Abstract
Understanding antibiotic use in dairy systems is critical to guide antimicrobial stewardship programs. We investigated antibiotic use practices in small-holder dairy farms, antibiotic quality, and antimicrobial resistance (AMR) awareness among veterinary drug retailers in a mixed farming community in the central Kenyan highlands. Data were collected from 248 dairy farms and 72 veterinary drug stores between February 2020 and October 2021. A scale was developed to measure knowledge about AMR and antibiotic use using item response theory, and regression models were used to evaluate factors associated with antibiotic use and AMR knowledge. The active pharmaceutical ingredient (API) content of 27 antibiotic samples was determined using high-performance liquid chromatography (HPLC). The presence and levels of 11 antibiotic residues in 108 milk samples collected from the study farms were also investigated using liquid chromatography tandem mass spectrometry (LC–MS/MS). Almost all farms (98.8%, n = 244) reported using antibiotics at least once in the last year, mostly for therapeutic reasons (35.5%). The most used antibiotics were tetracycline (30.6%), penicillin (16.7%), and sulfonamide (9.4%), either individually or in combination, and predominantly in the injectable form. Larger farm size (OR = 1.02, p < 0.001) and history of vaccination use (OR = 1.17, p < 0.001) were significantly associated with a higher frequency of antibiotic use. Drug retailers who advised on animal treatments had a significantly higher mean knowledge scores than those who only sold drugs. We found that 44.4% (12/27) of the tested antibiotics did not meet the United States Pharmacopeial test specifications (percentage of label claim). We detected nine antibiotics in milk, including oxytetracycline, sulfamethoxazole, and trimethoprim. However, only three samples exceeded the maximum residue limits set by the Codex Alimentarius Commission. Our findings indicate that antibiotics of poor quality are accessible and used in small-holder dairy systems, which can be found in milk. These results will aid future investigations on how to promote sustainable antibiotic use practices in dairy systems.
Similar content being viewed by others
Introduction
Animal husbandry currently accounts for approximately two thirds of the global consumption of antibiotics, and this is projected to increase1. The widespread use and misuse of antibiotics has raised concerns about potential development and dissemination of antimicrobial resistance (AMR). AMR infections have been estimated to lead to 1.27 million deaths globally in 2019, with most of the burden borne by low- and middle-income countries (LMICs), particularly in sub-Saharan Africa2. In these settings, antibiotics serve as ‘quick fixes’ for hygiene and productivity challenges, acting as substitutes for more costly interventions to improve the conditions within which health workers, farming communities, and animals work and live3. In the recent years, there are increasing calls for reduction in the use of antibiotics in the agri-food system4, with a growing emphasis on the importance of antimicrobial stewardship (AMS) and good animal husbandry practices5. In Kenya, as other countries, the government has developed a National Action Plan on AMR (AMR NAP), which aligns with the global AMR action plan, and prioritizes antimicrobial stewardship in livestock systems6.
Kenya's dairy sector, predominantly consists of smallholder farmers, owning between one to three cows, and contributes a substantial portion to the country's Gross Domestic Product (GDP) derived from livestock farming (14% of the agricultural GDP and 3.5% to the overall GDP)7,8. Use of antibiotics is widespread in these dairy systems, as they are plagued with mastitis, respiratory infections, enteric diseases; and “dry cow” therapy is a predominant reason for antibiotic use9. The extensive use of antibiotics raises concerns about the excretion of antibiotic residues in milk, which can pose health risks to humans and ecosystems10 and development of AMR in human and bovine pathogens. However, few studies have investigated how these antibiotics are accessed and used by farmers, levels of antibiotic residues in milk, and knowledge and practices related to on-farm antibiotic use.
Furthermore, most smallholder farmers are not able to afford veterinary services, which may also be scarce depending on the region, thus leading them to either self-diagnose or rely on veterinary drug stores for consultation, diagnosis, and prescription of drugs, including antibiotics11,12. However, irrational and inappropriate prescription of antibiotics is a common practice in these stores because most staff are not qualified to diagnose and prescribe treatment, some are legally not permitted to prescribe11,13, and sales of antibiotics are linked to profits for the veterinary drug stores business14. The situation is further exacerbated by the high reported prevalence of counterfeit and substandard veterinary medicines in Sub Saharan Africa15. A recent systematic review reported that 47.3% of medicines tested in 17 African countries were either substandard or falsified16. In a similar study from the Mekong Delta in Vietnam, 6.9% of the antibiotic sampled in veterinary drug stores contained levels of active pharmaceutical ingredients (APIs) outside the pharmacopeial limits17.
