Abstract
The severity of chest X-ray (CXR) findings is a prognostic factor in patients with coronavirus disease 2019 (COVID-19). We investigated the clinical and genetic characteristics and prognosis of patients with worsening CXR findings during early hospitalization. We retrospectively included 1656 consecutive Japanese patients with COVID-19 recruited through the Japan COVID-19 Task Force. Rapid deterioration of CXR findings was defined as increased pulmonary infiltrates in ≥ 50% of the lung fields within 48 h of admission. Rapid deterioration of CXR findings was an independent risk factor for death, most severe illness, tracheal intubation, and intensive care unit admission. The presence of consolidation on CXR, comorbid cardiovascular and chronic obstructive pulmonary diseases, high body temperature, and increased serum aspartate aminotransferase, potassium, and C-reactive protein levels were independent risk factors for rapid deterioration of CXR findings. Risk variant at the ABO locus (rs529565-C) was associated with rapid deterioration of CXR findings in all patients. This study revealed the clinical features, genetic features, and risk factors associated with rapid deterioration of CXR findings, a poor prognostic factor in patients with COVID-19.
Similar content being viewed by others
Introduction
The pandemic of coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in high morbidity and mortality rates worldwide. The disease rapidly spread throughout the world; as of December 29, 2022, there were 663,380,366 confirmed cases and 6,691,567 deaths worldwide1. COVID-19 severity varies according to several factors, including patient characteristics2,3,4,5 and laboratory findings6,7,8. Furthermore, the severity of chest X-ray (CXR) findings in patients with COVID-19 can predict various outcomes, including duration of hospitalization9,10, use of invasive mechanical ventilation (IMV)11,12,13, intensive care unit (ICU) admission11,12,13, and mortality14,15,16,17,18.
Chest imaging facilitates the diagnosis and management of patients19. In clinical settings, CXR can be easily performed using mobile CXR units in a dedicated, isolated room to reduce the transmission risk19. Although CXR is considered less sensitive for detecting pulmonary involvement in early-stage disease20, it is useful for monitoring the progression of lung abnormalities in COVID-19, especially in critically ill ICU patients21. The requirement for ventilatory support is associated with worsening findings early after admission22; moreover, mortality can be predicted based on CXR findings before and after ICU admission23. However, the impact of deteriorating CXR findings on outcomes other than ventilatory support needs and the clinical and genetic characteristics of patients with deteriorating CXR findings remain unclear.
A nationwide multi-center consortium was established to address the COVID-19 pandemic in Japan24,25. Since the pandemic's onset, the network has been collecting DNA, RNA, and plasma samples, as well as detailed clinical information, from patients with COVID-19 throughout Japan on a long-term basis. The first Japanese large-scale genome-wide association study (GWAS) on COVID-19 reported that genetic variants, including DOCK2, had a population-specific association with oxygenation requirements by patients with COVID-1924.
It is important for clinicians to be able to predict the severity of a patient's illness, especially during a pandemic, in order to use medical resources appropriately. We hypothesised that rapid deterioration in CXR findings after hospitalisation is associated with subsequent worsening of the disease. We aimed to investigate the prognosis, clinical characteristics, and genetic characteristics of patients with worsening CXR findings during early hospitalization.
Methods
Study design and settings
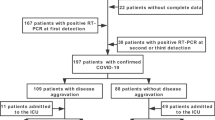
This retrospective cohort study recruited hospitalized COVID-19 cases through the Japan COVID-19 Task Force24. From February 2020 to May 2021, data obtained from consecutive patients aged ≥ 18 years, who were diagnosed with COVID-19 based on polymerase chain reaction tests and agreed to participate in the study, were entered into electronic case record forms by attending physicians at the affiliated research institutions. The exclusion criteria were as follows: (1) patients from other countries (2) patients with incomplete medical records, e.g., insufficient data regarding the presence/absence of chest radiographic deterioration within 48 h of admission or critical outcomes (Fig. 1). All patients provided written or oral informed consent. The study design was approved by the ethics committees of Keio University School of Medicine (20,200,061) and related research institutions. All methods were performed in accordance with the relevant guidelines and regulations.
