Abstract
Given the importance of diet in early life, assessing children’s diet is crucial to guide interventions. Using data from a nationally generalizable New Zealand (NZ) birth cohort we examined children’s dietary patterns at 9- (n = 6259), 24- (n = 6292), and 54-months (n = 6131), and their association with maternal sociodemographic and health behaviours. At each time-point, children's dietary patterns were identified using principal components analysis. We then used multivariate linear regression to examine associations between each pattern and maternal variables. At 9-, 24- and 54-months, two dietary patterns were identified, explaining 36.4%, 35.3% and 33.6% of children's intake variability, respectively. Refined high in sugar, salt and fat dietary pattern, at all time-points, was characterized by high positive loadings in white/refined breads and cereals, and items with high sugar, sodium, and fat content. At 24-months, Refined high in sugar, salt and fat also included a high positive loading with protein food groups. Fruit and vegetables dietary pattern, at all time-points, had high positive loadings for fruits and vegetables (with type varying across time-points). Fruit and vegetables also included high loading in whole grain options of breads and cereals at 24-months and the protein food group was part of this dietary pattern at 9- and 54-months. Children’s scores on the Refined high in sugar, salt and fat pattern had strong associations with maternal smoking habits, education level, ethnicity, and maternal scores in the “Junk” and “Traditional/White bread” dietary patterns (constructed from an antenatal interview). Children’s scores on the Fruit and vegetables pattern had strong associations with the maternal scores in the dietary pattern “Health Conscious”. Interventions to improve diet in early life in NZ need to be responsive to ethnicity and suitable for people of all education levels. Interventions that improve maternal health behaviours may also improve children’s diet.
Similar content being viewed by others
Introduction
Feeding practices in early life including optimal breastfeeding duration, timely introduction of foods, and intake of healthy foods are associated with higher diet quality in childhood and better short- and long-term health outcomes such as the decreased risk of developing obesity1,2,3,4,5,6,7,8,9,10,11,12,13. Several determinants influence feeding practices in the first years of life, with maternal health behaviours and sociodemographic inequalities playing an important role8,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29.
Intakes of many foods and nutrients are strongly correlated, making it difficult to isolate the effect (s) of a specific food or nutrient. This is where dietary patterns analyses can assist, as they make use of the correlated nature of dietary data to identify patterns of total food consumption11. Dietary pattern analyses represent a holistic assessment of diets which summarises the combined and potentially synergistic effects of different foods that contribute to the usual dietary intake of a population 9,11,30,31. For this reason, dietary patterns have been identified as a more realistic representation of dietary habits, as it can be translated into guidelines for the public more easily9,11, and are useful for understanding the associations between diet quality and health outcomes, including childhood obesity9,11,26,30,32,33,34. Previous studies have showed that dietary patterns energy-dense, high-sugar, high-fat, and low-fibre foods appear to be established in the early years of life and tend to track throughout the life course8,10,22,31,33,35,36. Therefore, this period of life represents a relevant window of opportunity for promoting dietary trajectories which have a positive and prospective impact on health and wellbeing2,11,33,35.
Childhood overweight/obesity is a public health problem in New Zealand (NZ), which has great ethnic and socioeconomic disparities37. NZ was placed second among the countries of the Organisation for Economic Cooperation and Development/European Union for the prevalence of overweight/ obesity in 5–19-year-olds (39.5%)38. Despite the importance of early feeding practices and their reported associations with child obesity9,10,11,12,13, NZ has limited nationally representative or generalizable information on children's diet quality, especially among children under five. Information is only available from a national child nutrition survey conducted two decades ago, which involved children aged 5–14 years39. Recently, information from the Growing up in NZ cohort study provided the first nationally generalizable information on breastfeeding indicators, timing of food introduction, and diet quality in the first 5 years of life (which used dietary indexes measuring adherence to national Food and Nutrition Guidelines)14,15,16,17. In this study, we used the Growing up in NZ cohort data to provide nationally generalizable information on (i) dietary patterns at three time-points in early life (9-, 24- and 54-months) and; (ii) determine associations between the identified dietary patterns and maternal health behaviours and sociodemographic characteristics. Findings from this study aim to guide interventions designed to improve and narrow inequities in diet quality in early life in NZ and, subsequently, to positively impact health and wellbeing indicators of future generations.
Methods
Data collection and population
This cross-sectional study used data from Growing Up in New Zealand, a contemporary NZ birth cohort which enrolled 6,822 pregnant women and their 6,853 children who survived to age 6 weeks40. The eligibility of a pregnant woman was determined by an estimated delivery date between 25 April 2009 and 25 March 2010, and residence in the region of NZ defined by boundaries of three contiguous district health boards. At birth, the cohort was broadly generalizable to the NZ births that took place from 2007 to 2010, allowing for robust analyses by ethnicity and socioeconomic position40.
This study used information from five data collection waves (antenatal, six weeks after birth, and at 9-, 24-, 31-, and 54-months). Information on maternal sociodemographic and health behaviour characteristics were obtained from the antenatal, 24- and 54- face-to-face computer-assisted personal interviews (CAPIs). Child perinatal information (sex, singleton/twin-triplet, birth weight and gestational age) was obtained from the six-week computer-assisted telephone interview (CATI). Information on children's dietary intake was obtained from the 9-, 24- and 54-month children’s CAPIs. Duration of any breastfeeding was estimated from data obtained at the six-week CATI and at the 9-month and 31-month CAPIs14.
