with ASA + CLOPI, platelet coverage was 17.4 ± 3.5, 5.6 ± 0.9, and 4.2 ± 0.8%, for APIX 0, 40 or 160 ng/mL (p < 0.01 and p < 0.001 respectively vs. APIX0). In patients treated with ASA + TICA, platelet surface coverages observed were 11.9 ± 1.3; 7.2 ± 0.5, and 5.1 ± 0.5% for APIX 0, 40, and 160 ng/mL (p < 0.001 vs. APIX0 for both concentrations).
Percentages of the perfused surface covered by platelets. Bars summarize percentages of the surface covered by platelets in microfluidic studies evaluated by confocal microscopy for the different study groups. The solid lines provide a visual estimate of the progressive inhibitory effects calculated as a percentage of the platelet coverage in the absence of apixaban (APIX0). APIX40 significantly reduced the association of platelets to the forming thrombi. Reductions were more significant in studies with APIX160. Reductions in the combination of APIX160 with antiplatelet regimens seem more intense in the group of patients exposed to APIX + TICA, less prominent in the group of patients treated with ASA, and somewhere intermediate in the group of patients treated with ASA + CLOPI. *p < 0.05; **p < 0.01; ***p < 0.001; vs. respective APIX0. Control (n = 9); ASA (n = 6) ASA + CLOPI (n = 4), ASA + TICA (n = 8).
The effects on fibrin coverage are presented in Fig. 6 showing a similar trend as previously described for platelet coverages. The Control group showed 59.2 ± 3.9, 43.3 ± 3, and 31 ± 4.9% for APIX 0, 40, and 160 ng/mL (p < 0.01 and p < 0.001 respectively vs. APIX0). Fibrin coverages in blood from patients treated with ASA were 50.4 ± 5.5, 38.1 ± 4.6, and 27.2 ± 5.1%, for APIX 0, 40, or 160 ng/mL (p < 0.01 vs. APIX0). The ASA + CLOPI group showed Fibrin coverages of 66.3 ± 1.9, 42.4 ± 6.5, and 28.7 ± 5.1% for APIX 0, 40, and 160 ng/mL (p < 0.01 and p < 0.001 respectively vs. APIX0). In patients treated with ASA + TICA, values were 60.7 ± 3.4, 47.3 ± 2.9, and 31.1 ± 3.1% for APIX 0, 40, and 160 ng/mL (p < 0.01 and p < 0.001 respectively vs. APIX0).
Percentages of the perfused surface covered by fibrin. Bars summarize percentages of the surface covered by fibrin in microfluidic studies evaluated by confocal microscopy for the different study groups. The solid lines provide a visual estimate of the progressive inhibitory effects calculated as a percentage of fibrin coverage in the absence of apixaban (APIX0). APIX40 significantly reduced the association of fibrin to the forming thrombi. Reductions were more significant in studies with APIX160 *p < 0.05; **p < 0.01; ***p < 0.001; vs. respective APIX0. Control (n = 9); ASA (n = 6) ASA + CLOPI (n = 4), ASA + TICA (n = 8).
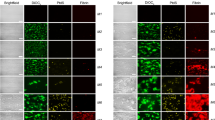
A detailed evaluation of the confocal images (Fig. 7) and the 3-D projections (Supplementary .MP4 clips) by confocal microscopy, revealed that APIX, in addition to its inhibitory action on fibrin formation, induced changes in the structure of the fibrin networks on the perfused surface. Fibrin fibers were thinner in the presence of APIX40, and their formation was delayed in studies with APIX160.
Confocal Images from representative frames from corresponding 3-D image compilations. (A) ASA + TICA APIX0, (B) ASA + TICA APIX40 and (C) ASA + TICA APIX160. Recruitment of platelets was reduced by the antiplatelet agents and further potentiated by the presence of APIX. Structural changes in fibrin fibers and fibrin networks are observed after exposure to APIX40 or APIX160. At the lower concentration (APIX40) apixaban interferes with the thickness and structure of the fibers formed. APIX160 delayed the generation of fibrin and caused more evident disorganization of the fibrin networks formed on thrombogenic surfaces in microfluidic studies. These patterns were consistently observed with the different antiplatelet agents investigated and were maximal for the combination of ASA + TICA.
