Abstract
Gestational diabetes mellitus (GDM) is an unique metabolic disorder that occurs during pregnancy. Both GDM and advanced age increase the risk of adverse pregnancy outcomes. This study used a GDM cohort study to investigate the role of age in the adverse pregnancy outcomes for pregnant women with GDM. From 2015 to 2021, 308,175 pregnant women were selected, and the data received from 22 hospitals by the Hebei Province Maternal Near Miss Surveillance System. There were 24,551 pregnant women with GDM that were divided into five groups by age (20–24, 25–29, 30–34, 35–39, 40–44 years old). Because the prevalence of adverse pregnancy outcomes was lower in pregnant women with GDM aged 25–29, they were used as a reference group (P < 0.05). Compared with GDM women aged 25–29 years, GDM women aged 35–44 years had a significant higher risk of cesarean delivery (aOR: 2.86, 95% CI 2.52–3.25) (P < 0.001), abnormal fetal position (aOR: 1.78, 95% CI 1.31–2.37) (P < 0.001), pre-eclampsia (aOR: 1.28, 95% CI 1.01–1.61) (P < 0.05), macrosomia (aOR: 1.25, 95% CI 1.08–1.45) (P < 0.05), and large for gestational age (LGA) (aOR: 1.16, 95% CI 1.02–1.31) (P < 0.05), GDM women aged 40–44 years had a higher risk of placenta previa (aOR: 2.53, 95% CI 1.01–6.35) (P < 0.05), anemia (aOR: 3.45, 95% CI 1.23–9.68) (P < 0.05) and small for gestational age (aOR: 1.32, 95% CI 1.01–1.60) (P < 0.05). Advanced maternal age was an independent risk factor for abnormal fetal position, pre-eclampsia, anemia, macrosomia, and LGA in pregnant women with GDM.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM) is a kind of hyperglycemia that initially appears or is detected during pregnancy, which is one of the most common obstetric complications1. In 2017, the global prevalence of GDM was 9.0%2. From 2011 to 2018, the average prevalence of GDM in China was 14.8%, ranging from 5.1 to 33.3% in various locations3.
GDM is a risk factor for adverse maternal and perinatal outcomes4. These adverse pregnancy outcomes include pre-eclampsia, preterm birth, caesarean section, stillbirth, macrosomia, large for gestational age (LGA), respiratory distress syndrome, fetal malformations, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admission4,5,6. Even from that, women with a history of GDM have an even greater risk to get type 2 diabetes mellitus, metabolic syndrome7 and cardiovascular diseases8. And their offspring are more likely to have metabolic illness9.
Advanced maternal age (AMA) is currently identified as a key risk factor for GDM and adverse pregnancy outcomes10. GDM is more common in women during pregnancy with AMA11. And AMA is related to the prevalence of stillbirth, preterm birth, small for gestational age (SGA), macrosomia12. The present situation in China is that the number of pregnant women with AMA and GDM has risen since the implementation of the two-child policy12. There will be more pregnant women in China with AMA and GDM as China's fertility strategy changes to encourage the birth of third children. Is there an impact of age on pregnancy outcomes of pregnant women with GDM? There were few studies on this issue currently. This study aimed to investigate the influence of age in adverse pregnancy outcomes in pregnant women with GDM.
Hebei Province is a populous province in northern China. The prevalence of GDM in Hebei Province was comparable to the national average, which ranged from 8.4 to 18.0%3. The database for our study was obtained from Hebei Province Maternal Near Miss Surveillance System (HBMNMSS) among 2015–2021, and the adverse pregnancy outcomes of pregnant women with GDM at different ages were analyzed.