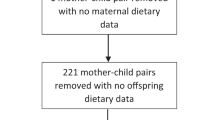
Of the 6,853 children enrolled in the cohort, the respective proportions of children for whom the 9, 24-, and 54-month interviews were completed were 92% (n = 6237), 90% (n = 6156) and 89.8% (n = 6156)41. To describe children's dietary patterns, those with no or incomplete information on dietary intake were excluded: 3.4% (n = 217), 0.6% (n = 35) and 0.4% (n = 25) of the children who took part of the 9-, 24-and 54-month interviews, respectively. Thus, dietary patterns were described for 96.6% (n = 6259), 99.4% (6292) and 99.6% (6131) of the children at the 9-, 24-and 54-month interviews, respectively. To examine associations between the children's scores for the dietary patterns at each time-point and maternal sociodemographic and health behaviour characteristics we excluded: (i) siblings of twins and two siblings of triplets: 1.1% (n = 75; 9-months); 1.2% (n = 79; 2- months) and 1.3% (n = 79; 54-months) and; (ii) children with missing information for all the maternal covariates examined: 0.3% (n = 17; at 9-months); 0.6% (n = 40; at 24-months) and 0.7% (n = 42; at 54-months). The analyses of associations included 95.2% (n = 6167), 97.6% (6173) and 97.6% (n = 6010) of the children who took part of the 9-, 24-and 54-month interviews, respectively (Fig. 1). Twins/triplets were included when identifying the dietary patterns because their dietary patterns will be used in future analyses within the Growing Up in New Zealand cohort study. We randomly excluded siblings of twins and two siblings of triplets from the analyses of associations to guarantee only the inclusion of independent observations.
Dietary patterns at 9, 24 and 54 months
Growing Up in New Zealand used semi-quantitative food frequency questionnaires (FFQs) to assess infant and children’s dietary intakes at the 9-, 24- and 54-month interviews. At the 9-month interview, a 25-food items FFQ was used, where mothers were asked to report their infants’ usual frequency of intake of foods and drinks15. At the 24- and 54-month interviews, a 62-food items FFQ was used; mothers were asked to report their children’s usual intake in the previous four weeks as well as the amount typically eaten, relative to a “standard” serving (two or more, one, ½ and ¼ servings). The only difference in the FFQs used at 24- and 54-month interviews was that “toddler formula milk” and “toddler prepared meals” were part of the 24-month FFQ but not of the 54-month FFQ. Similarly, “avocados” and “crackers” were part of the 54-month-FFQ but not of the 24-month-FFQ16.
The FFQs were administered to children's caregivers during the CAPI interviews at 9-, 24- and 54-months and show-cards containing the photos of usual servings of each food/food grouping listed in the FFQs were shown to help the caregivers16. Growing Up in New Zealand FFQs were designed to measure the cohort’s adherence to nationally recommended intakes of food groups for infants and preschoolers at the time of the interviews42,43, rather than to provide a full portrait of children’s usual intakes. To guarantee the inclusion of the foods commonly consumed by NZ infants and preschoolers, Growing Up in New Zealand FFQs’ food lists were revised based on studies conducted in NZ: an investigation conducted in Auckland-NZ with 6- to 23-month-olds44 and a nationally representative survey on diets for 5-to-14-year-olds39. We have previously created dietary indexes measuring Growing Up in New Zealand adherence to National Food and Nutritional Guidelines at 9-,24- and 54-months. These indexes were created using data from the FFQs administered at these time-points and the indexes convergent construct validity was demonstrated by their ability to discriminate the cohort's sociodemographic characteristics15,16.
For examining children's dietary patterns at 9-, 24- and 54-months, we grouped the FFQs' foods and food groups based on the national recommendation of food and food groups for infants and preschoolers 42,43. These food groups/items were grouped by adding the daily frequency of intake of foods at 9-months and the daily number of servings at 24- and 54-months. Thus, at 9-months food items/groups were grouped under 10 foods groups: Vegetables; Fruit; Breads and Cereals; Baby foods-iron enriched; Suitable infant formula milk; Other milk products; Protein Group: Meat, chicken or meat dishes, Fish or fish dishes (fresh and canned), Shellfish, Eggs, Soyfoods, Tofu, Soy desserts, Nuts or peanut butter; Inappropriate drinks—not recommended to infants; Inappropriate foods—high content of added sugars; Inappropriate foods—high content of sodium and fat. Similarly, at 24- and 54-months the food items/groups were grouped under 13 food groups: Green leafy vegetables; Other types of vegetables; Citrus fruit; Other types of fruit; Breads and cereals-white, refined options; Breads and cereals-brown, wholegrain options; Milk, milk products or suitable alternatives- whole milk, non-reduced fat options; Milk and milk products- skim/trim milk, low-fat options; Protein Group: Lean meat, poultry, fish, shellfish, eggs, legumes, nuts and seeds; Processed meats; Inappropriate drinks—high content of added sugars; Inappropriate foods—high content of added sugars; Inappropriate foods—high content of sodium and fat, especially saturated fat. At 24 months we also included toddler formula milk (further details on Table S1).
Mother and child covariates
The selection of maternal sociodemographic and health behaviour characteristics examined in this study was based on prior literature demonstrating their associations with children’s dietary patterns13,14,15,16,17,18,19,20,21,23. We also adjusted in the models examining the associations between maternal variables and children's dietary patterns the following children's covariates described in the literature as associated with diet quality in childhood: exact age at the time of 9-,24- and 54-month interviews (in months), sex (male/female), gestational age (in weeks-continuous), birth weight (in grams-continuous), and any breastfeeding duration (never breastfed/< 6 months/ ≥ 6 months)4,6,8,15,16,20,21,22,23,28 .
