Abstract
This study employs repeated, large panels of serological surveys to document rapid and substantial waning of SARS-CoV-2 antibodies at the population level and to calculate the extent to which infection and vaccination separately contribute to seroprevalence estimates. Four rounds of serological surveys were conducted, spanning two COVID waves (October 2020 and April–May 2021), in Tamil Nadu (population 72 million) state in India. Each round included representative populations in each district of the state, totaling ≥ 20,000 persons per round. State-level seroprevalence was 31.5% in round 1 (October–November 2020), after India’s first COVID wave. Seroprevalence fell to 22.9% in round 2 (April 2021), a roughly one-third decline in 6 months, consistent with dramatic waning of SARS-Cov-2 antibodies from natural infection. Seroprevalence rose to 67.1% by round 3 (June–July 2021), with infections from the Delta-variant induced second COVID wave accounting for 74% of the increase. Seroprevalence rose to 93.1% by round 4 (December 2021–January 2022), with vaccinations accounting for 63% of the increase. Antibodies also appear to wane after vaccination. Seroprevalence in urban areas was higher than in rural areas, but the gap shrunk over time (35.7 v. 25.7% in round 1, 89.8% v. 91.4% in round 4) as the epidemic spread even in low-density rural areas.
Similar content being viewed by others
Introduction
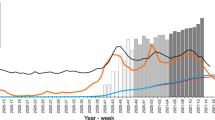
Knowledge of population-level immunity is critical for understanding the epidemiology of SARS-CoV-2 (COVID-19) and formulating an infection control policy, including both non-pharmaceutical interventions and vaccination campaigns. To facilitate this policymaking, the state government of Tamil Nadu, conducted population-level serological surveys four times, in October–November 2020, April 2021, June–July 2021, and December 2021–January 2022 (shaded sections of Fig. 1). In each round, the survey was conducted on representative populations in every district of the state, except Chennai in round 2. Tamil Nadu is the 6th most populous state in India, with roughly 72 million persons1. India, including Tamil Nadu, experienced three COVID-19 waves that peaked in September 2020, May 2021, and February 20222. Moreover, each state in India began a vaccination campaign in January 2021. Roughly half the population, 714 million persons, were vaccinated by February 1, 20223. Thus, the state’s four serological surveys span the period between the end of the first wave and the start of the third wave and mid-point of the country’s first vaccination campaign.
Daily new infections, new vaccinations (dose 1), and dates of serological survey rounds in Tamil Nadu. Notes: Figure plots daily confirmed cases (in 1000 s) in red on the left y-axis and cumulative number of first vaccine doses (in millions) on the right y-axis daily from February 2020 to May 2022. Dark grey intervals on x-axis indicate dates of each round of the serological survey in this study. Data source: www.covid19bharat.org.
This study reports seroprevalence estimates from these surveys over time, by district, by demographic groups, and by urban status. We employ these estimates to address three challenges of translating this surveillance into policy-relevant conclusions. First, not all changes to population-level immunity after a vaccination campaign starts are attributable to vaccination because the campaign takes time, during which the epidemic continues to infect the unvaccinated4. Moreover, spread of natural immunity can impact both the urgency and geographic focus of vaccine campaigns4. We address this problem by estimating the fraction of population immunity attributable to vaccination versus infection using a formula that compares changes in seroprevalence to changes in rates of infection and vaccination to across rounds of surveys.
Second, humoral immunity to SARS-CoV-2 from either infection or vaccination can be short-lived. Knowing how long it lasts is critical to determining the future value of non-pharmaceutical interventions and the urgency of both initial vaccination and boosters. There are many studies examining waning antibody levels in longitudinal samples, especially post-vaccination5,6,7,8,9,10,11,12,13,14. However, with few exceptions from Europe15,16,17, those studies do not example population representative samples18,19,20,21,22,23,24,25,26,27. Moreover, durability of humoral immunity may vary across the population28, so population-representative samples are critical for minimizing bias29. We estimate the extent to which antibodies decline following infection and vaccination by using data on changes in district-level seroprevalence across rounds and individual reports of the date of their own infection and vaccination, respectively.
Third, officially reported SARS-CoV-2 cases are typically not gathered from population-representative samples. Moreover, low testing rates may cause case counts to underestimate population-level immunity30. India reported 43 million COVID-19 cases and 524,000 COVID-19 deaths through May 31, 20222. Tamil Nadu has reported roughly 3.4 million COVID-19 cases and 38,000 deaths, ranked 4th highest among Indian states through May 31, 202231. Following similar exercises in other Indian states32,33, we compare the results of Tamil Nadu’s population-level serological surveys to these reported cases to measure the degree to which reported cases underestimate population immunity.