Data collection
The following characteristics were extracted from each electronic case record form: age, sex, height, weight, clinical symptoms and signs, laboratory findings on admission, comorbidities, and disease severity (ICU admission, IMV usage, and survival status). All laboratory tests were performed based on the patient’s clinical care needs. The recorded symptoms and signs included those observed at the times of referral and admission and during hospitalization. Laboratory and radiographic findings were collected within 48 h of the initial visit or admission. Rapid deterioration of CXR findings was defined as increased lung infiltrates in > 50% of the lung fields within 48 h compared with those at admission, which is based on the criteria for severe disease in patients with COVID-1926. One attending physician at each facility reviewed and assessed the initial and follow-up CXRs. The timing of CXR imaging was at the discretion of the attending physician. The collected data were reviewed by a team of respiratory clinicians. Missing core data were obtained by contacting the clinician. Missing background data were noted as unknown. We defined disease severity as follows: most severe: patients requiring high-flow oxygen device support, invasive mechanical ventilation, extracorporeal membrane oxygenation, or death; severe: patients requiring low-flow oxygen device support; mild: symptomatic patients not requiring oxygen support; and asymptomatic: asymptomatic patients not requiring oxygen support25.
Genotype characteristics of the patients with COVID-19
We performed GWAS genotyping of 2520 patients with COVID-19 using the Infinium Asian Screening Array (Illumina). We applied stringent quality control (QC) filters to the samples and variants. A total of 2393 COVID-19 cases passed the sample QC (details described elsewhere24) and underwent genome-wide genotype imputation (details described elsewhere24). Among them, 1,169 had information regarding chest radiographs. Among 18 known COVID-19-related risk variants, we evaluated the effects of 15 risk variants with imputation scores of > 0.7 on the rapid deterioration of CXR findings (See Supplementary Table 1 in the Supplementary Material)24,27,28,29,30,31,32,33,34,35.
Statistical analysis
Regarding baseline characteristics, categorical variables were presented as frequencies and proportions, while continuous variables were presented as means and standard deviations. We compared data according to the presence/absence of lung infiltrates in > 50% of the fields within 48 h of admission using the t-test and Chi-square test, as appropriate. The median hospitalization duration was estimated using the Kaplan–Meier method and compared using the log-rank test.
To investigate the association between rapid deterioration of imaging and radiographic findings, CXR findings (unilateral/bilateral ground-glass opacity [GGO]/consolidation) were adjusted, followed by multivariate logistic regression analysis. Additionally, a Cochran–Armitage trend test was performed to determine the rapid deterioration of CXR findings as well as the tendency of radiographic findings to exhibit no, unilateral, or bilateral shadows.
To assess the association between the rapid deterioration of CXR findings and clinical outcomes (death, most severe disease, IMV use, and ICU treatment), we performed multivariate logistic regression analyses with adjustment for baseline CXR findings, number of days from symptom onset to hospitalisation, and characteristics known as predictors of COVID-19 severity (age, body mass index [BMI], hypertension, diabetes mellitus, cardiovascular disease, malignancy, chronic obstructive pulmonary disease [COPD], asthma, chronic liver disease, and chronic kidney disease)2,3,4,5.
To identify the clinical characteristics of patients with rapid deterioration of CXR findings, we adopted a holdout method, where data from two-thirds of the cases were used as training data, while the remaining data were used as test data to validate the performance of the prediction model for patients with COVID-19 who have rapid deterioration of CXR findings. Receiver operating characteristic (ROC) curve analysis was performed to determine appropriate cut-off values of continuous variables for rapid deterioration of CXR findings using the Youden index. We performed multivariable analysis using a logistic regression model with a backward selection procedure to select the combinations of variables. The variables were selected based on a threshold p-value of 0.05. The Bayesian information criterion was also applied to select the optimal model among the existing models. We performed ROC curve analysis for the model, with the performance of the prediction models being assessed using the area under the curve (AUC).
The dosage effects of the variants on rapid deterioration of CXR findings were evaluated using logistic regression models, with age (included only in all age analyses) and sex as covariates.
To evaluate the association between the ABO blood groups and rapid deterioration of CXR findings, we performed a multivariate logistic regression analysis of the A/B/AB/O blood groups and other blood groups, with adjustment for age and sex.