The following antenatal maternal sociodemographic and health behaviour variables were examined: level of education completed, age, ethnicity, body-mass-index, physical activity before/during pregnancy, and maternal dietary patterns. Maternal smoking habits were examined at the three time-points. Before and during pregnancy smoking patterns were categorized as: continued smoking, stopped smoking during pregnancy and non-smokers. At the 24- and 54-month interviews, maternal cigarette smoking was assessed by asking the mothers if they were currently smoking regularly at least one cigarette/daily (yes/no). A NZ index of deprivation (NZDep) measured antenatally and at the 24-month and 54-month interviews was used. Children’s home addresses were geocoded and linked to the NZDep (in deciles) which are also used as the small area measure of neighbourhood deprivation45,46. The NZDep2006 was used at the 9- and 24-month interviews while the NZDep2013 was used at the 54-month interview. The NZDep2006 combines nine socioeconomic characteristics from the 2006 census data collected at aggregations of approximately 100 people and assigned to individual households based on geo-coded address data45. The NZDep2013 is derived similarly to NZDep2006, however, using 2013 census data46. Maternal ethnicity was prioritised using the Statistics NZ classification Level 1: Māori, Pacific Peoples, Asian, Middle Eastern, Latin American, and African (MELAA), Other, and European47. MELAA and Other were combined for analysis purposes. Maternal pre-pregnancy BMI was calculated based on self-reported weight and height and classified according to the World Health Organization (WHO) cut-offs48. Physical Activity (PA) before and/or during pregnancy was estimated using the International Physical Activity Questionnaire (IPAQ). Women who engaged in moderate PA for at least 30 min for at least 5 out of 7 days, or vigorous PA for at least 30 min on at least 2 out of 7 days were classified as participating in moderate/vigorous activity49 and this variable was categorized as: no moderate/vigorous PA before or during pregnancy, moderate/vigorous PA before and during pregnancy, and moderate/vigorous PA only before or only during pregnancy.
Maternal diet during pregnancy was assessed using a semi-quantitative 44-item FFQ. The FFQ's food list was developed based on the New Zealand Ministry of Health guidelines for healthy pregnant women50 and food intake was recalled over the 4 weeks preceding the antenatal interview (with frequencies of intake ranging from not at all, or less than once a month, to a number of times per month, week, or day). Show-cards containing photos of usual servings of food/food grouping listed were shown to participants51. A previous NZ study identified four distinct dietary patterns (DPs) during pregnancy that explained 23.4% of the total variance in dietary intake. High positive loadings for confectionary, snacks, takeaways, hot chips, soft/energy drinks, cakes/biscuits, processed meats, battered fried fish/seafood and ice cream characterized the “Junk” dietary pattern while the “Healthy Conscious” dietary pattern had high positive loadings for vegetables, brown whole meal bread, cheese, non-citrus fruits, yoghurt, dried fruits, high fibre cereal and Vegemite/Marmite. The “Traditional/White” dietary pattern had high positive loadings for whole/standard milk, white bread, margarine, jam/honey/marmalade, peanut butter/Nutella, and a high negative loading for skim/trim milk. High positive loadings for noodles/rice/pasta, seafood, chicken, green leafy vegetables, eggs, red meat, processed fish, and citrus fruits characterized the “Fusion” dietary pattern51.
Statistical analyses
Children's dietary patterns at the 9-,24-and 54-month interviews were identified using principal components analyses. Food/food groups were entered in the analyses as daily frequency of intake (9-months) and daily serving’s intake (24- and 54-months), as previously described, and were standardized using the correlation matrix. Rotated factor solutions ranging from 2 to 4 were generated before deciding which factors to retain. The numbers of patterns (components) identified at each time-point were based on factors with components with eigenvalues > 1.5, breaks in the scree plots and the interpretability and meaningfulness of the matrix after varimax rotation. We considered that food items had moderate to large correlations with the identified dietary patterns adopting the threshold of > 0.45 or < − 0.45 for the factor loadings. For each dietary pattern, naming was based on food groupings with high positive factor loadings.
Associations between the children’s factor scores for each dietary pattern identified at 9-, 24-and 54-month interviews (dependent variables) and the maternal socio-demographic and health behaviour characteristics (independent variables) were examined using multivariate linear regression models with associations described using β-coefficients and 95% confidence intervals (95% CI). At the three time-points, models were adjusted for children's sex and age. The 9-months models were also adjusted for children’s birth weight, gestational age, and duration of any breastfeeding. Analyses of covariance (unadjusted and adjusted) were also performed between the dependent variables and the independent variables with 2 or more categories, to get an omnibus test of the importance of that variable. All analyses were performed using SPSS software (version 25, IBM SPSS Statistics).
Ethics approval and consent to participate in the study
Growing up in New Zealand was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving human subjects were approved by the Ministry of Health’s Northern Y Regional Health and Disability Ethics Committee (NTY/08/06/055). Written informed consent was obtained from all children's mothers/caregivers40.
Results
Population
Table S2 presents the characteristics of the children and their mothers included in the analyses of dietary patterns at the 9-, 24-and 54-month interviews. The mean (SD) child’s age at the 9-, 24- and 54-month interviews were, 9.0 (0.9), 24.3 (2.0) and 54.0 (1.6) months, respectively. Most children were singletons and males represented 51.6%, 51.6% and 51.5% of the children at the 9-, 24- and 54-month interviews. Among the children included in the analyses at 9-months, approximately one-third were not breastfed or were breastfed for less than 6 months, had a mean (SD) of 3490 (583.6) grams for birth weight and 39.1 (1.9) weeks for gestational age. At all time-points, approximately 4 in 10 mothers had education level higher or equal bachelor’s degree and approximately 6% had no secondary school qualification. At the 9-, 24- and 54-month interviews, 37%, 35.9% and 33.2% of the children belonged to the most deprived neighbourhoods (deciles 8–10 of the NZDep), respectively. Approximately half of the mothers were of European ethnicity, most were aged 20–34 years and approximately 4 in 10 mothers had a pre-pregnancy BMI ≥ 25 kg/m2. Approximately 3 in 10 mothers reported practicing moderate/vigorous PA before and during pregnancy. Antenatally, 19.3% of mothers reported smoking before and during pregnancy and respectively at the 24- and 54-month interviews 13.4% and 13.1% of the mothers were smoking at least 1 cigarette/day.