This study reports the results of a cross-sectional survey in dairy farms and veterinary drug stores in a Kenyan mixed farming community to assess (1) on-farm antibiotic usage patterns and practices, (2) determine the levels of antibiotic residues in milk, (3) prescription practices amongst veterinary drug stores workers, and (4) determine the quality of selected antibiotics purchased from veterinary drug stores.
Methods
Study area and population
The study population was a crop-livestock mixed farming community in Kericho county located in the highlands of the Kenyan Rift Valley. Kericho County is characterized by high levels of rainfall and agricultural productivity, and most households grow a range of crops for subsistence and to sell. Dairy farming, with an average herd size of < 5 cows, is often integrated with crop farming, mainly tea and maize production, on small plots of 1–5 acres18.
All research reported here was performed in accordance with the Declaration of Helsinki and the legal requirements of the Government of Kenya. Ethical approval for human data collection was obtained from The International Livestock Research Institute (ILRI) Institutional Research Ethics Committee (ILRI-IREC2020-01). Animal sampling was conducted under the approval of the ILRI Institutional Animal Care and Use Committee (ILRI-IACUC2020-06), and permits were obtained from the Directorate of Veterinary Services. Written informed consent was obtained from all study participants.
Study design and selection of farms and veterinary drug stores
The study was cross-sectional, with the primary study unit being dairy farms in Kipkelion East, a sub-county of Kericho, while veterinary drug stores spanned the whole county. Kipkelion East sub-county was randomly selected from a list of sub-counties in Kericho. A sample size of 248 dairy farms was calculated to estimate the prevalence of antibiotic residues in milk on farms, assuming an expected prevalence of antibiotic residues 10% in milk19. Random selection of farms was stratified by sub-location, which is the smallest administrative unit in Kenya. Within each sub-location, farms were identified with the assistance of the local veterinary officers, as lists of farms were not available. For veterinary drug stores, a total of 72 stores were purposefully selected across the county for maximum spatial distribution. Due to the lack of a registry of veterinary drug stores, random sampling within each sub-county was not possible, but efforts were made to ensure consistent selection of study stores. The final distribution of sampled farms and veterinary drug stores is shown in Fig. S1. Data collection happened between February 2020 and October 2021.
Data collection on dairy farms
On each farm, the farmer (or a nominated farm worker) completed a questionnaire20,21 detailing livestock ownership (e.g., number of dairy cows), farm management practices (e.g., history of vaccination), antimicrobial use patterns and knowledge of antibiotic use. From selected healthy milking cows on each farm, milk samples were collected from each quarter after thoroughly cleaning and drying the teats. The samples were placed immediately into a cool box with ice and refrigerated at 4 °C and transferred to the lab within five hours.
Data collection at veterinary drug stores
A questionnaire was administered to veterinary drug store owners or workers to collect data on demographics, customer demographics, types of antibiotics sold, antibiotic prescribing practices, and knowledge of AMR and antibiotic use. In Kenya, veterinary drug stores are primarily managed by animal health technicians, also known as para-veterinarians, while only a small number are operated by veterinarians. According to the Kenyan regulations, animal health technicians are not authorized to prescribe antibiotics, only veterinarians. In this study, the term “pharmacist” refers to individuals involved in the sale of antibiotics, regardless of their level of clinical training.
Concurrently with the questionnaire survey, a mystery shopper, assumed the role of a farmer and visited veterinary drug stores, presenting two scenarios: Scenario 1 involved a dairy cow experiencing respiratory distress symptoms such as nasal discharge, lethargy, labored breathing, and lack of appetite; Scenario 2 involved a flock of broilers displaying symptoms of diarrhea, weight loss, weakness, lack of appetite, and ruffled feathers. One of the shoppers, who was a veterinarian, further requested a specific antibiotic brand. The mystery shoppers were not denied an antibiotic despite not having a prescription. The samples were purchased in their original containers, labelled with unique numbers, and the following information was collected on each sample: brand name, active pharmaceutical ingredients, manufacturer's name, package size, strength, and dosage form. Data from the questionnaires were recorded using Open DataKit (ODK) Collect software on electronic tablets and uploaded to databases at the International Livestock Research Institute (ILRI), Nairobi, Kenya.