Methods
The study was approved by the Directorate of Public Health and Preventive Medicine, Government of Tamil Nadu, and the Institutional Ethics Committee of Madras Medical College, Chennai, India. The study was performed in accordance with the guidelines of the Institutional Ethics Committee. In particular, only subjects who provided written informed consent were enrolled in the study, were surveyed, and asked for biosamples. The study was entirely funded by the Government of Tamil Nadu and the National Health Mission, Tamil Nadu.
Outcomes
The primary endpoints are (1) the fraction of the population that would obtain positive results on CLIA (chemiluminescent immunoassay) antibody tests for COVID, i.e., seropositivity, at the district-level, and (2) the fraction of the population that have antibodies for COVID, i.e., seroprevalence, district level.
The secondary endpoints are (1) seroprevalence (a) by age and sex, (b) by urban status, and (c) at the state level; (2) the difference between population immunity estimated by serological survey and by reported cases; and (3) self-reported infection and vaccination.
Survey timing, sample, and location
Data was gathered between 19 October–30 November 2020, 7–30 April 2021, 28 June to 7 July 2021, 27 December 2021–6 January 2022 in rounds 1, 2, 3 and 4, respectively. Individuals residing in Tamil Nadu and ages 18 years and older were eligible for rounds 1–3 of this study. In round 4, eligibility was expanded to ages 10 and older because the state government felt it safe enough to test for seropositivity among children. The exclusion criteria were refusal to consent and contraindication to venipuncture. In round 2, Chennai district was not surveyed because there was an outbreak that prevented sampling in that district.
Sample size
Sample sizes for rounds 1–3 were calculated assuming a seropositivity of 0.5 throughout the state, to maximize power. For round 4, the positivity rate estimated from round 3 (0.662) was used. Calculations sought a confidence level of 0.95. Because clustered sampling would be done, a design effect of 1.5 was applied in rounds 1–3 and of 2 in round 4. The resulting sample size was multiplied by 37, the number of districts in Tamil Nadu as of October 2020, for rounds 1 and 3. In round 2, the multiple was 36 because Chennai was not sampled. In round 4, the multiple was 38, as one of the districts was split into two by round 4. This implied state-wide sample size targets were 26,651 in rounds 1 and 3, 25,931 in round 2, and 32,664 in round 4.
Sampling strategy
The study selected participants in each district in five steps. First, districts were divided into rural and urban strata. District-wise sample-size targets were allocated to rural and urban strata in proportion to strata population. Second, rural and urban strata were divided into geographic clusters, defined as a village and street segments in rural and urban strata, respectively. Third, strata-wise sample-size targets were converted into cluster sample-size targets assuming 30 persons were sampled per cluster. Fourth, random sampling was used to select the targeted sample-size of clusters from each strata in each round. Fifth, up to 30 were sampled from each cluster using a random starting point, systematic sampling of households, and the Kish34 method to select one participant per household. (Additional details are in the Supplement).
Data collection
Each participant was asked to complete a health questionnaire (including questions on prior infections and vaccination) and provide 5 ml venous blood collected in EDTA vacutainers. Serum was analyzed for IgG antibodies to the SARS-CoV-2 spike protein using either the iFlash-SARS-CoV-2 IgG (Shenzhen YHLO Biotech; sensitivity of 95.9% and specificity of 95.7% per manufacturer)35 or the Vitros anti-SARS-CoV-2 IgG CLIA kit (Ortho-Clinical Diagnostics; sensitivity of 90% and specificity of 100% per manufacturer)36. We obtained data on each reported COVID-19 case and death through May 2022 from the Government of Tamil Nadu and Covid19Bharat.org31 and on the number of tests done through January 2022 from the Government of Tamil Nadu.
Statistical analysis
All statistics are calculated separately for each round unless otherwise indicated.
Seropositivity
The proportion of positive CLIA tests by district is obtained by estimating a logit regression of test result on district indicators and reporting the inverse logit of the coefficient for each district indicator. Observations are weighted by the inverse of sampling probability for their age and gender groups; the sampling probability here and below is based on population counts from the 2011 Indian Census. We reweight to match the 2011 Census because the Kish method ensures even (rather than representative) sampling by gender and age. Clustered standard errors are calculated at the cluster level.
Seroprevalence
Seroprevalence by district is estimated in two steps. First, we calculate the weighted proportion of positive tests at the district level. (We explain an exception for Chennai in round 1 and Virudhunagar in round 3 in the Supplement.) All samples in a district were tested using the same type of CLIA kit. We estimate a logit regression of test results on district indicators and take the inverse logit of the coefficient for each jurisdiction indicator. Observations are weighted by the inverse of sampling probability for their age and gender groups. Clustered standard errors are calculated at the cluster level. Second, for each jurisdiction, we predict seroprevalence using the Rogan-Gladen formula37, test parameters for the kit used in each jurisdiction, and regression estimates of seropositive proportion by jurisdiction.
State-level seroprevalence is obtained by aggregating the seroprevalence across districts weighted by 2011 Census data on the relative populations of districts.