Dietary patterns at 9-, 24- and 54-months
At each of the interviews, two distinct dietary patterns were identified and together explained 36.4%, 35.3% and 33.6% of the food intake variance at 9-, 24- and 54-months, respectively (scree plots are illustrated in Figure S1). At each time-point the dietary patterns were named based on the food items with higher factor loadings. The first dietary pattern was named Refined high in sugar, salt, and fat, and was characterized, at all time-points, by high positive loadings in white/refined breads and cereals and items with high content of sugar, sodium, and fat. At 24-months, Refined high in sugar, salt, and fat also had high positive loading in the protein group (which included lean meats, poultry, fish, shellfish, eggs, legumes, nuts, and seeds). The second dietary pattern was named Fruit and vegetables and at all time-points had high positive loadings for fruits and vegetables (with type varying at 9-, 24- and 54-months). Fruit and vegetables at 24- months had also high positive loadings for whole grain breads and cereals. At 9- and 54-month, Fruit and vegetables had also positive loading in the protein group (at 9-months this group included Meat, chicken, or meat dishes; Fish or fish dishes (fresh and canned); Shellfish; Eggs; Soyfoods-tofu, soy deserts, Nuts/peanut butter; at 54-months this group included lean meats, poultry, fish, shellfish, eggs, legumes, nuts and seeds) (Table 1).
Maternal covariates associated with children's dietary patterns at 9-, 24- and 54-months
The unadjusted associations between the children’s scores in the two dietary patterns at each interview and the maternal socio-demographic and health behaviour characteristics are presented in Supplementary Tables S3–S5.
In the adjusted models, for all time-points, lower maternal educational attainment was significantly associated with children's higher scores in the Refined high in sugar, salt, and fat dietary pattern. The largest difference was between mothers with a bachelor’s degree or higher, and those who had not completed a secondary qualification, with intermediate scores for secondary or trade qualifications. For all time-points, compared to children living in the least deprived neighbourhoods (deciles 1–3), those in the most deprived deciles (8–10) scored higher in the Refined high in sugar, salt, and fat pattern. Maternal ethnicity was also significantly associated with the Refined high in sugar, salt, and fat pattern at all time-points. In comparison to children from European mothers, children scored higher in the pattern if their mothers: were from Māori ethnicity (at 9-, 24- and 54-months), or Asian and Pacific ethnicities (at 24- and 54-months). Maternal age at pregnancy influenced children's score in the Refined high in sugar, salt, and fat pattern at 9- and 24-months, but this influence was attenuated at 54-months. Children scored higher in this pattern if their mothers were younger than 35 years of age (compared to children from mothers aged ≥ 35 years). At all time-points, children scored higher in the Refined high in sugar, salt, and fat pattern if their mothers were smokers and if their mothers had high antenatal scores in the “Junk” and “Traditional/White” dietary patterns. Conversely, children of mothers with high scores in the “Health conscious” dietary pattern scored lower in the Refined high in sugar, salt, and fat at all time-points (Tables 2, 3, 4). Children whose mothers had antenatal high score in the “Fusion” dietary pattern scored higher in the Refined high in sugar, salt, and fat pattern at 24-months (Table 3) but scored lower in this dietary pattern at 9-months (Table 2).
In the adjusted models, children whose mothers had educational attainment lower than Bachelor's degree scored lower in Fruit and vegetables dietary pattern at 9- and 24-months. Children living in neighbourhoods belonging to deciles 8–10 of the NZDep (at 9-months) and deciles 4–10 (at 24-months) also scored lower in the Fruit and vegetables pattern. Children also scored lower in this pattern if their mothers: were from Māori or Pacific ethnicities (at 9-months) or Asian ethnicity (at 9- and 24-months); did not practice moderate/vigorous PA before and during pregnancy (at 24- and 54-months) and; had high antenatal scores in the “Junk” (at 9-,24- and 54-months) and the “Traditional/White” dietary patterns (at 9-months). At 54-months, children scored higher in the Fruit and vegetables pattern when their mothers were younger than 35 years of age. Higher children's scores in this dietary were also observed among those whose mothers had antenatal high scores in the “Health conscious” dietary pattern (all time-points), “Fusion” patterns (at 9- and 54-months) and “Traditional/White” pattern (at 54-months) (Tables 2, 3, 4).
Discussion
This study provides the first NZ nationally generalizable information on the dietary patterns of under-five-year-olds. At 9-, 24-and 54-months two distinct dietary patterns were identified (Refined high in sugar salt and fat and Fruit and vegetables). At all time-points, the significant associations between the dietary patterns and maternal sociodemographic variables highlight the influence of inequities on children's dietary quality. Maternal health behaviours (diet quality in pregnancy and smoking habits) were also associated with children's poorer diet quality at all time-points.
The dietary patterns identified within Growing up in New Zealand cohort are similar to those described in the first years of life in other cohorts and surveys worldwide9,11,13,19,23,27,28,29,34,52,53,54,55,56. Most studies have identified at least two dietary patterns in this period of life, despite differences in naming and methods used to derive them. Overall, the patterns identified were those with: (1) high positive loading on fatty foods, refined grains, and highly processed and/or discretionary foods-with high contents of sodium, sugar and unhealthy fats and; (2) high loadings for fruits, vegetables and whole grains and low-fat protein products9,13,19,27,28,29,34,52,53,54,55,56. Studies involving infants and toddlers in the UK, Australia, Norway, and Japan identified two dietary patters, which were consistent with: core or recommended foods, and non-core or discretionary foods- high contents of sugar, sodium, and unhealthy fats27,28,29,53,54. The Southampton Women's Study identified a “Guidelines” pattern among 6- and 12-month-old children, which had high loadings in fruits, vegetables, and home prepared foods and “Adult Foods” pattern, with high consumption of chips, savoury snacks, and biscuits27. Secondary analysis of an Australian randomized trial and a longitudinal study found two patterns at ages 14- and 24-months. At 14- months, the “Core foods” pattern had high loadings in fruit, grains, vegetables, cheese, and nuts/seeds and at 24-months this pattern included fruit, vegetables, dairy, nuts/seeds, meat, and water. The “Basic combination” pattern had high loadings for white bread, milk, spreads, juice and ice-cream at 14-months and white bread, spreads, sweetened beverages, snacks, chocolate, and processed meat at 24-months28. Studies conducted with pre-schoolers and older children consistently identified a dietary pattern with high loadings in non-recommended foods-high amount of sugar, sodium, and healthy fats. The names commonly used to characterize the referred pattern included, but were not limited to “Junk”, “Processed” “Processed and fast food”, “High energy-dense foods”, “Discretionary”, “Snacking” and “Sweet and fat”8,11,13,19,23,34,55,56. Two NZ birth cohorts identified such a pattern among 3.5-, 6-, and 7-year-olds13,55. In the cohort nested within Auckland Birthweight Collaborative study, involving babies born small for gestational age (SGA) and appropriate for gestational age (AGA), the “Junk” dietary pattern identified at 3.5 and 7 years had high loadings for candy bars, hamburgers, soft drinks, and chips. Additional items with high loadings on foods where intakes were enquired only at 7 years were chocolate and lollies55. Among 1,142 six-year-olds who were part of the Screening for Pregnancy Endpoints (SCOPE) cohort- NZ, the 'Junk' dietary pattern was characterized by high loadings in candy bars, potato crisps, lollies or sweets, sausage rolls, and bought cake or muffin13.