To ensure the reliability of the data, two clinicians from different affiliations, M.W. (National Hospital Organization Tokyo Medical Center) and T.K. (Keio University School of Medicine), independently reviewed the initial and follow-up CXRs and assessed for rapid deterioration of CXR findings (n = 45). Agreement between the two clinicians’ readings was analysed using Cohen's kappa coefficient (κ), with κ values graded as slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and almost perfect (0.81–1.00) according to Landis and Koch criteria36.
Data were presented as adjusted odds ratios (aORs) with 95% confidence intervals (CIs). Statistical significance was set at p < 0.05. All statistical analyses were performed using the JMP 16 program (SAS Institute) and SAS software (version 9.4; SAS Institute).
Statement of Ethics
All patients involved in this study provided written or oral informed consent, and the study design was approved by the ethics committees of Keio University School of Medicine (20,200,061) and the affiliated research institutions.
Results
Patient baseline characteristics
Table 1 summarizes the baseline characteristics of the patients (n = 1656; 538 women). Comparison of two clinicians’ ratings of rapid deterioration of CXR findings showed almost perfect agreement, with a Cohen's kappa coefficient (κ) of 0.85 (95% CI 0.55–1.00). Among them, 168 (10.1%) patients experienced rapid deterioration of CXR findings; further, these patients were generally older, had a higher BMI, and had a longer interval between symptom onset and hospitalization (all P < 0.001). Rapid deterioration of CXR findings were associated with a higher rate of comorbidities, including hypertension (P = 0.044), diabetes mellitus (P < 0.001), cardiovascular disease (P = 0.017), COPD (P < 0.001), and chronic kidney disease (P = 0.002), as well as a higher frequency of symptoms, including fever (P < 0.001), cough (P < 0.001), sputum (P = 0.006), rhinorrhea (P = 0.029), shortness of breath (P < 0.001), abdominal pain (P = 0.022), diarrhea (P = 0.036), nausea (P < 0.001), and fatigue (P < 0.001). We analyzed patient characteristics according to baseline CXR findings; we found similar trends in the presence/absence of imaging findings and rapid deterioration of CXR findings (Supplementary Tables 2).
Compared with patients with two CXRs within 48 h, those without were generally younger (P < 0.001) and comprised more women (P = 0.001), and patients with less severity (P = 0.003) (Supplementary Table 3).
Supplementary Table 4 summarizes the clinical features of the patients. The vital signs of patients with rapid deterioration of CXR findings were characterized by increased body temperature (P < 0.001), heart rate (P < 0.001), respiratory rate (P < 0.001), decreased SpO2 (P < 0.001), and a high rate of a requirement for oxygen support (P < 0.001). Further, their blood test findings included increased white blood cell counts (P = 0.0477) and neutrophil ratios (P < 0.001) as well as increased blood urea nitrogen (P < 0.001), creatinine (P = 0.006), lactate dehydrogenase (P < 0.001), brain natriuretic peptide (P = 0.018), ferritin (P < 0.001), hemoglobin A1c (P < 0.001), fibrinogen (P < 0.001), D-dimer (P = 0.003), and C-reactive protein (CRP) (P < 0.001) levels. Moreover, they presented with decreased lymphocyte ratios (P < 0.001), platelet counts (P < 0.001), albumin (P < 0.001), and sodium (P < 0.001) levels.
Association between baseline CXR findings and rapid deterioration
Figure 2 shows the comparison of the baseline CXR findings between patients with and without rapid deterioration of CXR findings. For patients with GGO and consolidation on both modalities, the frequency of rapid deterioration of CXR findings increased in the following order: no shadow, unilateral shadow, and bilateral shadow; CXR GGO (P < 0.001), CXR consolidation (P < 0.001). Multivariate logistic regression analysis revealed that unilateral shadow, bilateral shadow, GGO, and consolidation were associated with an increased likelihood of rapid deterioration of CXR findings (Table Supplementary Table 5).