Seroprevalence by demographic group is estimated in three steps. First, we calculate the proportion of positive tests at the jurisdiction-by-demographic group level in that round using logit regressions of test results on jurisdiction-by-demographic group indicators. Demographic groups indicators are sex x age for 6 age bins. Standard errors are clustered at the cluster level. Second, we predict district-by-demographic group level seroprevalence using the Rogan–Gladen formula. Third, we compute the weighted average of seroprevalence for each demographic-group across all districts. The weight for any given district is the share of the state-wide population in a demographic group that resides in that district. These weights are computed using data from the 2011 Indian census.
Seroprevalence by vaccine status in each of rounds 2–4 (when vaccines were available) is estimated in the same manner we calculate seroprevalence by demographic group in a round, except we replace demographic group by vaccine status.
The population was largely vaccinated with either of two vaccines. Covishield was developed by Oxford University, AstraZeneca and the Serum Institute of India. It is a viral vector vaccine that uses a modified version of a Chimpanzee adenovirus to deliver SARS-Cov-2 genetic material. Covaxin was developed by Bharat Biotech, the Indian Council of Medical Research, and the National Institute of Virology. It is an inactivated vaccine. Seroprevalence by specific vaccine in each of rounds 3–4 (when vaccine names were requested from respondents) is estimated in the same manner we calculate seroprevalence by demographic group in a round, except we replace demographic group by vaccine brand.
Seroprevalence by urban status is obtained in the same manner as seroprevalence by demographic group, with two changes. First, we use the urban status of a cluster in lieu of demographic status of an individual at each step. Second, observations in our regression are weighted by inverse of the sampling probability for their urban status.
The size of a population that was seropositive by the end of a round is obtained by multiplying our seroprevalence estimates for the population in that round by the size of that population (as reported in the 2011 Census).
Undercounting of infections
The degree of undercounting of infections in round 1 is estimated by dividing the estimated number of people that are seropositive in the Tamil Nadu population by the number of government-reported cases in that population as of 1 week before the median sampling date of that round (23 October 2020). We focus on round 1 because vaccinations started after round 1 and some seropositivity in rounds 2–4 is due to vaccination, not infections. The lag accounts for the delay, both between infection and seropositive status and between infection and prevalence testing. We calculate the Pearson’s correlation coefficient between undercounting rate and testing rate (tests per million as of median date of testing) by district.
Waning antibodies
We estimate the decline of antibodies after infection and in the absence of vaccination using district-level observations and a linear regression of district-level seropositivity in round 2 on district-level seropositivity in round 1. We focus on round 1 because no participants were vaccinated before round 1, meaning all seropositivity is due to infection. Observations are weighted in proportion to the population of each district in the 2011 Census. To address the possibility that decay is masked by new infections or vaccinations, we estimate a second specification that includes as controls a measure of the percent of population infected between round 1 and round 2 and the fraction of respondents who self-report vaccination. The measure of infection, which we call the “adjusted cases rate”, is the number of new confirmed cases per capita between rounds 1–2, adjusted by the infection undercount rate in round 1 (seroprevalence rate in round 1 divided by cases per capita until round 1).
We estimate the decline of antibodies following two doses of vaccination in two steps. First, we restrict the sample to individuals from round 4 who had been vaccinated with their second dose at least 20 days prior to bio sample collection. The 20-day delay is intended to omit the period of time during which antibodies are climbing post-vaccination. We do not consider individuals from round 2 because we do not have their date of vaccination and from round 3 because so few individuals were vaccinated by that date. Second, we estimate a linear regression with an indicator for whether a person was seropositive as the dependent variable and the number of years (i.e., number of days/365) since dose 2 as the independent variable. Observations are weighted to match age and gender proportions in the 2011 Census. To obtain plausibly causal estimates, we use age as an instrumental variable (IV) for the number of days since vaccination. The logic for this instrument is that Tamil Nadu prioritized individuals for vaccination based on their age, with older age persons given greater priority; to validate this instrument, we create a binscatter of days since vaccination on age among individuals with only 1 dose of vaccine and confirm that days since vaccination rises with age. The drawback of this instrument is that it is possible that antibody decay is directly a function of age38.
Attribution to infection or vaccination
We attribute the change in seropositivity from round t − 1 to round t to changes in the levels of infections and of vaccination using the formula:
where st is the seropositivity rate in round t, pt is the fraction of the sample vaccinated by round t, svt is the seropositivity rate among those vaccinated by round t, and snvt is the seropositivity rate among those not vaccinated by round t. The first term captures the share of the change in seropositivity (st − st-1) attributable to changes in seropositivity rate among the previously vaccinated (svt − svt-1). (This rate can change over time because antibodies levels may depend on the number of days since vaccination.) The second term captures the share attributable to infections among the previously unvaccinated (1 − pt). The third term captures the share attributable to changes in the vaccination rate. This captures both the effect of the increase in the vaccination rate (pt − pt-1) and the change in seropositivity when one gets vaccinated (svt − snvt). We calculate these components for the change in seropositivity from rounds 2–3 and from rounds 3–4.