Laboratory analysis of antibiotic residues in milk
Details on sample extraction and testing are provided in the supplementary methods, but briefly, we used ultra-high performance liquid chromatography-tandem mass spectrometry (LC–MS/MS) to determine the concentration of antibiotic residues in milk. The LC–MS/MS experiments were performed using the Shimadzu LC–MS 8050 system (Shimadzu Corporation, Kyoto, Japan) consisting of LC-20AD solvent delivery pumps, a SIL-30AC autosampler, a CTO-30A column oven and the LC–MS 8050 triple quadrupole detectors. Detection by MS/MS was performed on a Shimadzu 8050 triple quadrupole mass spectrometer, fitted with an electrospray ionization source operating in positive ionization mode. Samples were tested for tetracycline, oxytetracycline, chlortetracycline, penicillin G, sulfamethoxazole, trimethoprim, gentamicin, ceftiofur, dihydrostreptomycin, ampicillin, and chloramphenicol. Antibiotic levels in milk were compared to Codex Alimentarius Commission maximum residue limits for veterinary drugs22.
Laboratory analysis of veterinary drugs
Details on sample preparation and testing are provided in the supplementary methods, but briefly, we used a validated LC–MS/MS method on a Shimadzu SPD-20A Prominence Diode Array (PDA) UV–VIS Detector DAD HPLC system to measure the active pharmaceutical ingredient (API) content in each sample. Duplicate homogeneous mixtures of the samples were prepared and analysed, with the analysis conducted blindly, without prior knowledge of the brand. The assessment of drug quality was based on the mean percentage of API per label and compliance with the United States Pharmacopeial 29 (USP 29) standard on content assay (percentage content) for the respective antibiotics. Samples that exceeded these limits were classified as non-compliant and further divided into two categories based on the extent of deviation from the USP 29 criteria: moderate deviations and extreme deviations (Table s1).
Modelling drivers of antibiotic use in dairy farms
Data analysis was performed using R. Descriptive analysis displayed frequencies and percentages for categorical variables and mean, standard deviation, and range for quantitative variables. Univariable analyses were performed to test for independent associations between the various predictor variables and the frequency of antibiotic use on dairy farms. The outcome of interest was the number of times antibiotics used on farm during the year before the study. The explanatory factors included history of vaccination, total number of livestock on the farm, utilization of laboratory services, participation in animal health campaigns, seeking professional veterinary help, frequency of milk sales, grazing type, participation in farmer trainings, utilization of household waste as feed, and use of commercial premixed feed. Variables with a p-value < 0.2 were included in a multivariable Poisson generalized linear model (GLM) to identify significant (p < 0.05) explanatory variables associated with farm-level antibiotic use. The multivariable models were fitted using the lme4 package in R, and significance was determined using Wald χ2-tests. The best-fitting model was selected based on the lowest Akaike Information Criterion (AIC) using the 'dredge' function in the MuMIn package23. Diagnostics plots of the models were generated using the DHARMa package24 to assess whether the model assumptions were violated.
Analysis of knowledge of antibiotics and antibiotic use amongst veterinary drug store retailers
Information on knowledge and practices related to AMR and antibiotic use were collected from veterinary pharmacists using a 3-point Likert scale (‘agree,’ ‘disagree,’ and ‘don't know’). The responses were dichotomized, with ‘agree’ recoded as one (1) and ‘disagree’ or ‘don't know’ as zero (0). Cronbach’s alpha was calculated to assess reliability, with a value greater than 0.70 indicating good consistency of item responses. The unidimensionality of a scale based on the knowledge questions was evaluated using confirmatory factor analysis (CFA) using the cfa function in lavaan package25. A two-parameter logistic item response theory (IRT) model was applied using the ltm26 package to estimate the latent level of knowledge among respondents. Four items with low discriminatory power were removed from the analysis. The remaining ten items were used to create item characteristic curves (ICC) and item information functions (IIF) to evaluate the measurement scale's performance. Knowledge scores for each respondent were derived using empirical Bayes predictions using factor.scores function in ltm26. A generalized linear model was used to examine the relationship between veterinary drug shop demographics and prescription practices with the latent knowledge score.