Discussion
The results of the present study demonstrate that APIX potentiates the antithrombotic effects of current antiplatelet regimens. The combination of antiplatelet therapy (whether single or dual) with low doses of APIX enhanced the antithrombotic effects of the association, but always with a more conservative profile for hemostasis, with less interference with fibrin formation, than the higher APIX concentrations. The highest APIX dose used in our study corresponds to the plasma levels achieved after the administration of the commonly recommended dose for thromboprophylaxis in patients with atrial fibrillation. These data provide experimental evidence supporting the present recommendations from the clinical guidelines recommending a more conservative (or less intense) alternative/approach for patients at higher bleeding risk and on antiplatelet therapy. The alternative approach would be either to reduce the duration of dual antiplatelet therapy or the dose of anticoagulant to decrease bleeding risk without significantly impacting the antithrombotic efficacy of the DOACs.
A large proportion of patients with atrial fibrillation on chronic DOACs undergoing stent-implantation will also require dual antiplatelet therapy for a certain period. The combination of the anticoagulant treatment due to coexistent atrial fibrillation or deep venous thrombosis with the antiplatelet therapy will entail a higher risk for bleeding complications in this group of patients.
There is limited information from studies exploring the interactions of DOACs with antiplatelet agents12. Several clinical studies have explored the safety/efficacy balance of the association of DOACs and antiplatelet therapies with variable results13. In an initial study, the APPRAISE-2 trial investigated the beneficial effect of APIX (5 mg BID, the full anticoagulant dose recommended for AF) which resulted in an increased rate of major bleeding events without benefits on the incidence of major cardiovascular events14, results which are consistent with those of our mechanistic study.
The PIONEER AF-PCI successfully treated patients with atrial fibrillation undergoing PCI with lower doses of rivaroxaban (2.5 mg twice daily) in combination with dual antiplatelet therapy15. The RE-DUAL PCI combined regular doses of dabigatran, adjusted per age and comorbidities, associated with a P2Y12 inhibitor, compared with dual antiplatelet therapy plus warfarin16. Those previous studies showed comparable efficacy in thromboembolic events prevention and a decreased bleeding risk with DOACs, specifically in intracranial hemorrhage, the most feared bleeding complication. Later on, the AUGUSTUS trial demonstrated APIX superiority compared to anti-vitamin K drugs in the prevention of ischemic cerebral events and a significant reduction of the bleeding risk in the group treated with APIX and the P2Y12 inhibitor alone, without aspirin17. Recent clinical trials are considering the reduction of APIX to lower doses when concomitant antiplatelet treatment is indicated18. Overall evidence from the previously mentioned studies suggests the possibility of balancing the risk/benefit ratio by individualizing doses of DOACs in those clinical conditions requiring dual or triple antithrombotic therapies.
Our results support the validity of an experimental approach similar to the one undertaken by us evaluating platelet and coagulation components of hemostasis can help to select the most adequate antithrombotic treatments for patients receiving both, DOACs and antiplatelet therapy. In summary, our data show that APIX at its standard dosage (5 mg BID), may exhibit synergistic activity with direct P2Y12 receptor blockers such as clopidogrel or ticagrelor. Moreover, the results from our study provide additional mechanistic insights into how these antithrombotic agents work in combination and how APIX or other DOACs may reduce the contribution of thrombin to platelet reactivity.
APIX caused an alteration of some viscoelastic parameters as assessed by ROTEM, characterized by a dose-related prolongation of the clotting time (CT) with a surprisingly minimal impact on maximum clot firmness (MCF). These findings confirm the results of previous studies revealing that DOACs prolong clotting time without impacting maximum clot strength in whatever the DOACs or the concentrations used11,19,20,21,22,23,24.