Statistical tests comparing groups are performed using a two-sided Wald test with 95%. All statistical analyses were conducted with Microsoft Excel 365 (Microsoft, USA) and Stata 16 (StataCorp, USA). All plots were generated in R.
Results
Sample
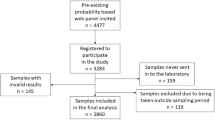
In round 1, the study obtained results for 26,135 persons in 882 clusters (Table 1). The study could not sample 6 clusters and was unable to consent 324 persons in sampled clusters. One person aged 16 was incorrectly consented and dropped from the analysis. In round 2, the study obtained results for 21,966 persons in 746 clusters. (Chennai was not sampled.) The study could not sample 118 clusters and was unable to consent 388 persons in sampled clusters. Twenty-six persons of age < 18 were incorrectly consented and dropped from the analysis. In round 3, the study obtained results for 26,592 persons. The study could not consent 48 persons in sampled clusters. In round 4, the study obtained results for 32,244 persons. The study was unable to sample 13 clusters and could not consent 56 persons in sampled clusters. The final sample size per round was within the allowable 20% non-response rate.
Table 1 reports the demographic characteristics of the sample in each round. The sample has substantially more females and fewer persons aged 10–17 and 18–29 and more elderly persons than the general population.
Seropositivity
State-level seropositivity was 33.0% (95% CI 32.0–34.0%), 23.1% (95% CI 22.2–24.0%), 67.5% (95% CI 66.7–68.4%), and 88.3% (CI 87.8–88.8%) in rounds 1, 2, 3 and 4, respectively (Fig. 2).
Proportion of positive CLIA tests by district. Notes: Each subfigure plots the mean (dot) and 95% confidence intervals (whiskers) of seropositivity on a CLIA test for antibodies to SARS-CoV-2 in each district (black) and in the state overall (red) in one round of the Tamil Nadu seroprevalence survey. Districts or state are indicated on the y-axis. Weighted proportion of sample is indicated on the x-axis, with weights indicated to make the sample representative of a district or state’s population, as appropriate. The date of each round is indicated in the subfigure titles.
Seropositivity varied dramatically across districts in the first 3 rounds: from 11.9% (The Nilgris) to 49.7% (Perambalur) in round 1, 11.3% (Ramanathapuram) to 49.5% (Tiruvallur) in round 2, and 37.5% (Erode) to 81.9% (Chennai) in round 3 (Fig. 2). Seropositivity converged by round 4, ranging from 82.9% (Tirupathur) to 94.3% (Thiruvarur).
Seroprevalence
State-level seroprevalence was 32.4% (95% CI 31.3–33.6%), 21.6% (95% CI 20.6–22.6%), 69.2% (95% CI 68.2–70.2%), and 90.6% (CI 90.1–91.1%) in rounds 1, 2, 3 and 4, respectively (Table 2). District-wise seroprevalence has a similar pattern to district-wise seropositivity (Fig. S1).
Seroprevalence was significantly greater in urban areas than rural areas in rounds 1 (35.7% v. 25.7%, p < 0.001) and round 3 (74.8% v. 64.1%, p < 0.001) (Table 2).
Urban classification of clusters was not available for round 2. By round 4, however, the gap has largely closed (91.4% v. 89.8%, p < 0.001).
Seroprevalence is not substantially different across sexes (females v. males: 30.8% v. 30.2% in round 1; 22.0% v. 21.3% in round 2; 67.5% v. 65.5% in round 3; 92.7% v. 88.5%, round 4) (Table 2). While the round 4 difference is significant (p < 0.001), it is still a small gap.
Seroprevalence is highest among older working-age populations in rounds 1 to 2 and among younger populations in rounds 3–4. Seroprevalence is significantly higher among older working-age populations than the elderly in rounds 1–3 (age 50–59 v. age 70 + : 32.2% v. 26.5%, p = 0.002 in round 1; 25.6% v. 21.5%, p = 0.006 in round 2; 66.6% v. 59.6%, p < 0.001 in round 3). Seroprevalence among young adult populations is significantly greater than among the elderly in rounds 3–4 (18–29 v. 70+: 67.7% v. 59.6%, p < 0.001 in round 3; 92.2% v. 83.5%, p < 0.001 in round 4) (Table 2). However, seroprevalence among the children aged 10–17 is lowest of all in round 4 (69.1%, p < 0.001 v. each other age group).