Results
Demographic characteristics of study participants
Out of the 248 dairy farmers who participated in the survey, 145 (58.5%) were men, and their average age was 46.7 years. The mean herd size was 7.4 (range, 0–40), with almost all farmers (96.8%) practicing zero grazing. Private animal health providers (AHPs), either paraveterinarians or veterinarians, served as the primary veterinary care providers (98%) for farmers in this study. A third of farmers had participated in animal health campaigns within the last year, while a quarter of them had attended trainings specifically focused on disease prevention.
A total of 72 veterinary drug stores were surveyed. More than two thirds (70.8%) of the veterinary drug store pharmacists were male, with a mean age of 34.1 years (range 19–60). Of the pharmacists surveyed, 45.8% were exclusively involved in retail business, while 54.2% were engaged in both retailing and private veterinary practice. Additionally, 23.6% had less than one year of experience, 24% had 1–5 years of experience, and 31% had more than five years of experience. Meanwhile, 25% of the respondents did not have animal health training but only completed secondary or primary school.
Antibiotic access and use amongst dairy farmers
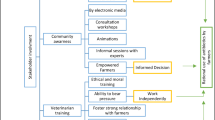
Of the 248 dairy farms surveyed, 245 (98.8%) used antibiotics at least once in the last year. Moreover, 35.5% of farmers reported only using antibiotics for therapeutic reasons, 4.9% used prophylactic reasons, and 5.6% for both purposes. Tetracycline (30.6%, n = 75), penicillin (16.7%, n = 41), and sulfonamide (9.4%, n = 23) were the most frequently used antibiotic classes, either individually or in combinations, and predominantly in the injectable form. Other antibiotics reported include aminoglycosides (6.1%, n = 15), macrolides (0.8%, n = 2), and fluoroquinolones (0.4%, n = 1). Most farmers (90.3%) reported buying antibiotics from animal health service providers, while 5.7% bought them directly from veterinary drug stores, and the remaining farmers sourced antibiotics from other farmers. Univariable analysis revealed significant associations between antibiotic use and several factors, including number of livestock on farm, vaccination status, utilization of laboratory services in the last year, participation in farmer training programs, frequency of milk sales, and the practice of using household waste as animal feed (Table S2). The multivariable analyses revealed that larger farms and those with history of vaccination use were significantly associated with a higher frequency of antibiotic use (OR = 1.02, p < 0.001, 95%CI [1.01–1.03]; OR = 1.17, p < 0.001, 95%CI [1.04–1.31], respectively) (Fig. 1).
Dairy farmers were the most frequent customers (77.8%) who purchased antibiotics in the stores followed by poultry farmers (22%). One-third of veterinary drug stores described antibiotics as the product that contributed the most to their sales, while only one store (1.4%) mentioned vaccines as their primary product. Eighty six percent of stores reported that they sold antibiotics without a prescription, relying on drug labels and the information provided by the farmers for making a diagnosis and deciding on treatment choices including the prescribing of antibiotics.
Antibiotic residues in milk
A total of 108 milk samples collected from farms and analyzed for nine antibiotics. All nine antibiotics were detected in varying rates above the detection limit with oxytetracycline (50%, n = 54), sulfamethoxazole (50%, n = 54) and trimethoprim (40.7%, n = 44) detected in more than a third of the samples (Table S4). However, only three samples, containing tetracycline (0.9%), oxytetracycline (2.8%), and penicilin G (4.6%) were above their respective MRLs.
Knowledge of AMR and antibiotic use amongst veterinary pharmacists
The internal reliability of the ten “knowledge statements” was Cronbach’s α of 0·72. Most of the questions had a similar discrimination level and difficulty level expect for Q8 which had a higher difficulty of 1.07 and Q5 which had a discrimination level of 2.35 (Table S3 and Fig. S2). Our final model indicated that respondents working in drug retail businesses who also advised on animal treatments had a significantly higher mean for knowledge than those who only sold drug (p = 0.03, GLM, Fig. 2). There was no significant difference between having knowledge and whether prescriptions are required or not, respondents age, gender, education level or duration in business (p > 0.05, GLM).
Quality of antibiotics purchased from veterinary drug stores
A total of 27 antibiotics were purchased and of those, 19 (70.3%) were single compound antibiotics and the remaining eight (29.6%) were combinations of two or more antibiotics. Oxytetracyline (66.7%, 18), was the most frequently sold antibiotic. The other antibiotics include sulfamethoxazole/trimethoprim (11.1%, 3), sulfadimidine/trimethoprim (3.7%, 1), penicillin G/dihydrostreptomycin (11.1%, 3), cefitour (3.7%, 1), and oxytetracycline/streptomycin (3.7%, 1). Of the 19 samples with one antibiotic, 13 (65%) were within the API-specific limit ranges of USP29 (% label claim), four (20%) showed extreme deviations and two (15%) showed moderate deviations. Of the eight antibiotic combination samples, two (25%) were within acceptable range, and remaining six (75%) failed (Fig. 3 and Table s4).