Thrombin generation in samples of plasma from the different groups of patients evaluated in our study revealed more consistent differences among treatment groups. Thrombin generation tests have been used to identify bleeding risks in patients exposed to dual antiplatelet therapy25. Results from our present study indicate that the time to initiation of thrombin generation was progressively delayed by the increasing concentrations of APIX. Thrombin peaks and total amounts of thrombin generated seemed to be affected by the potency of the antiplatelet therapies being more important in patients on ASA + TICA than in those on ASA + CLOPI and ASA. These results are in agreement with another study, where thrombin generation assays induced by tissue factor plus ADP in human platelet-rich plasma showed as a potential measurement to assess the effect of the concomitant use of an oral factor Xa inhibitor and P2Y12 receptor antagonists26. Although results of thrombin generation assays do not follow a strict correlation with levels of apixaban in treated patients27 these assays provide more accurate information on the dynamics of thrombin generation that is not offered by the viscoelastic technologies in ROTEM.
Confocal analysis of microfluidic studies revealed a progressive effect of the anticoagulant APIX and antiplatelet therapies. APIX reduced platelet and fibrin interactions with the thrombogenic surface exposed to circulating blood. Inhibition in platelets and fibrin formation has been reported for different DOACs in previous studies from our group and others20,21,28,29. Interestingly, one of the previous studies reported a delayed fibrin formation in DOAC-treated under static conditions29. Our present microfluidic studies with recalcified anticoagulated blood were performed for a relatively short time (3 min) to avoid clotting of the system. Thus, these conditions can only explore the initiation of a hemostatic plug. Results demonstrate that the presence of APIX significantly impaired both, the formation of fibrin and the recruitment of platelets into forming thrombi. APIX 160 ng/mL was more potent at suppressing platelet and fibrin interactions with the collagen-tissue factor substrata with all antiplatelet treatments, while APIX 40 ng/mL demonstrated a consistent antithrombotic action, but proved more respectful at preserving hemostatic parameters with all antiplatelet regimens.
Structural changes in fibrin fibers and fibrin network density have been associated with alterations in hemostasis. Gauer and cols30, reported that anticoagulants interfered with the fibrin clot structure, most likely through a reduction of thrombin generation. Alterations in the formation and stability of fibrin structures reported in patients with hemophilia can be effectively corrected by pro-hemostatic strategies31,32. Our present data indicate that APIX in addition to its inhibitory action on total fibrin formation may also interfere with structural patterns of fibrin networks formed on thrombogenic surfaces. A delayed formation with additional disorganization of fibrin networks found in microfluidic studies was always more evident with the highest concentration of APIX. Overall, reduced incorporation of platelets and delayed fibrin generation intensified by synergistic antithrombotic therapies would result in the formation of a less occlusive hemostatic plug that will likely translate into an enhanced hemorrhagic risk in patients under dual or triple antithrombotic therapies.
Our study has limitations. Individuals in the control group were in general healthier than those receiving antiplatelet treatments. As a real-life study, the antiplatelet regimes described here reflect the genuineness of antithrombotic treatment at our institution. Clinical protocols and guidelines have evolved during the development of our studies. Changes in clinical and therapeutic approaches have had an impact on the conformation and number of patients finally included in the different study groups. Another limitation of our study is not having studied the combined effect of APIX with single P2Y12 inhibitors, to reproduce conditions of clinical trials that have studied the combination of DOACs or anti-vitamin K with these agents. Occasional discrepancies of n values in tables with numbers of patients per group are related to technical issues with occasional tests. Confocal analysis of microfluidic studies was not performed in the full patient population due to difficulties in blood sampling volumes and timing.
Overall, our present data indicate that APIX potentiates the antiplatelet effects of current antiplatelet treatments investigated in our studies. The concentration of 40 ng/mL shows a significant antithrombotic action in combination with the different antiplatelet regimens but appears to be more conservative for hemostasis than the concentrations of 160 ng/mL. The latest concentration more closely assimilates to the Cmax reached after the standard 5 mg BID, recommended for thromboprophylaxis in patients with atrial fibrillation. The 40 ng/mL would be compatible with the Cmin after the same treatment regimen, (10) but also with the Cmax achieved after 2.5 mg twice daily approved as an antithrombotic treatment for subgroups of older patients and/or renal impairment33.
In summary, the experimental strategy used in our ex vivo setting may provide information on the intensity of the alterations of hemostasis by antithrombotic therapies before designing expensive clinical trials, thus avoiding the exposure of patients to hazardous combinations of antiplatelet and anticoagulant treatments. Newer anticoagulants and antiplatelet agents are being developed and the effects of their combined use will remain a critical problem. In vitro approaches such as the one described in our studies will be useful as a screening method for possible synergistic antithrombotic actions that could severely impair hemostasis in patients.