Association between rapid deterioration of CXR findings and outcomes
Table 2 shows the results of multiple logistic regression analysis using parameters that included rapid deterioration of CXR findings, GGO, consolidation on CXR, and number of days from symptom onset to hospitalisation, in addition to the previously reported prognostic factors2,3,4,5. Rapid deterioration of CXR findings was an independent risk factor for death (aOR [95% CI] = 3.12 [1.45–6.74]), most severe disease (aOR [95% CI] = 3.03 [1.98–4.62]), use of IMV (aOR [95% CI] = 1.96 [1.24–3.09]), and ICU treatment (aOR [95% CI] = 2.33 [1.60–3.41]).
Risk factors for rapid deterioration of CXR findings
Table 3 displays the results of the logistic regression analysis performed to clarify the risk factors for rapid deterioration of CXR findings, and Fig. 3 shows the ROC curve. The presence of consolidation (bilateral consolidation (aOR [95% CI] = 3.07 [1.91–4.93]), unilateral consolidation (aOR [95% CI] = 2.19 [1.11–4.32]) on CXR, coexisting cardiovascular disease (aOR [95% CI] = 2.00 [1.12–3.58]), coexisting COPD (aOR [95% CI] = 2.32 [1.12–4.79]), body temperature ≥ 37.7 °C (aOR [95% CI] = 2.53 [1.64–3.91]), aspartate aminotransferase (AST) ≥ 30 IU/L (aOR [95% CI] = 2.31 [1.38–3.87]), serum potassium ≥ 4.3 mEq/L (aOR [95% CI] = 1.75 [1.11–2.78]), and CRP ≥ 2.53 mg/dL (aOR [95% CI] = 3.16 [1.74–5.73]) were independent risk factors for rapid deterioration of CXR findings. The AUC (95% CI) was estimated to be 0.806 (0.753–0.858).
ROC curve of the multivariate logistic regression model for predicting rapid deterioration of CXR findings using CXR consolidation; comorbid cardiovascular disease; comorbid COPD; body temperature; and AST, K, and CRP levels in patients with COVID-19. Using test data, AUC = 0.806. ROC, receiver operating characteristic; CXR, chest X-ray; COPD, chronic obstructive pulmonary disease; AST, aspartate aminotransferase; K, potassium; CRP, C-reactive protein; COVID-19, coronavirus disease; AUC, area under the curve.
Association of COVID-19-associated risk variants with rapid deterioration of CXR findings
Table 4 shows the dosage effects of COVID-19-related risk variants on rapid deterioration of CXR findings. rs529565-C (ABO) was associated with rapid deterioration of CXR findings in patients of all ages (aOR [95% CI] = 1.67 [1.23–2.26]; P < 0.001) and those aged < 65 years (aOR [95% CI] = 2.00 [1.31–3.05]; P = 0.001).
Association of the ABO blood groups with rapid deterioration of CXR findings
Table 5 shows the association between rapid deterioration of CXR findings and the ABO blood groups. Blood group AB was associated with an increased risk of rapid deterioration of CXR findings compared with the non-AB blood types (aOR [95% CI] = 1.84 [1.15–2.95]), after adjustment for age and sex.
Discussion
Our study provided three novel findings with clinical relevance. First, patients with COVID-19 with rapid CXR deterioration had poorer clinical outcomes than those without; accordingly, and they may require more aggressive treatment. Second, we identified predictors of CXR deterioration upon admission. Therefore, clinicians should pay more attention to CXR deterioration after hospitalization of patients with these risk factors. Third, we identified the genetic risk factors for CXR deterioration in Japanese patients with COVID-19.
The CXR severity score on admission is a risk factor for death, severe disease, IMV use, and ICU treatment in patients with COVID-199,11,12,14,15. Deterioration of CXR findings after hospitalization influences the requirement for ventilatory support after admission22. Moreover, studies have investigated mortality prediction using CXR findings before and after ICU admission23 and the association of the worst CXR scores during hospitalization with discharge and death37. However, we found that rapid deterioration of CXR findings was an independent risk factor for death, most severe disease, ICU admission, and tracheal intubation. Furthermore, multivariate analysis using baseline CXR findings revealed that these relationships were robust. Compared with baseline CXR findings, rapid deterioration of CXR findings had a higher aOR of predicting worse outcomes. Our findings suggest clinicians should be aware of CXR deterioration, especially within 48 h of admission, regardless of the baseline CXR findings.