Content of the active pharmaceutical ingredient (API) determined for each antibiotic purchased. Each sample is represented by a dot, with red color indicating extreme deviation, amber indicating moderate deviation, and green indicating compliance within the quality range. EFT, ceftiofur; OXY, oxytetracycline; DSTR, dihydrostreptomycin; PEN, penicillin; SXT, sulfamethoxazole; TMP, trimethoprim; TET, tetracycline; SUL, sulfadiazine; STR, streptomycin.
Discussion
This study aimed to describe practices associated with antibiotic use in dairy farms and antibiotic sale and quality of antibiotics in veterinary drug stores within a mixed farming community in central Kenyan highlands. This study extends previous research on non-prescription antibiotic sales at veterinary drug stores in LMICs11,13,27. Despite legislation prohibiting sales of antibiotics without a prescription in Kenya28, this practice remains common, and is driven by factors such as animal health services either too costly and inaccessible for farmers, weak enforcement of regulations by the government, and financial motivations amongst pharmacists. Improving pharmacists’ knowledge and practice of antimicrobial stewardship has been posited as a possible and cost-effective strategy to reduce non-prescription antibiotic sale29. Similarly, veterinary drug stores should be encouraged to diversify their business to sell other products such as vaccines, vitamins, and provide veterinary services, which would provide an alternative source of income and reduce reliance on antibiotic sales30. However, we found that knowledge of antibiotics or AMR did not vary by level of animal health training, nor did it influence whether a pharmacist sold an antibiotic with or without a prescription. There is an increasing agreement that antimicrobial stewardship education programs alone may not be enough to sustain improvements in optimized antibiotic use31,32. Instead, these programs should be integrated with reinforced legislation, strong surveillance systems along the antibiotic supply chain, and consumer education for behavioural change.
Most dairy farmers in this study reported using antibiotics, mainly for therapeutic purposes, with higher usage observed in larger farms and those implementing routine vaccinations. Our finding that most farmers relied on animal health service providers for animal health management, which placed the responsibility of antibiotic stewardship on them, highlights the complexity of on-farm antibiotic decision-making processes. Animal health service providers have minimal access to diagnostic testing to support treatment (antibiotic) choice, and the definition of “normal” use is not well-defined. Targets that define acceptable amounts of antibiotics to be used and metrics of quantitative values of antibiotic used in farming systems are critically needed for AMS, but these are lacking in LMIC settings. We found antibiotic residues in milk that exceeded the MRLs for the three antibiotics, tetracycline, oxytetracycline and penicillin G. This finding is consistent with previous research documenting similar results in milk collected along the milk value chain33,34,35, and is reflective of imprudent antibiotic use practices particularly non-adherence to antibiotic withdrawal guidelines. To guide risk assessment and the development of evidence-based animal health policies, antibiotic use, and residue monitoring surveillance programs should be considered36. Antibiotic use was associated with herd size suggesting that poor animal management increase the likelihood of infections requiring antibiotic treatment. However, history of vaccination did not reduce the likelihood of antibiotic use, despite the widely accepted knowledge that the use of vaccines in livestock substantially decreases antibiotic use37. This discrepancy raises the possibility that farms that vaccinated their animals modified their behavior based on the perception of being protected, potentially neglecting adequate biosecurity measures that reduce infections and subsequent antibiotic use. Another possible explanation is potentially low vaccine efficacy, possibly due to poor handling along the supply chain, the use of the wrong vaccine, or poor administration38.