Methods
Study design
This exploratory clinical study was conducted on patients on single or dual antiplatelet therapies, prescribed by their cardiologists for pre-established conditions. All participants provided written informed consent before any study procedures. The study protocol was approved by the Ethics Committee of the Hospital Clinic de Barcelona (CV185-700) and the study complied with all local regulations and the ethical principles of the current Declaration of Helsinki.
Inclusion criteria were age > 18 years and receiving stable antiplatelet therapy for at least one month. The attending physician was responsible for the indication of the antiplatelet therapy to patients with a diagnosis of any cardiovascular condition requiring platelet aggregation inhibition. Exclusion criteria were: renal failure with GFR < 50 mL/min, anemia with hemoglobin levels < 13 g/dL, platelet counts < 140,000/mL or > 400,000/mL, previously known hemostasis disorders, current use of anticoagulants or any drugs potentially interfering with hemostasis (non-steroidal anti-inflammatory drugs and serotonin-reuptake inhibitors) or previous thromboembolic events of unknown etiology.
The study recruitment involved the identification of potential participants from consecutive patients admitted to the Cardiology Department with ACS or heart failure or needing structural procedures (TAVR, percutaneous mitral or tricuspid valve repair). Those requiring antiplatelet therapy were identified as potential qualifiers. Once the minimum period of 4 weeks of treatment stabilization was achieved, they were approached and enrolled for the study upon positive consent.
Figure 1 summarizes the study design and the distribution of control and treated groups. After screening, blood samples were collected from patients at the Cardiology Clinic during a follow-up visit. A group of healthy donors was used as a control. Apixaban was generously provided by Bristol Myers Squibb (Madrid, Spain). Following the manufacturer's advice, a stock solution was initially prepared in DMSO. This stock solution was further diluted in phosphate buffer saline (PBS) with a lower proportion of DMSO to reach the expected concentrations in blood. Volumes spiked to blood samples for control or treatment groups were equal (50 µL/mL of blood). Concentrations of APIX were corrected for the small volume change. Concentrations of DMSO were kept identical (< 0.05%) in all blood samples including controls. Blood samples were spiked with APIX at concentrations equivalent to 0 (APIX0), 40 (APIX40), and 160 ng/mL (APIX160) and incubated for 30 min. Patients or controls were never exposed to the effects of the DOACs.
The antithrombotic effects of the different doses of APIX were assessed on (1) clot formation, by thromboelastometry, using the ROTEM; (2) thrombin generation, applying a cell-based model of coagulation primed by platelets; and (3) platelet and fibrin interactions/depositions onto a thrombogenic substratum (Type I collagen + tissue factor) experimental model of thrombosis, with blood circulating at arterial shear-rates.
Screening procedures
All participants underwent screening including medical history, physical examination, routine blood works, drugs of abuse testing, EKG, and cardiac imaging techniques when appropriate. The main diagnoses were: ischemic heart disease, elective percutaneous coronary intervention, arrhythmia, heart failure, peripheral arterial revascularization, or ambulatory primary prevention. All concomitant medications taken by the patients were recorded to confirm adherence to the inclusion criteria. Patients were instructed to take their antiplatelet treatment 3–4 h before their study visit.
Blood collection and functional evaluation of antiplatelet treatments
Citrated blood samples (20 mL) were drawn in 129 M citrate tubes BD Vacutainer™ (ref 3653079) by a clean venipuncture from the study participants. All participants were tested for proper inhibitory response to the antiplatelet treatment they were prescribed. Platelet hemostatic performance in control individuals and patients was evaluated to verify that patients were adequately following or responding to their antiplatelet treatment34,35. For this purpose, aliquots of citrated blood were tested in the Platelet Function Analyzer (PFA-200 System, Siemens Healthineers, Barcelona Spain) using Col-Epi, Col-ADP, and P2Y (Innovance®) cartridges. Closure times were recorded. Cut-off values for closure times for the different cartridges were Col-Epi > 137 “(aspirin); Col-ADP < 105”; “Innovance P2Y > 106” (for clopidogrel or ticagrelor)36.