We identified the risk factors for rapid CXR deterioration. Several reported factors contribute to severe COVID-19 development2,3,4,5,6,7,8. In our study, patients with rapid CXR deterioration showed many of these risk factors for death and severe disease. CXR consolidation; concomitant cardiovascular disease and COPD; and elevated body temperature, AST, serum potassium, and CRP levels, were independent risk factors for rapid deterioration of CXR findings. A prediction model for CXR deterioration using these risk factors showed high accuracy (AUC = 0.806). These results may facilitate the prediction of rapid deterioration of CXR findings and prompt interventions.
Previous GWAS reports have suggested an association between the genetic characteristics of patients with COVID-19 and the severity of COVID-1924,38. We performed analyses using risk variants and ABO blood groups to characterize the genetic characteristics related to rapid deterioration of CXR findings. We identified whole-population and population-specific risk variants at the ABO locus (rs529565-C) among the 15 genes extracted from previous reports24,27,28,29,30,31,32,33,34,35. The DOCK2 locus has been associated with severe disease in patients with COVID-19 aged < 65 years24. Therefore, we performed an analysis of the association between DOCK2 and acute worsening of imaging in patients with COVID-19 aged < 65 years, but found no significant differences. Regarding the risk variant of the ABO locus, we observed a risk of rapid deterioration of CXR findings in patients with COVID-19 in the AB blood group. In patients with COVID-19, an association between the AB blood group and most severe disease has been reported39. The AB blood group is reported to be more susceptible to a variety of infections40 and at higher risk for thrombosis41, which may contribute to the same mechanisms as most severe disease and rapid deterioration of CXR findings. However, further studies are needed to explore this point.
Our study had three main limitations. First, we could not determine the mechanisms underlying rapid deterioration of CXR findings from a virological perspective. Therefore, our findings may not reflect the current clinical picture of COVID-19 since epidemics of different variants have become serious public health threats in Japan and other countries42,43. Second, since this was a multi-center study, the interpretation of radiograms may have differed among centers. Although previous studies have performed clinical investigations based on quantitative evaluation of CXR findings, such quantitative evaluations were unavailable in our study22,37. Specifically, although ‘deterioration of lung infiltrates in > 50% of the lung field’ is a qualitative parameter, it may involve an increased risk of ambiguity. However, one strength of this multi-center study was that we enrolled a larger sample size than previous CXR-related studies on patients with COVID-19. Moreover, our prediction model for clinical outcomes applied a very simple assessment index that can be easily used in daily clinical practice. One problem with the definition of rapid deterioration of CXR findings was that if the baseline CXR revealed a shadow more extensive than half of the lung field, it did not meet the definition, irrespective of the subsequent deterioration degree. Nonetheless, despite this bias, we still achieved significant results; moreover, we did not discuss the management of possible controversial cases. Third, we excluded 269 patients without two chest X-rays within 48 h. Clinically severe patients tended to undergo repeated CXRs; therefore, these missing data might have led to selection bias.
Conclusions
Rapid deterioration of CXR findings within 2 days of admission is a significant prognostic risk factor in patients with COVID-19. Accordingly, carefully monitoring changes in radiographic findings during this period is important. Specifically, patients with CXR consolidation, comorbid cardiovascular disease or COPD, fever with high inflammatory response, and elevated AST or blood potassium levels should be carefully monitored after admission.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- CXR:
-
Chest X-ray
- COVID-19:
-
Coronavirus disease
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- ICU:
-
Intensive care unit
- GWAS:
-
Genome-wide association study
- IMV:
-
Invasive mechanical ventilation
- QC:
-
Quality control
- GGO:
-
Ground-glass opacity
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- AST:
-
Aspartate aminotransferase
- CRP:
-
C-reactive protein
References
Worldometer COVID-19 coronavirus pandemic (2022). https://www.worldometers.info/coronavirus/.
Docherty, A. B. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ 22(369), m1985 (2020).
Deng, G., Yin, M., Chen, X. & Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 24(1), 179 (2020).
Cunningham, J. W. et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern. Med. 181(3), 379–381 (2020).