Seroprevalence is significantly greater among vaccinated populations with any number of doses (25.7% v. 20.9%, p < 0.001; 80.0% v. 62.3%, p < 0.001; and 93.1% v. 68.1%, p < 0.001 in rounds 2, 3, and 4, respectively). Rounds 3 and 4 suggest that seroprevalence is increasing in number of doses taken (0 doses v. 1 dose: 62.3% v. 77.5% (p < 0.001) in round 3, and 68.1% v. 87.4% (p < 0.001) in round 4; 1 dose v. 2 doses: 77.5% v. 85.9% (p < 0.001) in round 1 and 87.4% v. 95.0% (p < 0.001) in round 4) (Table 2).
Seroprevalence varied by vaccine platform. Most sample members in rounds 3 and 4 received the Covishield vaccine (89.21 v. 10.59 Covaxin, with remainder getting other vaccines). Seroprevalence is greater among individuals who received the Covishield, as opposed to the Covaxin, vaccine (round 3 and 4 combined: 89.18% v. 80.08%, p < 0.001).
Undercounting
The ratio of the number of infections implied by seroprevalence to confirmed cases is both high and varies widely across districts, from 10 to 148 in round 1 (Table S1). There is a significant negative correlation (\(\rho\)= − 0.58, p < 0.00) between COVID testing rate per thousand and the undercount rate in round 1 (Fig. S2).
Waning antibodies
On average, district-wise seroprevalence rate in round 2 is 68.4% of the seroprevalence rate in round 1 in a district (Table 3), implying a 31.6% decline in seroprevalence, perhaps due to antibody waning. Across districts, the average adjusted cases rate is 9.35% between rounds 1 and 2 and on average 17.8% of sample members report being vaccinated with at least 1 dose by round 2. Adding (a) the district-level adjusted case rate to the regression to control for seropositivity due to new infection and (b) the self-reported vaccination rate to the regression to control for seropositivity due to vaccination yields a lower seroprevalence rate of 42.7% of round 1, implying a significantly larger 57.3% decline (p < 0.001) in seroprevalence after infection.
The annual rate of antibody decay after vaccination among individuals given 2 vaccine doses by round 4 is 16.3 percentage points (p.p.) after 1 year (Table 4). The seropositivity rate falls to zero within a year with the use of age as an instrumental variable for time since dose 2 to obtain causal estimates.
Attribution to infection or vaccination
Seropositivity increased by 42 p.p. between rounds 2, just before India’s second COVID wave, and round 3, after that wave (Table 5). Infections accounted for 74% of this increase. Increased seropositivity among those vaccinated by round 2 accounted for 23%, and new vaccinations accounted for just 2%. Seropositivity increased 23 p.p. from round 3–4, the period between India’s second and third wave. New vaccinations accounted for 65% of this increase. New infections and greater seropositivity among those vaccinated by round 3 accounted for 22% and 13% of this change.
Discussion
Our estimates of seroprevalence rates across the four rounds of our survey are in line with other cotemporaneous surveys conducted in Tamil Nadu. Serological surveys before our round 1 survey in October–November 2021 are all lower than estimated seroprevalence (32.4%) that round39,40,41. Our round 1 estimated seroprevalence is in the middle of the range of estimates from late 202039,42,43. Likewise our round 3 estimate of 69.2% seroprevalence in June–July 2021 is similar to the one other study in mid-202142. This is reassuring as our survey is the only state-wide survey conducted; all prior ones in Tamil Nadu focused on single districts. (No other surveys were conducted in Tamil Nadu in the time range of our round 2 and 4 surveys.)
Our estimates of seroprevalence depart from the literature in two respects. First, our survey is the first population-representative, repeated cross-sectional survey to show a decline in seroprevalence, consistent with antibody waning. Second, our survey suggests that Tamil Nadu had higher seroprevalence than India as a whole44,45,46,47,48.
Our seroprevalence estimates suggests that officially confirmed cases dramatically underestimate the number of infections before vaccination. Statewide seroprevalence in round 1 implies that at least 22.6 million persons in Tamil Nadu were infected after Tamil Nadu’s first wave (by 30 November 2020). This estimate of actual infections is roughly 35 times larger than the number of confirmed cases by round 1 (674,802 cases by 16 October 2020)49.
Our findings suggest that humoral immunity following infection declines rapidly. Seropositivity declined between 31.6 and 57.3% across districts over the roughly 6 months (170 days) between rounds 1 and 2 of Tamil Nadu’s survey, before the state’s vaccination campaign made substantial progress. An implication is that vaccination remained critical for humoral immunity despite India’s devastating Delta wave of infections.
Our study also finds declining seropositivity—implying declining antibody counts—after vaccination. However, we only observe individuals for at most 6 months after last dose. Therefore, one should not extrapolate from these data beyond one-half year. Moreover, participants with the greatest time since vaccination are also older, and older people may experience more rapid waning of antibodies38. Therefore, caution should be taken before extrapolating to younger populations.