Assessment of the anticoagulant action of apixaban in thromboelastometry studies
We evaluated the inhibitory effects of APIX on the dynamics of whole blood coagulation, using the ROTEM Thromboelastometry Analyzer (PentapharmGmbH, München, Germany)37. Citrated blood was recalcified with the starTEM reagent and activated with the r-EXTEM reagent (Biometa, Spain) containing tissue factor as the clotting activator. Dynamics of clot formation were followed for 45 min. Coagulation time (CT), the time (sec) elapsed from the start until the amplitude of the forming clot reaches 2 mm, and maximum clot firmness (MCF) the maximum amplitude of the tracing reached (in mm), were assessed in our ROTEM studies.
Evaluation of thrombin generation
The contribution of platelets to local thrombin generation was evaluated in a cell-based model of thrombin generation primed by platelets, using a fluorogenic assay (Technoclone GmBH, Austria) as previously described11. Briefly, this cell-based model consists of isolated platelets at 1 × 106 platelets/µL from controls or treated blood samples resuspended in Hanks' balanced salt solution, with platelets or plasma always from the same participant. Thrombin generation was initiated by the addition of 1.1 pM tissue factor exposed on phospholipids micelles (Thromborel®S, Dade Behring, Marburg GmbH, Germany), and a fluorogenic substrate that also contains CaCl2 to favor the activation of coagulation mechanisms. The fluorescence generated was evaluated at a wavelength of 390 nm/450 nm (excitation/emission) for 90 min (at intervals of 1 min) and fluorescence units were analyzed with the Thermo Fluoroskan Ascent Software (Technoclone GmbH). Parameters assessed in our studies were lag time (min), maximum thrombin peak (nM), time to achieve this peak (min), and the total amount of thrombin generated as the area under the curve (arbitrary units).
Measurement of platelets and fibrin deposition in microfluidic studies
Microfluidic studies were carried out using commercially available slide-based chambers (μ-SlideVI 0.4, IBIDI) coated with fibrillar collagen type I and tissue factor (TF)38. Aliquots of citrated whole blood were incubated with APIX concentrations calculated to give rise to concentrations 0 (APIX0), 40 (APIX40), and 160 ng/mL (APIX160) for 30 min, then recalcified with a CaCl2 solution, and immediately perfused through the slide micro-channels at a shear rate of 600 s−1, for 3 min. Perfused channels were rinsed with 0.15 M PBS, fixed with paraformaldehyde 1% for 15 min at 4 ºC, and further incubated with glycine 1% for 10 min to reduce high background staining due to free unreactive aldehyde groups. Thereafter, perfused channels were exposed to 1% bovine serum albumin (BSA) for 15 min before incubation with specific antibodies to discriminate the contribution of platelets and fibrin, a combination of indirect and direct immunofluorescence. First, platelets were labeled using a mouse anti-CD36 primary antibody for 1 h at RT in a humidified chamber. Then, a secondary antibody anti-mouse Alexa fluor 488 was incubated together with a conjugated antibody anti-fibrinogen Alexa fluor 594 to stain fibrin deposition for 1 h at RT in a humidified chamber.
Evaluation of platelets and fibrin deposition using confocal microscopy
Fibrin and platelet interactions with the collagen-TF surface were evaluated using confocal microscopy38. Perfused channels were observed on a Leica TCS-SP5 Laser Scanning Confocal Microscope. Alexa fluor 488 (green) and Alexa Fluor 594 (red) and reflection images were acquired sequentially using 488 and 561 nm laser lines, AOBS (Acoustic Optical Beam Splitter) as a beam splitter, and emissions from the different channels were acquired through internal photomultipliers at the emission ranges of 500–550 nm, 571–625 nm, 555–565 nm, for reflection, respectively39. The confocal pinhole was set at 1 Airy unit. Transmitted light bright field images were acquired simultaneously through a transmitted light detector. The percentage of the surface covered by platelets and fibrin was quantified using Image-J software40.
Statistics
Results are expressed as mean ± standard error of the mean (SEM). Comparisons between different concentrations of APIX vs. baseline values or the control healthy groups were evaluated. ANOVA and Friedman’s or Kruskall–Wallis tests with Dunn’s correction for multiple comparisons were used for statistical comparisons before and after exposure to APIX treatment. The minimal level of statistical significance was set at p < 0.05.