Despite anecdotal evidence suggesting that poor-quality veterinary antibiotics are common in Sub-Saharan Africa, prevalence data is lacking16,39,40. The presence of poor-quality antibiotics poses significant risks to animal health and welfare, food and nutritional safety, farmers’ profits and raising concerns about the potential emergence and spread of AMR41,42. In our study, we found that 44.4% (12/27) of the tested antibiotics did not meet the United States pharmacopeial test specifications for assay (% label claim). These findings align with previous research conducted in Vietnam, where 6.9% of antibiotic were found to have concentrations less than half of the labeled content17. The underlying reasons for the presence of poor-quality antibiotics include poor manufacturing practices, falsification of contents, poor pharmacovigilance, degradation during the supply chain, and inadequate storage conditions43,44. We could not test these hypotheses using our data; however, a combination of epidemiological surveys with robust methodology, larger sample sizes, and chemical analyses including MS fingerprinting may help distinguish among these factors45. A multi-pronged approach combining regulatory improvements that focus on pharmacovigilance, strengthening laboratory capacity, and increasing public awareness about the importance of using high-quality antibiotics are needed to enhance health of antibiotic supply chains.
The limitations of this study are inherent to the epidemiological methods used in our cross-sectional survey, which focused on describing antibiotic sales, antibiotic use, and antibiotic quality in a specific rural dairy farming community. Therefore, generalization of the findings to urban populations, where these practices may differ, is limited. Additionally, the data obtained from veterinary drugs stores relied on information provided by pharmacy staff and was not cross validated with veterinary or sales records. Furthermore, the analysis of antibiotic quality was based on a sample size of 27 samples, and storage conditions or periods in the veterinary store could have influenced our findings.
Conclusion
We characterized antibiotic sale, use, and quality patterns in a dairy farming community in Kenya. Our findings indicate that antibiotics, often of poor quality, are frequently sold without prescription, commonly used without proper diagnostics, and can contaminate food products. With the growing threat of AMR, it is crucial for veterinary medicine stakeholders to reevaluate the role of community veterinary drug stores in antibiotic stewardship programs. In addition, we highlight the urgency to develop interventions aimed at enhancing the quality of antibiotics including stricter regulation, post-market surveillance surveys utilizing public–private partnerships, and public education.
Data availability
The datasets generated and analysed in the current study are available from the corresponding author upon reasonable request.
References
Boeckel, T. P. V. et al. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U. S. A. 112, 5649–5654. https://doi.org/10.1073/pnas.1503141112 (2015).
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. The Lancet 399, 629–655. https://doi.org/10.1016/S0140-6736(21)02724-0 (2022).
Laurie Denyer, W. & Clare, C. Quick fix for care, productivity, hygiene and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Glob. Health 4, e001590. https://doi.org/10.1136/bmjgh-2019-001590 (2019).
World Health Organization. World leaders and experts call for significant reduction in the use of antimicrobial drugs in global food systems. 2021. https://www.who.int/news/item/24-08-2021-world-leadersand-experts-call-for-significant-reduction-in-the-use-of-antimicrobial-drugs-in-global-food-systems. Accessed August 2023.
Allerton, F. & Russell, J. Antimicrobial stewardship in veterinary medicine: A review of online resources. JAC-Antimicrob. Resistance 5, dlad058. https://doi.org/10.1093/jacamr/dlad058 (2023).
Wesangula, E., Githii, S. & Ndegwa, L. Implementing the national action plan on antimicrobial resistance in Kenya: Global expectations, national realities. Int. J. Infect. Dis. 101, 41 (2020).
Ajwang, F. & Munyua, C. The influence of state of road and ownership of means of transport to smallholder dairy farmers’ choice of milk marketing outlet in kipkaren division of nandi county in Kenya. Asian J. Agric. Extens. Econ. Sociol. 9, 1–6 (2016).
Odero-Waitituh, J. Smallholder dairy production in Kenya; a review. Livestock Res. Rural Dev. 29, 139 (2017).
Farrell, S. et al. Factors influencing dairy farmers’ antibiotic use: An application of the COM-B model. J. Dairy Sci. 106, 4059–4071. https://doi.org/10.3168/jds.2022-22263 (2023).
Virto, M., Santamarina-García, G., Amores, G. & Hernández, I. Antibiotics in dairy production: Where is the problem?. Dairy 3, 541–564 (2022).
Muloi, D. et al. A cross-sectional survey of practices and knowledge among antibiotic retailers in Nairobi, Kenya. J. Glob. Health 9, 145. https://doi.org/10.7189/jogh.09.020412 (2019).
Kiambi, S. et al. Understanding antimicrobial use contexts in the poultry sector: Challenges for small-scale layer farms in Kenya. Antibiotics 10, 896. https://doi.org/10.3390/antibiotics10020106 (2021).