Material and methods
Study population and data collection
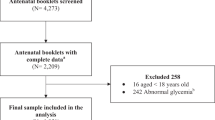
The data, which included 328,273 births, was collected in a random cluster sample from 22 hospitals (tertiary, secondary, and primary) in Hebei China from January 1, 2015 to December 31, 2021. Every year, more than 1000 infants are born at each of the hospitals. The data was collected by HBMNMSS, which was a sub-database of China's National Maternal Near Miss Surveillance System (NMNMSS). The database was entered and processed by special personnel, which was collected by trained doctors based on random stratified cluster sampling. We obtained authorization from Hebei Women and Children's Health Center to use the database. This study included singleton pregnant women with GDM aged 20–44 years, with a gestation week ≥ 28 weeks and a newborn weight ≥ 1000 g. Figure 1 depicts the flow chart of case enrollment. Finally, 24,551 pregnant women with GDM were enrolled. The adverse pregnancy outcomes of pregnant women with GDM at different age were analyzed. The 24,551 pregnant women with GDM were divided into five groups for analysis: 20–24 years old, 25–29 years old, 30–34 years old, 35–39 years old, and 40–44 years old. Between 24 and 28 weeks of pregnancy, oral glucose tolerance tests were performed on pregnant women. GDM was detected as fasting levels of 5.1 mmol/L, one-hour postprandial levels of 10.0 mmol/L, and two-hour postprandial levels of 8.5 mmol/L in oral glucose tolerance test1.
Outcomes
The adverse pregnancy outcomes including maternal and infant outcomes. The adverse maternal outcomes were defined as: cesarean section delivery, abnormal fetal position, pre-eclampsia, anemia, placenta previa, placental abruption, uterine atony, and postpartum hemorrhage. The adverse infant outcomes were defined as: preterm birth, macrosomia, LGA, SGA, NICU admission, and neonatal death. Pregnant women aged ≥ 35 years was defined as AMA. The Kessner Index was used to determine that prenatal care was adequate, intermediate, and inadequate13. Placenta previa was described as placenta attached to the lower uterine segment lower than the fetal presentation, with the placental margin reaching or covering the cervical os after 28 weeks of gestation14. Pre-eclampsia was defined as hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg), proteinuria (≥ 300 mg/day), or liver, kidney functional failure, or nerve, blood system abnormalities after 20 weeks of gestation15. Anemia was defined as hemoglobin level ≤ 110 g/L. Placental abruption was defined as the separation of the placenta from the uterine wall while the fetus remained still in the uterine cavity after 20 weeks of gestation16. Postpartum hemorrhage was defined as blood loss ≥ 500 mL after vaginal birth or ≥ 1000 mL after caesarean delivery17. Preterm birth was defined as delivery from 28 to 36 + 6 weeks gestation18. Macrosomia was defined as birth weight ≥ 4000 g. Birth weight below the 10th percentile at each gestational week was classified as SGA, while birth weight over the 90th percentile at each gestational week was defined as LGA19,20.
Statistical analysis
For qualitative data analysis, the Chi-square test was used. For normally distributed data, one-way analysis of variance (ANOVA) was used, and for non-normally distributed variables, the Kruskal–Wallis H test was used. The influence of age on the risk of adverse pregnancy outcomes in GDM pregnant women was analyzed using univariable and multivariable unconditional logistic regression analysis. Prenatal care, maternal education, gravidity, parity, delivery place, gestational week, and previous cesarean delivery were adjusted using multivariable logistic regression. The reference group was women aged 25–29 years. The result was presented as odds ratio (OR) or adjusted odds ratio (aOR) and 95% confidence interval (CI). P < 0.05 was considered as statistically significant. SPSS Version 17.0 and GraphPad Prism 8.0.1 statistical tools were used.
Ethics approval
The data obtained from the NMNMSS were anonymised and did not infringe on patients’ privacy, therefore subjects could not be identified. The ethics committee of Hebei Reproductive Health Hospital and Hebei Center for Women and Children’s Health waived the need for informed consent from patients due to the retrospective nature of the study. All methods in our study were approved by Hebei Women and Children’s Health Center (Supplementary Material 2).