Harrison, S. L., Fazio-Eynullayeva, E., Lane, D. A., Underhill, P. & Lip, G. Y. H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 17(9), e1003321. https://doi.org/10.1371/journal.pmed.1003321 (2020).
Petrilli, C. M. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 22(369), m1966 (2020).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180(7), 934–943 (2020).
Liao, D. et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 7(9), e671–e678 (2020).
Masood, L. et al. Progression and resolution of COVID-19 pneumonia on chest radiograph. J. Coll. Phys. Surg. Pak. 31, 258–261 (2021).
Kim, H. W. et al. The role of initial chest X-ray in triaging patients with suspected COVID-19 during the pandemic. Emerg. Radiol. 27(6), 617–621 (2020).
Shen, B. et al. Initial chest radiograph scores inform COVID-19 status, intensive care unit admission and need for mechanical ventilation. Clin. Radiol. 76(473), e1-7 (2021).
Kaleemi, R. et al. The association of chest radiographic findings and severity scoring with clinical outcomes in patients with COVID-19 presenting to the emergency department of a tertiary care hospital in Pakistan. PLoS One 16, e0244886 (2021).
Cozzi, D. et al. Chest X-ray in new coronavirus disease 2019 (COVID-19) infection: findings and correlation with clinical outcome. Radiol. Med. 125, 730–737 (2020).
Homayounieh, F. et al. Clinical and imaging features predict mortality in COVID-19 infection in Iran. PLoS One 15, e0239519 (2020).
Galloway, J. B. et al. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: An observational cohort study. J. Infect. 81, 282–288 (2020).
Mushtaq, J. et al. Initial chest radiographs and artificial intelligence (AI) predict clinical outcomes in patients with COVID-19: Analysis of 697 Italian patients. Eur. Radiol. 31, 1770–1779 (2021).
Borghesi, A. et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: A study of 302 patients from Italy. Int. J. Infect. Dis. 96, 291–293 (2020).
Balbi, M. et al. Chest X-ray for predicting mortality and the need for ventilatory support in patients with COVID-19 presenting to the emergency department. Eur. Radiol. 31, 1999–2012 (2021).
Larici, A. R. et al. Multimodality imaging of COVID-19 pneumonia: From diagnosis to follow-up. A comprehensive review. Eu. J. Radiol. 131, 109217 (2020).
Rubin, G. D. et al. The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the Fleischner Society. Chest 158, 106–116 (2020).
Borghesi, A. & Maroldi, R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol. Med. 125, 509–513 (2020).
Plasencia-Martínez, J. M. et al. Early radiological worsening of SARS-CoV-2 pneumonia predicts the need for ventilatory support. Eur. Radiol. 32, 3490–3500 (2022).
Cheng, J. et al. COVID-19 mortality prediction in the intensive care unit with deep learning based on longitudinal chest X-rays and clinical data. Eur. Radiol. 32, 4446–4456 (2022).
Namkoong, H., Edahiro, R., Fukunaga, K., Shirai, Y., Sonehara, K., Tanaka, H., et al. Japan COVID-19 Task Force: a nation-wide consortium to elucidate host genetics of COVID-19 pandemic in Japan. Medrxiv 2021.05.17.21256513. Accessed 7 August 2022, https://www.medrxiv.org/content/https://doi.org/10.1101/2021.05.17.21256513v1
Tanaka, H. et al. Clinical characteristics of patients with coronavirus disease (COVID-19): Preliminary baseline report of Japan COVID-19 task force, a nation-wide consortium to investigate host genetics of COVID-19. Int J Infect Dis. 113, 74–81 (2021).
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242 (2020).
Roberts, G.H., Partha, R., Rhead, B., Knight, S.C., Park, D.S., Coignet, M.V. et al. Novel COVID-19 phenotype definitions reveal phenotypically distinct patterns of genetic association and protective effects. Medrxiv 2021.01.24.21250324. Accessed 7 August 2022, https://www.medrxiv.org/content/https://doi.org/10.1101/2021.01.24.21250324v1.
COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature 600, 472–7 (2021).
Wang, F. et al. Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discov. 6, 83 (2020).