Ethics approval
The study protocol was approved by the Ethics Committee (CV185-700) of our Institution and the study complied with all local regulations and the ethical principles of the current Declaration of Helsinki.
Consent to participate
Informed consent to participate in the study was obtained from all individual participants included.
Data availability
All authors agree that all data and materials support the published claims and comply with field standards.
Code availability
The authors affirm that software application or custom code is not applicable in this study.
Abbreviations
- APIX:
-
Apixaban
- APIX0:
-
Apixaban 0 ng/mL
- APIX40:
-
Apixaban 40 ng/mL
- APIX160:
-
Apixaban 160 ng/mL
- ASA:
-
Aspirin (acetyl salicylic acid)
- ASA + CLOPI:
-
Aspirin + clopidogrel
- ASA + TICA:
-
Aspirin + ticagrelor
- CLOPI:
-
Clopidogrel
- MCF:
-
Maximum clot firmness
- PFA:
-
Platelet function analyzer
- ROTEM:
-
Rotational thromboelastometry
- TICA:
-
Ticagrelor
- VASP:
-
Vasodilator-stimulated phosphoprotein
References
The-Affirm-Investigators. Baseline characteristics of patients with atrial fibrillation: The AFFIRM study. Am. Heart J. 143, 991–1001. https://doi.org/10.1067/mhj.2002.122875 (2002).
Rathore, S. S. et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: Prevalence and outcomes. Circulation 101, 969–974. https://doi.org/10.1161/01.cir.101.9.969 (2000).
Kralev, S., Schneider, K., Lang, S., Suselbeck, T. & Borggrefe, M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS ONE 6, e24964. https://doi.org/10.1371/journal.pone.0024964 (2011).
Rubboli, A. et al. Periprocedural management and in-hospital outcome of patients with indication for oral anticoagulation undergoing coronary artery stenting. J. Interv. Cardiol. 22, 390–397. https://doi.org/10.1111/j.1540-8183.2009.00468.x (2009).
Holmes, D. R. Jr. et al. Stent thrombosis. J. Am. Coll. Cardiol. 56, 1357–1365. https://doi.org/10.1016/j.jacc.2010.07.016 (2010).
Shah, Z., Masoomi, R. & Tadros, P. Managing antiplatelet therapy and anticoagulants in patients with coronary artery disease and atrial fibrillation. J. Atr. Fibrillation 8, 1318. https://doi.org/10.4022/jafib.1318 (2015).
Kumar, S. et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Cardiol. Rev. 24, 218–223. https://doi.org/10.1097/crd.0000000000000088 (2016).
Hawiger, J. Formation and regulation of platelet and fibrin hemostatic plug. Hum. Pathol. 18, 111–122. https://doi.org/10.1016/s0046-8177(87)80330-1 (1987).
Coughlin, S. R. Thrombin signalling and protease-activated receptors. Nature 407, 258–264. https://doi.org/10.1038/35025229 (2000).
Frost, C. et al. Apixaban, an oral, direct factor Xa inhibitor: Single-dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br. J. Clin. Pharmacol. 75, 476–487 (2012).
Pujadas-Mestres, L. et al. Differential inhibitory action of apixaban on platelet and fibrin components of forming thrombi: Studies with circulating blood and in a platelet-based model of thrombin generation. PLoS ONE 12, e0171486. https://doi.org/10.1371/journal.pone.0171486 (2017).
Ringwala, S. M., Dibattiste, P. M. & Schneider, D. J. Effects on platelet function of a direct acting antagonist of coagulation factor Xa. J. Thromb. Thrombolysis. 34, 291–296 (2012).
Carreras, E. T. & Mega, J. L. Role of oral anticoagulants in patients after an acute coronary syndrome. Arterioscler. Thromb. Vasc. Biol. 35, 520–524 (2015).
Alexander, J. H. et al. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: Results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 119, 2877–2885 (2009).