Results
Figure 1 shows the case screening process from HBMNMSS. From 2015 to 2021, data on 328,273 pregnant women were collected from 22 hospitals within 10 areas in Hebei, China. A total of 308,175 pregnant women were selected, and 24,551 pregnant women with GDM were analyzed. From 2015 to 2021, the prevalence of GDM in pregnant women was 8.1% in our study. Pregnant women's age was linked to an increased prevalence of GDM. GDM was found in around 20% of pregnant women aged over 35 years, and in only 3.6% of pregnant women aged 15–19 years, with a total of 52 cases (Fig. 2). In this study, 24,548 GDM pregnant women aged 20–44 years were analyzed.
The clinical baseline characteristics of 24,548 pregnant women with GDM are shown in Table 1. There were significant differences between primipara and multipara pregnant women with GDM at different ages (p < 0.001). The frequency of primipara was higher in pregnant women with GDM aged 20–24 years and 25–29 years, with 82.5% and 64.4%, respectively. The majority of AMA with GDM were multipara, and the rate of multipara in pregnant women with GDM aged 35–39 years and 40–44 years was 86.8% and 90.5%, respectively. Pregnant women with GDM aged 35–39 years and 40–44 years with less than 6 years of education had a higher ratio of 2.9% and 3.6%, respectively (P < 0.001). The frequency of pregnant women with GDM who were delivered in primary hospitals was associated with maternal age. In primary hospitals, the delivery rate of pregnant women with GDM of the five groups was 66.5%, 73.7%, 76.5%, 78.9%, and 82.1%, respectively (P < 0.001). The rate of pregnant women with GDM aged 40–44 years who had inadequate prenatal care was 7.0% (P < 0.001). There were no significant differences in the marital status and sex ratio of newborn infants in different age groups of pregnant women with GDM (P > 0.05).
As shown in Table 2 and Fig. 3(A), the total cesarean delivery rate was strongly associated with maternal age in pregnant women with GDM aged 20–44 years. Furthermore, the cesarean delivery rate of primipara and multipara without previous cesarean section pregnant women with GDM aged 20–44 years was strongly related to maternal age (P < 0.001). The cesarean delivery rate among pregnant women with GDM in the five groups was 50.5%, 52.1%, 61.9%, 70.9%, and 78.6%, respectively. The cesarean delivery rate was lower in primipara (47.6%) and multipara without previous cesarean section (20.8%) women with GDM aged 25–29 years. Whereas primipara (89.7%) and multipara without previous cesarean section (56.0%) with GDM aged 40–44 years had the higher cesarean delivery rates. The prevalence of placenta previa, abnormal fetal position, and SGA was the highest in pregnant women with GDM aged 40–44 years, at 1.0%, 4.6%, and 19.7%, respectively. In primipara with GDM aged 40–44 years, the prevalence of SGA was 28.7% (P < 0.05). Pregnant women with GDM aged 25–29 years had the lowest rate of pre-eclampsia (4.5%) and preterm birth (6.9%), and who aged 40–44 years had the highest rate of pre-eclampsia (7.9%) and preterm birth (13.9%) (P < 0.001). Especially, the prevalence of preterm birth in primipara with GDM aged 40–44 years was 19.5% (P < 0.05) (Fig. 3B). Pregnant women with GDM aged 20–24 years had the lowest frequency of macrosomia (9.7%), and who aged 35–39 years had the highest rate (13.2%) (P < 0.01) (Fig. 3C). The primipara with GDM aged 40–44 years had the highest rate of SGA, which was 28.7% (P < 0.001) (Fig. 3D). The overall prevalence of LGA was associated with maternal age in pregnant women with GDM aged 20–44 years, however, the rate of LGA in primipara who aged 25–29 years was the lowest, at 10.3% (P < 0.001) (Fig. 3E). In pregnant women with GDM aged 40–44 years, the frequency of NICU admission and neonatal death increased, although the difference was not statistically significant (P > 0.05).