The associations found in this study indicate that there were ethnic and sociodemographic disparities in diet quality in early life in NZ and that children whose mothers were smokers or had lower diet quality also had low dietary quality during the first 5 years. We demonstrated that lower maternal education attainment was associated with higher scores in the Refined high in sugar, salt, and fat pattern at 9-, 24-and 54-months and lower scores in the Fruit and vegetables pattern at 9-and 24-months. These are not surprising findings and corroborate with those reported for 6-year-olds New Zealanders who scored higher in a “Healthy” pattern when their mothers had higher educational attainment and they scored higher in the “Junk” pattern when mothers had lower level of education13. Other cohorts have reported that children of mothers with lower education attainment scored higher in dietary patterns characterized by high intakes of foods with high amounts of energy, sugar and fat, and low amounts of fibre18,22,53 as well as lower in dietary patterns composed of fruit, vegetables, whole grains, and other relevant core foods18,22,53. Lower maternal education level was also associated with high trajectories of discretionary food intake in the first 3 years of life in the Healthy Smiles Healthy Kids (HSHK) birth-cohort study in South-Western Sydney, Australia8 The literature suggests that lower parental education may be related to lower literacy and knowledge about nutrition and healthier eating behaviours23.
Within Growing Up in New Zealand cohort, overall, despite of the variations on the magnitudes of associations across the three time-points, it was observed higher scores in the Refined high in sugar, salt, and fat pattern and lower in the Fruit and vegetables pattern among children from ethnicities other than European. These findings align with results from the NZ SCOPE cohort which identified that children of mothers Pacific or Indian ethnicities scored higher in the “Junk” dietary patterns at age 6 years while those from Indian and Māori mothers were less likely to score highly in the “Healthy” dietary pattern13. Maternal non-English speaking ethnicity was also associated with high trajectories of discretionary food intake among Australian children during the first 3 years of life8. Children whose mothers were younger at pregnancy scored higher in the Refined high in sugar, salt, and fat pattern at 9-and 24-months within Growing Up in New Zealand cohort, validating observations in NZ, Australia, and Brazil8,20,22,28,53,55. In this study, maternal smoking habits and diet quality in pregnancy were also associated with children's diet quality at all time-points. Significant associations between maternal smoking habits with infants' non-timely food introduction and lower diet quality at 9-,24-and 54- months15,16,17 have also been reported within the Growing up in New Zealand cohort and within others two NZ cohorts13,55. Wall et al.55 and Flynn et al13 demonstrated that 6-year-olds New Zealanders were more likely to have higher scores in dietary patterns with high loading in foods high in energy, sugar, and fat when their mothers were smokers13,55. Previous observation suggests that smoking results from behavioural, psychosocial, and biological factors and that smoking mothers may be less likely to be health conscious and more likely to experience stress and lower social support57.
Another relevant finding of our study were the strong associations found between maternal diet quality in pregnancy and children diet quality at all time-points, where maternal high scores in the “Junk” pattern was positively associated with children's high scores in the Refined high in sugar salt and fat patterns and maternal high scores in the “Health conscious” pattern associated positively with children's scores in the Fruit and vegetables pattern. The strong influence of maternal dietary patterns on comparable dietary patterns for their children have been also reported in cohorts from the UK, Australia, and Portugal7,19,23,27 and authors have suggested that this may be due to the influence of maternal eating behaviours on children's food habits19,23,27. Role modelling and the food environment at home is likely to be even more important in early life given higher dependency of children on their mothers23.
The identification of a dietary pattern loading high in refined foods and foods high in sugar, sodium and unhealthy fats within Growing up in New Zealand at the three time-points in early life is concerning as previous studies have showed significant associations between children's low diet quality in early life and adiposity later in childhood and adolescence9,11,13,32,34,56. This dietary pattern has also moderately stable trajectories through childhood and adolescence, with the intakes of the foods that load high on this pattern increasing with age8,10,22,31,33,36,58.