Gibson, C. M. et al. An open-label, randomized, controlled, multicenter study exploring two treatment strategies of rivaroxaban and a dose-adjusted oral vitamin K antagonist treatment strategy in subjects with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI). Am. Heart J. 169, 472-478.e475. https://doi.org/10.1016/j.ahj.2014.12.006 (2015).
Cannon, C. P. et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 377, 1513–1524. https://doi.org/10.1056/NEJMoa1708454 (2017).
Lopes, R. D. et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N. Engl. J. Med. 380, 1509–1524. https://doi.org/10.1056/NEJMoa1817083 (2019).
Biagioni, R. B. et al. Rationale and design for the study Apixaban versus ClopidoGRel on a background of aspirin in patient undergoing InfraPoPliteal angioplasty for critical limb ischemia: AGRIPPA trial. Am. Heart J. 227, 100–106. https://doi.org/10.1016/j.ahj.2020.06.010 (2020).
Seyve, L., Richarme, C., Polack, B. & Marlu, R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int. J. Lab. Hematol. 40, 84–93. https://doi.org/10.1111/ijlh.12744 (2018).
Escolar, G. et al. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: Significance of studies in vitro with circulating human blood. PLoS ONE 8, e78696. https://doi.org/10.1371/journal.pone.0078696 (2013).
Arellano-Rodrigo, E. et al. Idarucizumab, but not procoagulant concentrates, fully restores dabigatran-altered platelet and fibrin components of hemostasis. Transfusion 59, 2436–2445. https://doi.org/10.1111/trf.15259 (2019).
Pailleret, C. et al. Modified ROTEM for the detection of rivaroxaban and apixaban anticoagulant activity in whole blood: A diagnostic test study. Eur. J. Anaesthesiol. 36, 449–456. https://doi.org/10.1097/EJA.0000000000000903 (2019).
Sahli, S. D. et al. The impact of direct oral anticoagulants on viscoelastic testing—A systematic review. Front. Cardiovasc. Med. 9, 991675. https://doi.org/10.3389/fcvm.2022.991675 (2022).
Taune, V. et al. Rapid detection of apixaban by a ROTEM-based approach and reversibility with andexanet alfa or DOAC-Stop. TH Open 6, e238–e247. https://doi.org/10.1055/s-0042-1751072 (2022).
de Breet, C. et al. Thrombin generation as a method to identify the risk of bleeding in high clinical-risk patients using dual antiplatelet therapy. Front. Cardiovasc. Med. 8, 679934. https://doi.org/10.3389/fcvm.2021.679934 (2021).
Honda, Y. & Morishima, Y. Thrombin generation induced by tissue factor plus ADP in human platelet rich plasma: A potential new measurement to assess the effect of the concomitant use of an oral factor Xa inhibitor edoxaban and P2Y12 receptor antagonists. Thromb. Res. 135, 958–962. https://doi.org/10.1016/j.thromres.2015.03.001 (2015).
Meihandoest, T. et al. Automated thrombin generation assay for rivaroxaban, apixaban, and edoxaban measurements. Front. Cardiovasc. Med. 8, 717939. https://doi.org/10.3389/fcvm.2021.717939 (2021).
Escolar, G. et al. Reversal of rivaroxaban-induced alterations on hemostasis by different coagulation factor concentrates—in vitro studies with steady and circulating human blood. Circ. J. 79, 331–338. https://doi.org/10.1253/circj.CJ-14-0909 (2015).
Brinkman, H. J. M. et al. Reversing direct factor Xa or thrombin inhibitors: Factor V addition to prothrombin complex concentrate is beneficial in vitro. Res. Pract. Thromb. Haemost. 6, e12699. https://doi.org/10.1002/rth2.12699 (2022).
Gauer, J. S. et al. Effect of anticoagulants on fibrin clot structure: A comparison between vitamin K antagonists and factor Xa inhibitors. Res. Pract. Thromb. Haemost. 4, 1269–1281. https://doi.org/10.1002/rth2.12443 (2020).
Gray, L. D. et al. Recombinant factor VIIa analog NN1731 (V158D/E296V/M298Q-FVIIa) enhances fibrin formation, structure and stability in lipidated hemophilic plasma. Thromb. Res. 128, 570–576. https://doi.org/10.1016/j.thromres.2011.04.009 (2011).