Compared with GDM women aged 25–29 years, GDM women aged 35–44 years had a significantly higher risk of cesarean delivery, abnormal fetal position, pre-eclampsia, anemia, macrosomia, and LGA (P < 0.05). There had the highest risk of cesarean delivery (aOR: 5.40, 95% CI 4.36–6.69), abnormal fetal position (aOR: 2.44, 95% CI 1.60–3.73), pre-eclampsia (aOR: 2.19, 95% CI 1.59–3.03), anemia (aOR: 3.45, 95% CI 1.23–9.68), placenta previa (aOR: 2.53, 95% CI 1.01–6.35) in GDM women aged 40–44 years. Compared with GDM women aged 25–29 years, GDM women aged 20–24 years had a significantly higher risk of pre-eclampsia (aOR: 1.31, 95% CI 1.03–1.17) and anemia (aOR: 1.30, 95% CI 1.17–1.46), but there were no statistically significant difference in the effects of re-eclampsia (aOR: 0.88, 95% CI 0.61–1.29) and anemia (aOR: 0.88, 95% CI 0.54–1.44) after adjusting the confounding factors. There was no difference in the risk of placental abruption, uterine atony, and postpartum hemorrhage after adjusted for confounding factors (Fig. 4). For more information, see supplementary Table 1.
Forest plots of adjusted odds ratio for adverse maternal outcomes with GDM at different ages. Maternal aged 25–29 years as the reference group. The adjusted odds ratio and 95%CI by multiple logistic regression model after adjusted by prenatal care, maternal education, gravidity, parity, delivery place, gestational week, and previous cesarean delivery were adjusted using multivariable logistic regression.
Compared with GDM women aged 25–29 years, GDM women aged 30–44 years had a significant higher risk of macrosomia and LGA, GDM women aged 35–44 years had a significant higher risk of preterm birth, and who aged 40–44 years had a significant higher risk of SGA (P < 0.05). Compared with GDM women aged 25–29 years, GDM women aged 20–24 years had a significant lower risk of macrosomia (aOR: 0.82, 95% CI 0.68–0.99). There was no difference in the risk of NICU admission and neonatal death after adjusted for confounding factors (Fig. 5). For more information, see supplementary Table 2.
Forest plots of adjusted odds ratio for adverse infant outcomes with GDM at different ages. Maternal aged 25–29 years as the reference group. The adjusted odds ratio and 95%CI by multiple logistic regression model after adjusted by prenatal care, maternal education, gravidity, parity, delivery place, gestational week, and previous cesarean delivery were adjusted using multivariable logistic regression.
Discussion
This study showed a significantly higher prevalence of GDM in AMA, and it is critical to focus on the negative pregnancy outcomes of AMA with GDM.
This study found that the prevalence of GDM in AMA was significantly higher, in which was around 20%, that was similar to the result in mainland China3. The metabolic dysfunctions were caused by abnormal pancreas islet function of pregnant women with AMA which could induce chronic diseases during pregnancy21. In this study, we noticed that the incidence of adverse pregnancy outcomes such as pre-eclampsia, uterine atony, abnormal fetal position, preterm birth, SGA, and NICU admission was lower in pregnant women with GDM aged 25–29 years, so that this group was selected as the reference group.
The World Health Organization (WHO) advises limiting the cesarean delivery rate between 10 and 15%22. From 2008 to 2014, the prevalence of cesarean delivery ranged from 28.8 to 34.9% in China, which was greater than the WHO recommendation, and it was continuously growing23. The prevalence of cesarean delivery has been shown to be strongly connected with maternal age, and pregnant women with AMA and GDM were risk factors for cesarean delivery12,21. The cesarean delivery rate in pregnant women with AMA ranged from 24.4 to 74.1%, and from 50.8 to 59.9% in pregnant women with GDM12,24. Our study had the similar results of the cesarean delivery rate, AMA with GDM was more than 70%, in which primipara had the highest cesarean delivery rate (> 90%), multipara without previous caesarean section who aged over 40 years had a rate of more than 50%. The risk of cesarean delivery in pregnant women with GDM was found to be positively correlated with AMA in this study. Pregnant women with GDM aged over 35 years had twofold increased risk of cesarean delivery compared to who aged 25–29 years. Furthermore, the risk of cesarean delivery was fivefold higher in pregnant women with GDM aged over 40 years than in who aged 25–29 years. Insufficient perfusion of the uterus and placenta caused by vascular dysfunction of pregnant women with AMA would increase the risks of pregnancy complications, which increased the risk of cesarean delivery25.