This study has limitations important to be mentioned. First, we do not have individual level deprivation measures. Data on deprivation are based on fined grained neighbourhood measures. It may explain why education presented a more consistent association with children’s dietary patterns. Second, the observational data used here make inference about causality difficult, because we have not looked at interplay of education, ethnicity, deprivation, and ‘health conscious’ maternal intake. It means that the ethnic differences observed in our study could be partially accounted for education, deprivation and vice-versa. At all time-points, the FFQs data were examined for potential implausible outliers of intake before running PCA. However, as the FFQs were not designed to capture the cohort's total energy intake, we could not with 100% certainty classify some outlier values of food servings as “implausible” and opted to keep all values of intake in the datasets. Additionally, the FFQs were administered to the child’s mother and represent an indirect measure of the child’s intake, which may be influenced by measurement error. Lastly, another limitation to consider is that PCA analysis uses the correlation matrix of food intake variables to identify common patterns of food consumption within the data to account for the largest amount of variation in diet within the studied population. Although widely employed to characterize children's dietary patterns, in PCA analyses the selection of the patterns is somewhat arbitrary and subjectivity relies on the author's interpretation59. As strengths of our study, we highlight the nationally generalizable characteristic of our cohort, representing the children from New Zealand. Comparisons between the Growing up in New Zealand cohort with contemporary national data for 2007–2010 indicated that there were only small differences between them (prevalence of low birth weight and preterm) which are unlikely to limit the external validity of findings to the wider NZ population. The large size of the cohort means that small variations in percentages (< 1%) between the cohort and national births reached statistical significance, but this statistical difference is unlikely to be of public health or clinical relevance40. The ability of Growing up in New Zealand cohort to track antenatal diet and diet in early life can provide insights into the potential long-term effects of early dietary choices on health outcomes in NZ. Our findings echo other previously published studies, adding further evidence for the need to intervene across several maternal determinants to improve infant and young child health outcomes through food choices and dietary patterns. Although the effects of maternal determinants on children’s dietary patterns appears to be quite robust, most studies describing this relationship have concentrated analysis on a single time-point, not assessing dietary intake patterns through the first years of life as presented in this work.
Conclusion
Findings from this study indicate that diet quality in early life may be improved if sociodemographic disparities are narrowed in NZ. This may include the facilitation of maternal access to education and promoting culturally safe and integrated structural approaches to achieve improvements in early life diet quality. Examples of structural approaches include government led interventions for better affordability of healthy diets and to limit the availability and the marketing of junk foods (high content of added sugar, sodium and unhealthy fats) to children. NZ has currently no government-led mandatory actions or legislation to improve the healthiness of the food environments and to limit children's exposure to unhealthy foods60. Maternal smoking cessation and promotion of healthy diets among women of reproductive may also elicit long-term impacts on children’s diet quality, and health and wellbeing.
Data availability
The anonymized data that support the findings of this study are available from the Growing up in New Zealand Cohort Study, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author (Dr Teresa Gontijo de Castro) upon reasonable request and with permission of the Growing Up in New Zealand Data Access Committee (email: dataaccess@growingup.co.nz).
References
Hawkes, C., Ruel, M., Salm, P., Sinclair, B. & Branca, F. Double-duty actions: Seizing programme and policy opportunities to address malnutrition in all its forms. Lancet 395, 142–155 (2020).
Skouteris, H. et al. Reframing the early childhood obesity prevention narrative through an equitable nurturing approach. Matern Child Nutr 17(1), e13094. https://doi.org/10.1111/mcn.13094 (2020).
Grimm K.A., Kim S.A., Yaroch A.L., Scanlon K.S. Fruit and vegetable intake during infancy and early childhood. Pediatrics, 134 Suppl 1(Suppl 1):S63–9. doi: https://doi.org/10.1542/peds.2014-0646K (2014).
Bielemann, R. M., Santos, L. P., Costa, C. D. S., Matijasevich, A. & Santos, I. S. Early feeding practices and consumption of ultraprocessed foods at 6 y of age: Findings from the 2004 Pelotas (Brazil) Birth Cohort Study. Nutr J 47, 27–32. https://doi.org/10.1016/j.nut.2017.09.012 (2018).
Lauzon-Guillain, B. et al. The influence of early feeding practices on fruit and vegetable intake among preschool children in 4 European birth cohorts. Am J Clin Nutr 98(3), 804–812. https://doi.org/10.3945/ajcn.112.057026 (2013).
Perrine, C. G., Galuska, D. A., Thompson, F. E., Scanlon, K. S. Breastfeeding duration is associated with child diet at 6 years. Pediatrics 134 Suppl 1(Suppl 1):S50–5. https://doi.org/10.1542/peds.2014-0646I (2014).
Jones, L., et al. The influence of early feeding practices on healthy diet variety score among pre-school children in four European birth cohorts. Public Health Nutr. 18(10):1774–84. https://doi.org/10.1017/S1368980014002390 (2015).
Manohar, N. et al. Early life and socio-economic determinants of dietary trajectories in infancy and early childhood—results from the HSHK birth cohort study. Nutr J 20, 76. https://doi.org/10.1186/s12937-021-00731-3 (2021).
Liberali, R., Kupek, E. & Assis, M. A. A. Dietary patterns and childhood obesity risk: A systematic review. Child Obes. 16(2), 70–85. https://doi.org/10.1089/chi.2019.0059 (2020).
Ambrosini, G. L., Emmet, P. M., Northstone, K. & Jebb, S. A. Tracking a dietary pattern associated with increased adiposity in childhood and adolescence. Obesity 22, 458–465 (2014).
Ambrosini, G. Childhood dietary patterns and later obesity: A review of the evidence. Proc Nutr Soc 1, 137–146. https://doi.org/10.1017/S0029665113003765 (2014).
Woo Baidal, J. A. et al. Risk factors for childhood obesity in the first 1000 days: A systematic review. Am J Prev Med 50(6), 761–779. https://doi.org/10.1016/j.amepre.2015.11.012 (2016).
Flynn, C. A. et al. Childhood dietary patterns and body composition at age 6 years: the Children of Screening for Pregnancy Endpoints (SCOPE) study. Br J Nutr 124(2), 1–21. https://doi.org/10.1017/S0007114520000628 (2020).
Castro, T. G. et al. Breastfeeding indicators among a nationally representative multi-ethnic sample of New Zealand children. NZMJ 130, 34–44 (2017).
Castro, T. G. et al. An index measuring adherence to New Zealand infant feeding guidelines has convergent validity with maternal socio-demographic and health behaviours and with children’s body size. Br J Nutr 127(7), 1073–1085. https://doi.org/10.1017/S0007114521001720 (2022).
Castro, T. G., et al. Child feeding indexes measuring adherence to New Zealand nutrition guidelines: Development and assessment. Matern Child Nutr, 18, e13402.https://doi.org/10.1111/mcn.13402 (2022).