Both infection and vaccination can contribute to seroprevalence. Between rounds 2–3, India experienced a second COVID-19 wave due to the Delta variant50. As a result, a majority of the increase in seropositivity from 23.1 to 67.5% was attributable to new infections. Between rounds 3–4, India ramped up its vaccination campaign and did not experience another COVID-19 wave. Therefore, most of the increase in seropositivity from 67.5 to 88.3% was attributable to vaccination. This suggests that, as of the last round of Tamil Nadu’s survey, the majority of humoral immunity was attributable to natural immunity from the Delta wave. The potential value of India’s vaccine campaign, while critical, was limited by the timing of the Delta wave. Our analysis also reveals that vaccination is associated with higher rates of humoral immunity than natural infection, and that this can explain a non-trivial portion of the growth in humoral immunity, even in round 3 (23%), when the vaccination rate was still low.
Our data suggest that additional vaccine doses may contribute to seroprevalence, but with effects diminishing in the number of doses. Moreover, the Covishield vaccine is associated with greater population seroprevalence than Covaxin, which is consistent with studies suggesting Covishield had greater vaccine efficacy51,52.
Our study has several limitations. First, because antibody concentrations in infected persons decline over time53, our estimate of seroprevalence in round 1 may underestimate the level of prior infection and perhaps natural immunity. Moreover, evidence that cellular memory persists beyond humoral immunity54 suggests that, because of waning antibodies, our serological surveillance may also underestimate population immunity to COVID-19. Second, we may overestimate the degree to which humoral immunity has waned if the assays we employ are imperfectly sensitive55. Indeed, it is possible that more sensitive assays are only feasible in smaller samples that are less representative of the population55. Third, our estimate of antibody decline due to natural infection may be incorrect if our adjusted reported case rate does not accurately estimate the infection rate across districts. In that case, our control for infections in Eq. (1) is inadequate. The fact that a 1 percentage point (p.p.) increase in that adjusted rate is associated with a 1 p.p. higher seropositivity rate, however, suggests that the adjusted rate is a reasonable measure of infections. Fourth, we may not accurately untangle seropositivity in round 3 that is due to infection versus due to vaccination. Our estimate of seropositivity among the vaccinated and among the unvaccinated during round 3 may be biased if there is selection into vaccination status that is correlated with seropositivity.
This study demonstrates the growth of seroprevalence in a large, South Indian state through two waves of the COVID-19 pandemic. Seroprevalence estimates demonstrate that officially reported infections dramatically underestimated total infections. It illustrates that humoral immunity wanes amongst those who acquire immunity via infection or vaccination. Finally, it provides a formula to decompose changes in humoral immunity into those attributable to infection versus vaccination. Going forward, estimates of seroprevalence should be updated to account for the effect of India’s third COVID-19 wave in early 2022. Moreover, complementary work that track cellular immunity over the pandemic would be valuable because cellular immunity may outlast humoral immunity54,56,57,58,59.
Data availability
De-identified versions of the datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
01 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-55994-7
References
Wikipedia. List of states and union territories of India by population. https://en.wikipedia.org/wiki/List_of_states_and_union_territories_of_India_by_population (2020).
Johns Hopkins University & Medicine. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). (2021).
Mathieu, E. et al. Coronavirus Pandemic (COVID-19). https://ourworldindata.org/coronavirus (2020).
Duarte, N. et al. Adapting serosurveys for the SARS-CoV-2 vaccine era. Open Forum Infect. Dis. 9, ofab632. https://doi.org/10.1093/ofid/ofab632 (2022).
Tré-Hardy, M., Cupaiolo, R., Wilmet, A., Beukinga, I. & Blairon, L. Waning antibodies in SARS-CoV-2 naive vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J. Infect. 83, 381–412. https://doi.org/10.1016/j.jinf.2021.06.017 (2021).
Abdullahi, A. et al. SARS-COV-2 antibody responses to AZD1222 vaccination in West Africa. Nat. Commun. 13, 6131. https://doi.org/10.1038/s41467-022-33792-x (2022).
Evans, J. P. et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 14, 8057. https://doi.org/10.1126/scitranslmed.abn8057 (2022).
Hatzakis, A. et al. Modelling SARS-CoV-2 binding antibody waning 8 months after BNT162b2 vaccination. Vaccines 10, 285 (2022).
Perreault, J. et al. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood 136, 2588–2591. https://doi.org/10.1182/blood.2020008367 (2020).
Özbay Kurt, F. G. et al. Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2. Front. Immunol. 13, 1012526 (2022).
Bertrand, D. et al. Waning antibody response and cellular immunity 6 months after third dose SARS-Cov-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Am. J. Transplant. 22, 1498–1500. https://doi.org/10.1111/ajt.16954 (2022).
Peng, Q. et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. eBioMedicine https://doi.org/10.1016/j.ebiom.2022.103904 (2022).
Newman, J. et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 7, 1180–1188. https://doi.org/10.1038/s41564-022-01163-3 (2022).