Kemp, S. A., Pinchbeck, G. L., Fevre, E. M. & Williams, N. J. A cross-sectional survey of the knowledge, attitudes, and practices of antimicrobial users and providers in an area of high-density livestock-human population in Western Kenya. Front. Vet. Sci. 8, 56. https://doi.org/10.3389/fvets.2021.727365 (2021).
Dione, M. M., Amia, W. C., Ejobi, F., Ouma, E. A. & Wieland, B. Supply chain and delivery of antimicrobial drugs in smallholder livestock production systems in Uganda. Front. Vet. Sci. 8, 611076 (2021).
Jaime, G., Hobeika, A. & Figuié, M. Access to veterinary drugs in sub-saharan Africa: Roadblocks and current solutions. Front. Vet. Sci. 8, 74. https://doi.org/10.3389/fvets.2021.558973 (2022).
Vayouly, V., Konnie, B., Paul, N. N. & Céline, C. The quality of veterinary medicines and their implications for One Health. BMJ Global Health 7, e008564. https://doi.org/10.1136/bmjgh-2022-008564 (2022).
Luu-Quynh, H., Nguyen-Thi-Bich, T., Ta-Hoang, L., Erickson, V. I. & Padungtod, P. Quality testing of veterinary antimicrobial products used for livestock in Vietnam, 2018–2019. PLoS One 16, e0247337. https://doi.org/10.1371/journal.pone.0247337 (2021).
Kiplangat, N. E. & Vincent, N. Farm gate dairy milk marketing channel choice in Kericho County, Kenya. J. World Econ. Res. 7, 92 (2018).
Ahlberg, S., Korhonen, H., Lindfors, E. & Kang, E. Analysis of antibiotic residues in milk from smallholder farms in Kenya. Afr. J. Dairy Farm. Milk Prod. 3, 152–158 (2016).
Wieland, B. et al. AMUSE Livestock, version 2—Antimicrobial use in livestock production: A tool to harmonise data collection on knowledge, attitude and practices. https://hdl.handle.net/10568/107443 (2019).
Nohrborg, S. et al. Geographic and socioeconomic influence on knowledge and practices related to antimicrobial resistance among smallholder pig farmers in Uganda. Antibiot.-Basel 11, 251. https://doi.org/10.3390/antibiotics11020251 (2022).
Codex Alimentarius. Maximum residue limits and risk management recommendations for residues of veterinary drugs in foods. https://www.fao.org/fao-who-codexalimentarius/codex-texts/maximum-residue-limits/en/ (2021).
Barton, K. http://CRAN.R-project.org/package=MuMIn (2010).
Hartig, F. & Hartig, M. F. DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package. (2022).
Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 48, 1–36. https://doi.org/10.18637/jss.v048.i02 (2012).
Rizopoulos, D. ltm: An R package for latent variable modeling and item response analysis. J. Stat. Softw. 17, 1–25. https://doi.org/10.18637/jss.v017.i05 (2006).
Wilkinson, A., Ebata, A. & MacGregor, H. Interventions to reduce antibiotic prescribing in LMICs: A scoping review of evidence from human and animal health systems. Antibiotics 8, 2 (2019).
Government of Kenya. Veterinary Surgeons and Veterinary Paraprofessionals Act. https://faolex.fao.org/docs/pdf/ken112140.pdf (Kenya, 2011).
Lloyd-David, H. & Page-Stephen, W. Antimicrobial Stewardship in Veterinary Medicine. Microbiol. Spectr. 6, 3. https://doi.org/10.1128/microbiolspec.arba-0023-2017 (2018).
Carmo, L. P. et al. Veterinary expert opinion on potential drivers and opportunities for changing antimicrobial usage practices in livestock in Denmark, Portugal, and Switzerland. Front. Vet. Sci. 5, 859. https://doi.org/10.3389/fvets.2018.00029 (2018).
Sangwan, R., Neels, A. J., Gwini, S. M., Saha, S. K. & Athan, E. Is Education Alone Enough to Sustain Improvements of Antimicrobial Stewardship in General Practice in Australia? Results of an Intervention Follow-Up Study. Antibiot. Basel 12, 594. https://doi.org/10.3390/antibiotics12030594 (2023).
Satterfield, J., Miesner, A. R. & Percival, K. M. The role of education in antimicrobial stewardship. J. Hospital Infect. 105, 130–141. https://doi.org/10.1016/j.jhin.2020.03.028 (2020).