This study found that AMA was an independent risk factor for anemia and pre-eclampsia in pregnant women with GDM. This was similar to the previous study, that inadequate prenatal care and the lack of professional nutrition advice in AMA during pregnancy were linked to a rise in the prevalence of anemia26. For the reasons mentioned above, AMA with GDM had an elevated risk of anemia. The endothelial dysfunction, dyslipidemia, and inflammation of pregnant women with AMA increased the risk of pre-eclampsia27.
This study found that AMA was an independent risk factor for anemia and pre-eclampsia in pregnant women with GDM. This was similar to the previous study, that AMA was a risk factor for anemia and pre-eclampsia25. Several studies have shown that inadequate prenatal care and the lack of professional nutrition advice during pregnancy were linked to a rise in the prevalence of anemia26. For the reasons mentioned above, AMA with GDM had an elevated risk of anemia. And it was considered that inadequate prenatal care and the endothelial dysfunction, dyslipidemia, and inflammation of pregnant women with AMA increased the risk of pre-eclampsia27. This may explain this study’s result, that AMA with GDM was an independent risk factor for pre-eclampsia.
The prevalence of preterm birth in pregnant women with GDM was found to be positively associated with AMA in this study, and who aged 40–44 years was found to be an independent risk factor for preterm birth. The previous findings suggested that pregnant women with AMA and GDM were at a higher risk of preterm birth25, which were consistent with the findings of this study. This was due to the high incidence of pregnancy complications in GDM and AMA, which was related the risk increased of preterm birth28. Pregnant women with GDM had a higher risk of spontaneous preterm birth28, and pre-eclampsia had a higher risk of medically required preterm birth29.
This study found that pregnant women with GDM aged 20–24 years was a protective factor for macrosomia, and AMA with GDM was a risk factor for macrosomia and LGA. This was consistent with the previous research findings, which found that macrosomia and LGA were always more common in pregnant women with GDM5,30, and AMA was also a risk factor for macrosomia and LGA12,28. Pregnant women with AMA and GDM was lower for metabolize glucose, and excessive placental transport of glucose and other nutrients from pregnant woman to fetus caused abnormal fetal growth, including macrosomia and LGA30. The length of the biparietal diameter of macrosomia increased, and the fetal position appeared abnormally31, and pregnant women with AMA were at increased risk of breech presentation32. These findings were consistent to the result of this study, that was, AMA with GDM had the higher risk of abnormal fetal position.
This study was a multi-center cohort study which used data from 22 hospitals in Hebei, China, and covered ten cities. The number of pregnant women with AMA and GDM had significantly rise as China’s twice-birth in 2016 and will even increasing after third-birth policies in 2021. Our study investigated the impact of age on adverse pregnancy outcomes in GDM pregnant women, which was beneficial in managing pregnant women with GDM and AMA. However, because this was a retrospective study, it had certain limitations. The data did not include information on body mass index, pregnancy weight increase, blood glucose levels during pregnancy, and blood glucose management (diet, exercise or insulin treatment), which might be attributed to the results’ bias.
Conclusion
In pregnant women with GDM, AMA was an independent risk factor for caesarean section, anemia, abnormal fetal position, pre-eclampsia, macrosomia, and LGA. Pregnant women with GDM aged over 40 years was an independent risk factor for SGA. This shows the necessary of paying greater attention to GDM with AMA and improving prenatal care to enhance pregnancy outcomes of them.
Data availability
Data is available from the corresponding author on a reasonable request.
References
Zhu, W. W. & Yang, H. X. Diagnosis of gestational diabetes mellitus in China. Diabetes Care 36(6), e76. https://doi.org/10.2337/dc12-2624 (2013).
Brussels, Belgium. International Diabetes Federation. Hyperglycemia in pregnancy. IDF Diabetes Atlas 8th edn 2017.
Gao, C. H., Sun, X., Lu, L., Liu, F. W. & Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig. 10(1), 154–162. https://doi.org/10.1111/jdi.12854 (2019).