Thibord, F., Chan, M. V., Chen, M. H., Johnson, A. D. et al. A year of Covid-19 GWAS results from the GRASP portal reveals potential SARS-CoV-2 modifiers. Medrxiv 2021.06.08.21258507. Accessed 7 August 2022https://www.medrxiv.org/content/https://doi.org/10.1101/2021.06.08.21258507v2.
Thevarajan, I. et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 26, 453–455 (2020).
Nikoloudis, D., Kountouras, D. & Hiona, A. The frequency of combined IFITM3 haplotype involving the reference alleles of both rs12252 and rs34481144 is in line with COVID-19 standardized mortality ratio of ethnic groups in England. PeerJ. 8, e10402 (2020).
Alghamdi, J. et al. Interferon-induced transmembrane protein-3 genetic variant rs12252 is associated with COVID-19 mortality. Genomics 113, 1733–1741 (2021).
Kuo, C. L. et al. APOE e4 genotype predicts severe COVID-19 in the UK Biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 75, 2231–2232 (2020).
Hou, Y. et al. New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 18, 216 (2020).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33(1), 159–174 (1977).
Maroldi, R. et al. Which role for chest x-ray score in predicting the outcome in COVID-19 pneumonia?. Eur Radiol. 31, 4016–4022 (2020).
Severe Covid-19 GWAS Group et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 383, 1522–1534 (2020).
Kusumoto, T. et al. Association between ABO blood group/genotype and COVID-19 in a Japanese population. Ann. Hematol. 102(11), 3239–3249 (2023).
Garratty, G. Blood groups and disease: A historical perspective. Transfus. Med. Rev. 14(4), 291–301 (2000).
Jukic, I. et al. ABO blood groups and genetic risk factors for thrombosis in Croatian population. Croat. Med. J. 50(6), 550–558 (2009).
Ito, K., Piantham, C. & Nishiura, H. Predicted dominance of variant Delta of SARS-CoV-2 before Tokyo Olympic Games, Japan, July 2021. Euro. Surveill. 26, 2100570 (2021).
Lee, H. et al. Characteristics of hospitalized patients with COVID-19 during the first to fifth waves of infection: A report from the Japan COVID-19 Task Force. BMC Infect. Dis. 22, 935 (2022).
Acknowledgements
We would like to thank all the study participants and all members of the Japan COVID-19 Task Force engaged in clinical and research work on COVID-19 daily. All members contributed to this study.
Funding
This work was supported by AMED [Grant Number JP20nk0101612, JP20fk0108415, JP21km0405211, JP21km0405217, JP21wm0325031], JST CREST [Grant Number JPMJCR20H2], JST PRESTO [Grant Number JPMJPR21R7], and MHLW [Grant Number 20CA2054].
Author information
Authors and Affiliations
Contributions
Conceptualisation: T.K., S.C., H.N., T.A., K.M., H.K., M.I., N.H., and K.F. Data Curation: T.K., H.T., H.L., S.O., K.N., T.F., A.M., and M.W. Formal analysis: T.K., S.C., and H.N. Methodology: T.K., S.C., and H.N. Supervision: T.K., S.C., N.H., T.A., K.M., H.K, M.I., N.H., N.H., T.U., S.U., T.I., K.A., F.S., T.Y., Y.N., Y.M., Y.S., R.E., K.M., Y.S., Y.O., R.K., Y.K., K.T., A.K., S.I., S.M., S.O., T.K., and K.F. Visualisation: T.K., S.C., and H.N. Writing—original draft: T.K., S.C., and H.N. Writing—review and editing: T.K., S.C., N.H., T.A., K.M., H.K, M.I., N.H., N.H., T.U., S.U., T.I., K.A., F.S., T.Y., Y.N., Y.M., Y.S., R.E., K.M., Y.S., Y.O., R.K., Y.K., K.T., A.K., S.I., S.M., S.O., T.K., and K.F. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kusumoto, T., Chubachi, S., Namkoong, H. et al. Characteristics of patients with COVID-19 who have deteriorating chest X-ray findings within 48 h: a retrospective cohort study. Sci Rep 13, 22054 (2023). https://doi.org/10.1038/s41598-023-49340-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49340-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.