Brown, K., Mugoh, M., Call, D. R. & Omulo, S. Antibiotic residues and antibiotic-resistant bacteria detected in milk marketed for human consumption in Kibera Nairobi. PLoS ONE 15, e0233413. https://doi.org/10.1371/journal.pone.0233413 (2020).
Kosgey, A., Shitandi, A. & Marion, J. W. Antibiotic residues in milk from three popular kenyan milk vending machines. Am. J. Trop. Med. Hyg. 98, 1520–1522. https://doi.org/10.4269/ajtmh.17-0409 (2018).
Orwa, J. D., Matofari, J. W., Muliro, P. S. & Lamuka, P. Assessment of sulphonamides and tetracyclines antibiotic residue contaminants in rural and peri urban dairy value chains in Kenya. Int. J. Food Contam. 4, 5. https://doi.org/10.1186/s40550-017-0050-1 (2017).
Babu-Rajendran, N. et al. EPI-Net One Health reporting guideline for antimicrobial consumption and resistance surveillance data: A Delphi approach. Lancet Reg. Health Europe 26, 100563. https://doi.org/10.1016/j.lanepe.2022.100563 (2023).
Micoli, F., Bagnoli, F., Rappuoli, R. & Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 19, 287–302. https://doi.org/10.1038/s41579-020-00506-3 (2021).
Nuvey, F. S. et al. Effectiveness and profitability of preventive veterinary interventions in controlling infectious diseases of ruminant livestock in sub-Saharan Africa: A scoping review. BMC Vet. Res. 18, 332. https://doi.org/10.1186/s12917-022-03428-9 (2022).
Newton, P. N. et al. Falsified medicines in Africa: All talk, no action. Lancet Glob. Health 2, e509–e510. https://doi.org/10.1016/S2214-109X(14)70279-7 (2014).
Clifford, K. et al. Antimicrobial resistance in livestock and poor quality veterinary medicines. Bull. World Health Organ 96, 662–664. https://doi.org/10.2471/blt.18.209585 (2018).
Pyzik, O. Z. & Abubakar, I. Fighting the fakes: Tackling substandard and falsified medicines. Nat. Rev. Dis. Prim. 8, 55. https://doi.org/10.1038/s41572-022-00387-1 (2022).
World Health Organization. WHO Global Surveillance and Monitoring System for substandard and falsified medical products. Geneva: World Health Organization. https://www.who.int/publications/i/item/9789241513425 (2017).
Schäfermann, S. et al. Substandard and falsified antibiotics and medicines against noncommunicable diseases in Western Cameroon and Northeastern Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 103, 894–908. https://doi.org/10.4269/ajtmh.20-0184 (2020).
Herrington, J. E., Nayyar, G. M. & Breman, J. G. The Global Pandemic of Falsified Medicines: Laboratory and Field Innovations and Policy Perspectives (American Society of Tropical Medicine and Hygiene, 2015).
Bakker-’t Hart, I. M. E., Ohana, D. & Venhuis, B. J. Current challenges in the detection and analysis of falsified medicines. J. Pharmaceut. Biomed. Anal. 197, 113948. https://doi.org/10.1016/j.jpba.2021.113948 (2021).
Acknowledgements
This study was supported by the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI); we acknowledge the CGIAR Fund Donors (https://www.cgiar.org/funders/). CM was funded by African Academy of Sciences. This study also received support from the CGIAR One Health initiative “Protecting Human Health Through a One Health Approach,” which was supported by contributors to the CGIAR Trust Fund (https://www.cgiar.org/funders/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are grateful to the farmers and respondents in the veterinary drug store in Kericho for taking part in the study. We thank Henry Kiara for insightful discussions and help with study design, and the field enumerators for assistance with fieldwork. We thank the regional Veterinary Investigative Laboratory (RVIL) in Kericho for their cooperation in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization—P.K., A.M., C.M.; field investigation—P.K., C.M.; laboratory investigation—P.K., A.M., .L.O, F.N., F.G.; statistical analysis D.M.M., G.S., F.G.; writing—original draft—D.M.M.; writing—reviewing and editing—all; supervision—J.M.M., D.G., M.D., A.M. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muloi, D.M., Kurui, P., Sharma, G. et al. Antibiotic quality and use practices amongst dairy farmers and drug retailers in central Kenyan highlands. Sci Rep 13, 23101 (2023). https://doi.org/10.1038/s41598-023-50325-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50325-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.