Malaza, N., Masete, M. & Adam, S. A systematic review to compare adverse pregnancy outcomes in women with pregestational diabetes and gestational diabetes. Int. J. Environ. Res. Public Health 19(17), 10846. https://doi.org/10.3390/ijerph191710846 (2022).
Rekawek, P. et al. Large-for-gestational age diagnosed during second-trimester anatomy ultrasound and association with gestational diabetes and large-for-gestational age at birth. Ultrasound Obstet Gynecol. 56(6), 901–905. https://doi.org/10.1002/uog.21930 (2020).
Preda, A. & Pădureanu, V. Analysis of maternal and neonatal complications in a group of patients with gestational diabetes mellitus. Medicina (Kaunas) 57(11), 1170–1178. https://doi.org/10.3390/medicina57111170 (2021).
Vounzoulaki, E. et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 369, m1361. https://doi.org/10.1136/bmj.m1361 (2020).
Kramer, C. K., Campbell, S. & Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 62(6), 905–914. https://doi.org/10.1007/s00125-019-4840-2 (2019).
Mansell, T. et al. The newborn metabolome: Associations with gestational diabetes, sex, gestation, birth mode, and birth weight. Pediatr Res. 91(7), 1864–1873. https://doi.org/10.1038/s41390-021-01672-7 (2022).
Bahl, S. et al. Burden, risk factors and outcomes associated with gestational diabetes in a population-based cohort of pregnant women from North India. BMC Pregnancy Childbirth 22(1), 32. https://doi.org/10.1186/s12884-022-04389-5 (2022).
Li, Y. et al. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 162, 108044. https://doi.org/10.1016/j.diabres.2020.108044 (2020).
Cao, J. et al. Trends in maternal age and the relationship between advanced age and adverse pregnancy outcomes: A population-based register study in Wuhan, China, 2010–2017. Public Health 206, 8–14. https://doi.org/10.1016/j.puhe.2022.02.015 (2022).
Delgado-Rodríguez, M., Gómez-Olmedo, M., Bueno-Cavanillas, A. & Gálvez-Vargas, R. A comparison of two indices of adequacy of prenatal care utilization. Epidemiology 7(6), 648–650. https://doi.org/10.1097/00001648-199611000-00016 (1996).
Jain, V., Bos, H. & Bujold, E. Guideline No. 402: Diagnosis and management of placenta previa. J. Obstet. Gynaecol. Can. 42(7), 906–917. https://doi.org/10.1016/j.jogc.2019.07.019 (2020).
Tranquilli, A. L. Introduction to ISSHP new classification of preeclampsia. Pregnancy Hypertens. 3(2), 58–59. https://doi.org/10.1016/j.preghy.2013.04.006 (2013).
Monika, B. et al. Epidemiology, risk factors, and perinatal outcomes of placental abruption-detailed annual data and clinical perspectives from polish tertiary center. Int. J. Environ. Res. Public Health. 19(9), 5148–5161. https://doi.org/10.3390/ijerph19095148 (2022).
Girard, T., Mörtl, M. & Schlembach, D. New approaches to obstetric hemorrhage: The postpartum hemorrhage consensus algorithm. Curr. Opin. Anaesthesiol. 27(3), 267–274. https://doi.org/10.1097/ACO.0000000000000081 (2014).
Monika-Bączkowska, K.K.-K., Magdalena, Z., Robert-Brawura, B. S., Beata, R. & Michał, C. Epidemiology of late and moderate preterm birth. Semin. Fetal Neonatal Med. 17(3), 120–125. https://doi.org/10.1016/j.siny.2012.01.007 (2012).
Chen, Y., Wu, L., Zou, L., Li, G. & Zhang, W. Update on the birth weight standard and its diagnostic value in small for gestational age (SGA) infants in China. J. Matern. Fetal. Neonatal. Med. 30(7), 801–807. https://doi.org/10.1080/14767058.2016.1186636 (2017).
Chen, Y. et al. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth 13, 242. https://doi.org/10.1186/1471-2393-13-242 (2013).