Ferreira, S. et al. Prevalence and maternal determinants of early and late introduction of complementary foods: Results from the Growing Up in New Zealand cohort study. Br J Nutr 129(3), 491–502. https://doi.org/10.1017/S000711452200112X (2023).
Rashid, V., Weijs, P. J. M., Engberink, M. F., Verhoeff, A. P. & Nicolaou, M. Beyond maternal education: Socio-economic inequalities in children’s diet in the ABCD cohort. PLoS ONE 15(10), e0240423. https://doi.org/10.1371/journal.pone.0240423 (2020).
Durão, C. et al. Associations of maternal characteristics and behaviours with 4-year-old children’s dietary patterns. Matern Child Nutrn 13, e12278. https://doi.org/10.1111/mcn.12278 (2017).
Santos, L.P., et al. Dietary intake patterns of children aged 6 years and their association with socioeconomic and demographic characteristics, early feeding practices and body mass index. BMC Public Health 16, 1055.https://doi.org/10.1186/s12889-016-3725-2 (2016).
Sørensen, L.M.N., et al. Diet quality of Norwegian children at 3 and 7 years: Changes, predictors and longitudinal association with weight. Int. J. Obes. 46, 10–20.https://doi.org/10.1038/s41366-021-00951-x (2022).
Lioret, S. et al. Dietary patterns track from infancy to preschool age: cross-sectional and longitudinal perspectives. J Nutr 145, 775–782. https://doi.org/10.3945/jn.114.201988 (2015).
Lioret, S. et al. Association between maternal education and diet of children at 9 months is partially explained by mothers’ diet. Matern Child Nutr 11, 936–947. https://doi.org/10.1111/mcn.12031 (2015).
Patrick, H., Nicklas TA. A review of family and social determinants of children's eating patterns and diet quality. J Am Coll Nutr 24 (2):83–92. https://doi.org/10.1080/07315724.2005.10719448. (2005)
Choudhury, S., Headey, D. D., Masters, D. A. First foods: Diet quality among infants aged 6–23 months in 42 countries, Food Policy 88: https://doi.org/10.1016/j.foodpol.2019.101762. (2019)
Craig, L., McNeill, G., Macdiarmid, J., Masson, L., & Holmes, B. Dietary patterns of school-age children in Scotland: Association with socio-economic indicators, physical activity and obesity. Br J Nutr 103 (3), 319–334. https://doi.org/10.1017/S0007114509991942 (2010)
Robinson, S., et al. Southampton Women's Survey Study Group. Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. Br J Nutr 98 (5):1029–37. https://doi.org/10.1017/S0007114507750936 (2007).
Bell, L., et al. Dietary patterns of Australian children aged 14 and 24 months, and associations with socio-demographic factors and adiposity. Eur J Clin Nutr 67, 638–45. https://doi.org/10.1038/ejcn.2013.23 (2013)
Okubo, H., et al. Osaka Maternal and Child Health Study Group. Dietary patterns in infancy and their associations with maternal socio-economic and lifestyle factors among 758 Japanese mother-child pairs: the Osaka Maternal and Child Health Study. Matern Child Nutr 10(2), 213–25. https://doi.org/10.1111/j.1740-8709.2012.00403.x (2014).
Ambrosini, G. L. et al. Identification of a dietary pattern prospectively associated with increased adiposity during childhood and adolescence. Int J Obes 36, 1299–1305 (2012).
Northstone, K. & Emmett, P. Are dietary patterns stable throughout early and mid-childhood? A birth cohort study. Br J Nutr 100, 1069–1076 (2008).
Fernández-Alvira, J. M. et al. Prospective associations between dietary patterns and body composition changes in European children: The IDEFICS study. Public Health Nutr 20(18), 3257–3265 (2017).
Mikkila, V., Rasanen, L., Raitakari, O. T., Pietinen, P. & Viikari, J. Consistent dietary patterns identified from childhood to adulthood: the cardiovascular risk in Young Finns Study. Br J Nutr 93, 923–931 (2005).
Pala, V. et al. Dietary patterns and longitudinal change in body mass in European children: A follow- up study on the IDEFICS multicentre cohort. Eur J Clin Nutr 67, 1042–1049 (2013).
Dalrymple, K. V. et al. Longitudinal dietary trajectories from preconception to mid-childhood in women and children in the Southampton Women’s Survey and their relation to offspring adiposity: A group-based trajectory modelling approach. Int J Obes 46, 758–766. https://doi.org/10.1038/s41366-021-01047-2 (2022).
Veronica, L. et al. Unhealthy dietary patterns established in infancy track to mid-childhood: The EU childhood obesity project. J Nutr 148(5), 752–759. https://doi.org/10.1093/jn/nxy025 (2018).
Ministry of Health. Tier 1 statistics 2019/20: New Zealand Health Survey. Wellington: Ministry of Health (2020).
United Nation Children’s Fund. The State of the World’s Children 2019. Children, Food and Nutrition: Growing well in a changing world. UNICEF, New York (2019).
Ministry of Health. New Zealand food, New Zealand children: Key results of the 2002 National Children's Nutrition Survey. Ministry of Health: Wellington (2003).
Morton, S. M. et al. Growing up in New Zealand cohort alignment with all New Zealand births. Australian N. Z. J. Public Health 39, 82–87 (2015).
Morton, S. M, et al. Growing up in New Zealand: A longitudinal study of New Zealand children and their families. Now We Are Four: Describing the preschool years. Growing Up in New Zealand (2017).
Ministry of Health. Food and Nutrition Guidelines for Healthy Infants and Toddlers (Aged 0–2): a Background Paper, 4th ed. Wellington: Ministry of Health (2008).
Ministry of Health. Food and Nutrition Guidelines for Healthy Children and Young People (Aged 2–18 years): A background paper—Revised February 2015. Wellington: Ministry of Health (2012).