Wei, J. et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 6, 1140–1149. https://doi.org/10.1038/s41564-021-00947-3 (2021).
Ward, H. et al. Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: Serial cross-sectional studies of 365,000 adults. Lancet Reg. Health - Europe 4, 100098. https://doi.org/10.1016/j.lanepe.2021.100098 (2021).
Aldridge, R. W. et al. SARS-CoV-2 antibodies and breakthrough infections in the Virus Watch cohort. Nat. Commun. 13, 4869. https://doi.org/10.1038/s41467-022-32265-5 (2022).
den Hartog, G. et al. Persistence of antibodies to severe acute respiratory syndrome coronavirus 2 in relation to symptoms in a nationwide prospective study. Clin. Infect. Dis. 73, 2155–2162. https://doi.org/10.1093/cid/ciab172 (2021).
Choe, P. G. et al. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg. Infect. Dis. 27, 928–931. https://doi.org/10.3201/eid2703.204543 (2021).
Stone, M. et al. Evaluation of commercially available high-throughput SARS-CoV-2 serologic assays for serosurveillance and related applications. Emerg. Infect. Dis. 28, 672–683. https://doi.org/10.3201/eid2803.211885 (2022).
Buss, L. F. et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science 371, 288–292. https://doi.org/10.1126/science.abe9728 (2021).
Lau, E. H. Y. et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 12, 63. https://doi.org/10.1038/s41467-020-20247-4 (2021).
Lumley, S. F. et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin. Infect. Dis. 73, e699–e709. https://doi.org/10.1093/cid/ciab004 (2021).
Forgacs, D. et al. The effect of waning on antibody levels and memory B cell recall following SARS-CoV-2 infection or vaccination. Vaccines 10, 696 (2022).
Piñana, J. L. et al. SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination. Bone Marrow Transplant. 58, 567–580. https://doi.org/10.1038/s41409-023-01946-0 (2023).
Siller, A. et al. Seroprevalence, waning and correlates of anti-SARS-CoV-2 IgG antibodies in Tyrol, Austria: Large-scale study of 35,193 blood donors conducted between June 2020 and September 2021. Viruses 14, 568 (2022).
Whitcombe, A. L. et al. Comprehensive analysis of SARS-CoV-2 antibody dynamics in New Zealand. Clin. Transl. Immunol. 10, e1261. https://doi.org/10.1002/cti2.1261 (2021).
Krutikov, M. et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in England. Lancet Healthy Longev. 3, e13–e21. https://doi.org/10.1016/S2666-7568(21)00282-8 (2022).
Bartsch, Y. C. et al. Discrete SARS-CoV-2 antibody titers track with functional humoral stability. Nat. Commun. 12, 1018. https://doi.org/10.1038/s41467-021-21336-8 (2021).
Lopman, B. A. et al. A framework for monitoring population immunity to SARS-CoV-2. Ann. Epidemiol. 63, 75–78. https://doi.org/10.1016/j.annepidem.2021.08.013 (2021).
Stringhini, S. et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet 396, 313–319. https://doi.org/10.1016/S0140-6736(20)31304-0 (2020).
DataKind Bengaluru & Development Data Lab. Covid19Bharat.org. https://covid19bharat.org/ (2022).
Malani, A. et al. Seroprevalence of SARS-CoV-2 in slums versus non-slums in Mumbai, India. The Lancet Global Health (2020). https://doi.org/10.1016/S2214-109X(20)30467-8
Mohanan, M., Malani, A., Krishnan, K. & Acharya, A. Prevalence of SARS-CoV-2 in Karnataka, India. JAMA 325, 1001–1003. https://doi.org/10.1001/jama.2021.0332 (2021).
Kish, L. A procedure for objective respondent selection within the household. J. Am. Stat. Assoc. 44, 380–387. https://doi.org/10.1080/01621459.1949.10483314 (1949).
Shenzhen YHLO Biotech Co. Ltd. Customer Notification: Sensitivity and Specificity of iFlash-SARS-Cov-2 IgG and IgM kits from Clinical Trials (2020).
Ortho Clinical Diagnostics. INSTRUCTIONS FOR USE—CoV2G (Version 4.2) (2020).
Rogan, W. J. & Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 107, 71–76 (1978).
Levin, E. G. et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N. Engl. J. Med. 385, e84. https://doi.org/10.1056/NEJMoa2114583 (2021).
Isaac, R. et al. The burden of COVID-19 infection in a rural Tamil Nadu community. BMC Infect. Dis. 21, 1110. https://doi.org/10.1186/s12879-021-06787-0 (2021).
Selvaraju, S. et al. Population-based serosurvey for severe acute respiratory syndrome coronavirus 2 transmission, Chennai, India. Emerg. Infect. Dis. 27, 586–589. https://doi.org/10.3201/eid2702.203938 (2021).