Njogu, P. K., Makunyi, E. G. & Musau, J. Risk factors for caesarean delivery and fetal macrosomia among women with gestational diabetes in Nyeri County, Kenya: A cross-section study. Pan. Afr. Med. J. 41, 322. https://doi.org/10.11604/pamj.2022.41.322.29734 (2022).
Betran, A. P., Torloni, M. R., Zhang, J. J. & Gülmezoglu, A. M. WHO statement on caesarean section rates. BJOG. 123(5), 667–670. https://doi.org/10.1111/1471-0528.13526 (2016).
Li, H. T. et al. Geographic variations and temporal trends in cesarean delivery rates in China, 2008–2014. JAMA. 317(1), 69–76. https://doi.org/10.1001/jama.2016.18663 (2017).
Marconi, A. M., Manodoro, S., Cipriani, S. & Parazzini, F. Cesarean section rate is a matter of maternal age or parity?. J. Matern. Fetal. Neonatal. Med. 35(15), 2972–2975. https://doi.org/10.1080/14767058.2020.1803264 (2022).
Frick, A. P. Advanced maternal age and adverse pregnancy outcomes. Best Pract. Res. Clin. Obstet. Gynaecol. 70, 92–100. https://doi.org/10.1016/j.bpobgyn.2020.07.005 (2021).
Sunguya, B. F., Ge, Y., Mlunde, L. B., Mpembeni, R. & Leyna, G. H. Targeted and population-wide interventions are needed to address the persistent burden of anemia among women of reproductive age in Tanzania. Int. J. Environ. Res. Public Health. 19(14), 8401–8412. https://doi.org/10.3390/ijerph19148401 (2022).
Košir-Pogačnik, R. et al. The effect of interaction between parity, gestational diabetes, and pregravid obesity on the incidence of preeclampsia. J. Matern. Fetal. Neonatal. Med. 33(6), 931–934. https://doi.org/10.1080/14767058.2018.1509311 (2020).
Attali, E. & Yogev, Y. The impact of advanced maternal age on pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 70, 2–9. https://doi.org/10.1016/j.bpobgyn.2020.06.006 (2021).
Liu, K. et al. Association of maternal obesity with preterm birth phenotype and mediation effects of gestational diabetes mellitus and preeclampsia: A prospective cohort study. BMC Pregnancy Childbirth 22(1), 459. https://doi.org/10.1186/s12884-022-04780-2 (2022).
Bordin, P. et al. Gestational diabetes mellitus yesterday, today and tomorrow: A 13 year italian cohort study. Diabetes Res. Clin. Pract. 167, 108360. https://doi.org/10.1016/j.diabres.2020.108360 (2020).
Song, J. et al. The birth weight of macrosomia influence the accuracy of ultrasound estimation of fetal weight at term. J. Clin. Ultrasound. 50(7), 967–973. https://doi.org/10.1002/jcu.23236 (2022).
Zhou, Z., Wu, J., Zhang, G. & Yang, J. Spontaneous cephalic version and risk factors for persistent breech presentation: A longitudinal retrospective cohort study. J. Matern. Fetal. Neonatal. Med. 35(25), 9452–9459. https://doi.org/10.1080/14767058 (2022).
Acknowledgements
We thank the doctors who collected the data as well as all of the staff who compiled the data.
Funding
This work was supported by the Youth Science and technology project of Hebei Health Commission (20190294, 20200001).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data processing and analysis were performed by T.Z., M.L.T., L.Y.D. and Y.K.Z. The first draft of the manuscript was written by T.Z. and all authors commented on previous versions of the manuscript. P.Z., and X.Y.M. prepared figures. Z.J.T. oversaw the process and gave critical comments to the study design.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, T., Tian, M., Zhang, P. et al. Risk of adverse pregnancy outcomes in pregnant women with gestational diabetes mellitus by age: a multicentric cohort study in Hebei, China. Sci Rep 14, 807 (2024). https://doi.org/10.1038/s41598-023-49916-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49916-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.