Wall, C. R., Brunt, D. R. & Grant, C. C. Ethnic variance in iron status: Is it related to dietary intake?. Public Health Nutr 12, 1413–1421 (2009).
Salmond, C., Crampton, P., & Atkinson, J. NZDep2006 index of deprivation (Final Research Report). University of Otago (2021).
Atkinson, S. C., & Crampton, P. NZDep2013 index of deprivation. University of Otago (2021).
Statistics New Zealand. Ethnicity standard classification: Findings from public consultation November 2019. Statistics New Zealand Tatauranga Aotearoa (2020).
World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. WHO Technical Report Series no 854. Geneva: WHO (1995).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35, 1381–1395 (2003).
NZ Ministry of Health. Food and nutrition guidelines for healthy pregnant and breastfeeding women: a background paper. Wellington: Ministry of Health (2006).
Wall, C. R. et al. Dietary patterns in pregnancy in New Zealand—influence of maternal socio-demographic, health and lifestyle factors. Nutrients 8(5), 300. https://doi.org/10.3390/nu8050300 (2016).
Shi, Z., Makrides, M., Zhou, S. J. Dietary patterns and obesity in preschool children in Australia: A cross-sectional study. Asia Pac. J. Clin. Nutr. 27(2):406–412. https://doi.org/10.6133/apjcn.032017.19. (2018)
Smithers, L. et al. Associations between dietary patterns at 6 and 15 months of age and sociodemographic factors. Eur J Clin Nutr 66, 658–666. https://doi.org/10.1038/ejcn.2011.219 (2012).
Ystrom, E., Niegel, S., Vollrath, M.E. The impact of maternal negative affectivity on dietary patterns of 18-month-old children in the Norwegian Mother and Child Cohort Study. Matern Child Nutr, 5: 234–42. https://doi.org/10.1111/j.1740-8709.2008.00177.x (2009)
Wall, C.R., Thompson, J.M., Robinson, E., Mitchell, E.A. Dietary patterns of children at 3.5 and 7 years of age: a New Zealand birth cohort study. Acta Paediatr 102(2):137–42. https://doi.org/10.1111/apa.12065. Epub 2012 Dec 7 (2013).
Minn, K., et al. Dietary patterns and their associations with overweight/obesity among preschool children in Dongcheng District of Beijing: a cross-sectional study. BMC Public Health 21, 223 https://doi.org/10.1186/s12889-021-10240-x (2021)
Maxson, P. J. et al. Psychosocial differences between smokers and non-smokers during pregnancy. Addict Behav 37, 153–151 (2012).
A. Lovell, P. et al Longitudinal dietary patterns and nutrient intakes in the second year of life: A secondary analyses of the GUMLi RCT. J Academ Nutr and Dietet 118, 10: Page A148, https://doi.org/10.1016/j.jand.2018.08.100.(2018)
Zhao, J., et al. A review of statistical methods for dietary pattern analysis. Nutr J 20, 37, https://doi.org/10.1186/s12937-021-00692-7 (2021)
Mackay, S., Garton, K., Gerritsen, S., Sing, F., Swinburn, B. How healthy are Aotearoa New Zealand's food environments? Assessing the impact of recent food policies 2018–2021. The University of Auckland. Report (2021). https://doi.org/10.17608/k6.auckland.16968478. Accessed in 10 Nov 2023.
Acknowledgements
The authors would like to thank the participating families of the Growing Up in New Zealand cohort study who have given their time and shared the information that allowed them to conduct this study and to the interviewers and researchers who designed, collected, cleaned, and prepared the data for use. The Growing Up in New Zealand study has been designed and conducted by the Growing Up in New Zealand team, led by the University of Auckland. The authors would like to acknowledge the contributions of the original study investigators: Susan M. B. Morton, Polly E. Atatoa Carr, Cameron C. Grant, Arier C. Lee, Dinusha K. Bandara, Jatender Mohal, Jennifer M. Kinloch, Johanna M. Schmidt, Mary R. Hedges, Vivienne C. Ivory, Te Kani R. Kingi, Renee Liang, Lana M. Perese, Elizabeth Peterson, Jan E. Pryor, Elaine Reese, Elizabeth M. Robinson, Karen E. Waldie, and Clare R. Wall. The Growing Up in New Zealand study has been funded by the New Zealand Ministries of Social Development, Health, Education, and Justice; the former Ministry of Science Innovation, and the former Department of Labour (now both part of the Ministry of Business, Innovation, and Employment); the former Ministry of Pacific Island Affairs (now the Ministry for Pacific Peoples); the former Ministry of Women's Affairs (now the Ministry for Women); the Department of Corrections; the Families Commission (now known as the Social Policy Evaluation and Research Unit); Te Puni Kokiri; New Zealand Police; Sport New Zealand; Housing New Zealand Corporation; and the former Mental Health Commission (now part of the Office of the Health and Disability Commissioner); and the University of Auckland and Auckland UniServices Limited. Another support for the study has been provided by the Health Research Council of New Zealand, Statistics New Zealand, the Office of the Children's Commissioner, and the Office of Ethnic Affairs (now the Office of Ethnic Communities).
Funding
TGC received salary support to conduct this study from the Discipline of Nutrition and Dietetics (Faculty of Medical Sciences, University of Auckland, New Zealand).
Author information
Authors and Affiliations
Contributions
TGC and CW designed the study. All authors provided input in the analyses plan. TGC prepared the data and conducted the data analyses under advice of BJ. TGC, AL, LPS, BJ and CW contributed to the data interpretation. TGC, AL and LPS drafted the manuscript. BJ and CW revised the manuscript draft critically. TGC had primary responsibility for the final content. All listed authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gontijo de Castro, T., Lovell, A., Santos, L.P. et al. Maternal determinants of dietary patterns in infancy and early childhood in the Growing up in New Zealand cohort. Sci Rep 13, 22754 (2023). https://doi.org/10.1038/s41598-023-49986-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49986-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.