Kumar, M. S. et al. Monitoring the trend of SARS-CoV-2 seroprevalence in Chennai, India, July and October 2020. Trans. R. Soc. Trop. Med. Hyg. 115, 1350–1352. https://doi.org/10.1093/trstmh/trab136 (2021).
Dayanand, D. et al. Community seroprevalence and risk factors for SARS-CoV-2 infection in different subpopulations in Vellore, India, and their implications for future prevention. Int. J. Infect. Dis. 116, 138–146. https://doi.org/10.1016/j.ijid.2021.12.356 (2022).
Laxminarayan, R. et al. SARS-CoV-2 infection and mortality during the first epidemic wave in Madurai, south India: A prospective, active surveillance study. Lancet Infect. Dis. 21, 1665–1676. https://doi.org/10.1016/S1473-3099(21)00393-5 (2021).
Murhekar, M. V. et al. Prevalence of SARS-CoV-2 infection in India: Findings from the national serosurvey, May-June 2020. Indian J. Med. Res. 152, 48–60. https://doi.org/10.4103/ijmr.IJMR_3290_20 (2020).
Murhekar, M. V. et al. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: Findings from the second nationwide household serosurvey. Lancet Glob. Health 9, e257–e266. https://doi.org/10.1016/S2214-109X(20)30544-1 (2021).
Murhekar, M. V. et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020–January 2021. Int. J. Infect. Dis. 108, 145–155. https://doi.org/10.1016/j.ijid.2021.05.040 (2021).
Murhekar, M. V. et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June–July 2021: A population-based cross-sectional study. PLoS Med. 18, e1003877. https://doi.org/10.1371/journal.pmed.1003877 (2021).
Jahan, N. et al. Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020 to August 2021: A systematic review and meta-analysis. Int. J. Infect. Dis. 116, 59–67. https://doi.org/10.1016/j.ijid.2021.12.353 (2022).
Covid19India. COVID19INDIA (Catalogue No. January 30, 2021, 2021).
World Health Organization. Vol. 49 (2021).
Thiruvengadam, R. et al. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: A test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect. Dis. 22, 473–482. https://doi.org/10.1016/S1473-3099(21)00680-0 (2022).
Desai, D. et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: A test-negative, case-control study. Lancet Infect. Dis. 22, 349–356. https://doi.org/10.1016/S1473-3099(21)00674-5 (2022).
Post, N. et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 15, e0244126. https://doi.org/10.1371/journal.pone.0244126 (2021).
Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 23, 186–193. https://doi.org/10.1038/s41590-021-01122-w (2022).
Meyer, B. Waning antibodies to SARS-CoV-2—Don’t panic. Lancet Reg. Health - Europe https://doi.org/10.1016/j.lanepe.2021.100115 (2021).
Grifoni, A. et al. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe 30, 1788. https://doi.org/10.1016/j.chom.2022.10.017 (2022).
Nguyen, T. H. O. et al. T cells targeting SARS-CoV-2: by infection, vaccination, and against future variants. Front. Med. (Lausanne) 8, 793102. https://doi.org/10.3389/fmed.2021.793102 (2021).
Kedzierska, K. & Thomas, P. G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 3, 100562. https://doi.org/10.1016/j.xcrm.2022.100562 (2022).
Almendro-Vázquez, P., Laguna-Goya, R. & Paz-Artal, E. Defending against SARS-CoV-2: The T cell perspective. Front. Immunol. 14, 1107803. https://doi.org/10.3389/fimmu.2023.1107803 (2023).
Author information
Authors and Affiliations
Contributions
The study design and sampling were conceived, led and carried out by the Directorate of Public Health and Preventive Medicine, Government of Tamil Nadu, for the Government of Tamil Nadu’s Department of Health and Family Welfare, especially S. T.S. and S. S. S. A., J. M. S., S. P., V. V., A. B. K. C., P. K., R. S., A. R., P. V., Y. N., G. S., R. A., D. D.N., and S. I. helped administer the survey and study. Data cleaning was performed by V. T., R. P., and S. R. Statistical analysis was conducted by S. R. and A. M. Writing was done by A. M., S. R., and S. S. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the institutions with which the authors are affiliated.
Corresponding author
Ethics declarations
Competing interests
A. M. reports consulting for the World Bank, the Asian Development Bank and IDFC Institute (under a Rockefeller Foundation grant) during this study conducting seroprevalence studies or training in locations other than Tamil Nadu during the time of this study. The remaining authors report no potential or actual conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, Vaidehi Tandel was incorrectly affiliated with Henley Business School, University of Reading, Reading, UK. The correct affiliation is as follows: University of Manchester, Manchester, UK.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Selvavinayagam, T.S., Somasundaram, A., Selvam, J.M. et al. Contribution of infection and vaccination to population-level seroprevalence through two COVID waves in Tamil Nadu, India. Sci Rep 14, 2091 (2024). https://doi.org/10.1038/s41598-023-50338-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50338-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.