Wolberg, A. S. et al. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. Br. J. Haematol. 131, 645–655. https://doi.org/10.1111/j.1365-2141.2005.05820.x (2005).
Frost, C. et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 76, 776–786. https://doi.org/10.1111/bcp.12106 (2013).
Cattaneo, M. High on-treatment platelet reactivity—definition and measurement. Thromb. Haemost. 109, 792–798. https://doi.org/10.1160/th12-10-0758 (2013).
Tantry, U. S. et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 62, 2261–2273. https://doi.org/10.1016/j.jacc.2013.07.101 (2013).
Jover, E. et al. High on-treatment platelet reactivity in patients with ischemic cerebrovascular disease: Assessment of prevalence and stability over time using four platelet function tests. Blood Coagul. Fibrinolysis 25, 604–611. https://doi.org/10.1097/mbc.0000000000000118 (2014).
Caballo, C. et al. Impact of experimental haemodilution on platelet function, thrombin generation and clot firmness: Effects of different coagulation factor concentrates. Blood Transfus. 11, 391–399. https://doi.org/10.2450/2012.0034-12 (2013).
Lopez-Vilchez, I. et al. Microfluidic systems with vascular biomimetic surfaces for assessment of platelets and fibrin components of hemostasis: Comparison with a classic annular device in the evaluation of the antithrombotic effects of apixaban. Res. Pract. Thromb. Haemost. 1, 1–1451. https://doi.org/10.1002/rth2.12012 (2017).
Zafar, M. U. et al. Effects of electret coating technology on coronary stent thrombogenicity. Platelets 33, 312–319. https://doi.org/10.1080/09537104.2021.1912313 (2022).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. https://doi.org/10.1038/nmeth.2089 (2012).
Acknowledgements
We would like to thank the nurses’ team at the Department of Cardiology of Hospital Clinic de Barcelona for their support and collaboration in the study.
Funding
This study was supported by an ERISTA grant sponsored by Bristol Myers Squibb (BMS) and Pfizer. The study was partially supported by Instituto de Salud Carlos III (FIS PI19/00888), Fundació Marató de TV3 (202026-10), Deutsche José Carreras Leukämie-Stiftung (03R/ 2019), Generalitat de Catalunya (2017-SGR675, CERCA).
Author information
Authors and Affiliations
Contributions
J.M.-S.: performed laboratory tests and confocal studies, validated methodologies and results, participated in data collection and curation, and wrote the initial draft. L.C.: coordinated the study at the Department of Cardiology, organized patient availability and blood sampling, and participated in data collection and curation. D.J.: performed laboratory tests and confocal studies, validated results, and participated in data collection and curation. S.T.-M.: performed confocal studies and morphometric evaluations, validated results, and participated in data collection and curation. M.P.: organized and coordinated laboratory tests, and validated results. G.M.: recruited patients and organized clinical and laboratory studies at the Department of Cardiology. M.U.Z.: participated in interpreting the results, performed new statistic tests, and provided concepts and a bibliography related to the study, reviewing, and editing the text. A.B.M.-C.: coordinated laboratory tests, validated methodologies, and participated in data collection and statistical analysis. P.S.: performed a morphometric analysis in a confocal study, validated results, and participated in data collection and curation. J.J.B.: contributed with investigational concepts, bibliography analysis, reviewed and edited the text. M.D.-R.: designed the study, and participated in conceptualization, project administration, resources, and data analysis. G.E.: participated in conceptualization, and data analysis, and wrote the original draft. M.R.: designed the study, participated in conceptualization, project administration, resources, and data Analysis, and wrote the original draft. All authors have approved the final version of the manuscript. The authors affirm that all participants signed informed consent regarding publishing their data.
Corresponding author
Ethics declarations
Competing interests
Gines Escolar received honoraria consultant fees from Bayer, BMS, Boehringer Ingelheim, CSL Behring, and Novo Nordisk. All the other authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinez-Sanchez, J., Castrillo, L., Jerez, D. et al. Antithrombotic and prohemorrhagic actions of different concentrations of apixaban in patients exposed to single and dual antiplatelet regimens. Sci Rep 13, 22969 (2023). https://doi.org/10.1038/s41598-023-50347-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50347-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.