Abstract
Heart failure (HF) presents manifestations in both cardiac and vascular abnormalities. Pulmonary hypertension (PH) is prevalent in up 50% of HF patients. While pulmonary arterial hypertension (PAH) is closely associated with pulmonary artery (PA) stiffness, the association of HF caused, post-capillary PH and PA stiffness is unknown. We aimed to assess and compare PA stiffness and blood flow hemodynamics noninvasively across HF entities and control subjects without HF using CMR. We analyzed data of a prospectively conducted study with 74 adults, including 55 patients with HF across the spectrum (20 HF with preserved ejection fraction [HFpEF], 18 HF with mildly-reduced ejection fraction [HFmrEF] and 17 HF with reduced ejection fraction [HFrEF]) as well as 19 control subjects without HF. PA stiffness was defined as reduced vascular compliance, indicated primarily by the relative area change (RAC), altered flow hemodynamics were detected by increased flow velocities, mainly by pulse wave velocity (PWV). Correlations between the variables were explored using correlation and linear regression analysis. PA stiffness was significantly increased in HF patients compared to controls (RAC 30.92 ± 8.47 vs. 50.08 ± 9.08%, p < 0.001). PA blood flow parameters were significantly altered in HF patients (PWV 3.03 ± 0.53 vs. 2.11 ± 0.48, p < 0.001). These results were consistent in all three HF groups (HFrEF, HFmrEF and HFpEF) compared to the control group. Furthermore, PA stiffness was associated with higher NT-proBNP levels and a reduced functional status. PA stiffness can be assessed non-invasively by CMR. PA stiffness is increased in HFrEF, HFmrEF and HFpEF patients when compared to control subjects.
Trial registration The study was registered at the German Clinical Trials Register (DRKS, registration number: DRKS00015615).
Similar content being viewed by others
Background
Heart failure (HF) is a systemic syndrome affecting both the heart and the vascular system with an increasing prevalence1,2. While established treatment strategies have improved the prognosis in patients with HF with reduced ejection fraction (HFrEF) and recent studies also showed first effective therapies in patients with HF with mildly reduced ejection fraction (HFmrEF) or preserved ejection fraction (HFpEF), the prognosis of patients with HF remains poor and is heterogeneous across the population1,3,4. A major pillar of HF therapy is the pharmacological reduction of the increased afterload and vascular resistance, primarily driven by the activation of the renin–angiotensin–aldosterone system (RAAS)1. Pulmonary hypertension is highly prevalent among patients with HF, present in approximately 50% of those with HFrEF or HFpEF, and it is associated with worst outcomes5,6,7,8. PA stiffness is a manifestation of PH, the feasibility to detect PA stiffness by CMR and its predictive value for an early diagnosis pulmonary arterial hypertension (PAH) has already been shown9,10,11. The prognostic role of PA stiffness in HF and post-capillary PH is yet unknown12,13,14,15.
Currently, right-heart catheterization is the reference standard method to evaluate PA stiffness and flow hemodynamics. Echocardiographic attempts to assess PA stiffness and hemodynamic flow parameters are limited by frequent suboptimal acoustic windows. Cardiovascular magnetic resonance imaging (CMR) is a comprehensive technique, increasingly accessible and provides not only high resolution information on functional cardiac parameters but also details on vascular contraction and blood flow characteristics. In particular, the phase contrast magnetic resonance (PCMR) technique allows for structural and functional evaluation of blood vessels.
CMR-based assessment of PA stiffness and flow hemodynamics has been demonstrated to be accurate in patients with chronic obstructive pulmonary disease and PAH9,11,16,17,18. The applicability in a high-risk group for post-capillary PH with HF has not been shown.
In this study, we aimed to (a) assess PA stiffness and parameters of pulmonary hemodynamics by CMR in both patients with HF and subjects without HF, to (b) analyze their association with symptom burden, and to (c) compare the results between HF and control subjects groups.
Methods
Study population and design
This study was a prospective study conducted at two centers in Berlin, Germany, namely at the Charité—University Medicine Berlin and the German Heart Centre Berlin, between 2017 and 2018. Its rationale and design of the study as well as data and primary results of other research questions from the described patient cohort have been previously published19,20,21,22,23.
Briefly, subjects with a history of HF and an age of at least 45 years could be included. The initial diagnosis of HF had to be made at least 30 days ago; patients were required to be in a stable state with no changes in their HF medication and no HF hospitalization within the previous 7 days. HFrEF was defined by LVEF < 40%, HFmrEF by an increased NT-proBNP (> 220 pg/mL) and LVEF ≥ 40% and < 50% as well as HFpEF by an increased NT-proBNP (> 220 pg/mL) and LVEF ≥ 50% at the time of study inclusion19. We did not distinguish between the causes of HF for recruiting patients19.
Additionally, we recruited subjects without HF or advanced cardiovascular (CV) diseases as controls. All studies complied with the Declaration of Helsinki, the protocols were approved by the responsible ethics committees, and all patients gave written informed consent. The study was registered at the German Clinical Trials Register (DRKS, registration number: DRKS00015615). Detailed inclusion and exclusion criteria are listed on the webpage of the DRKS.
CMR protocol
All examinations were performed in the supine position using a 1.5 T MR scanner (Achieva, Philips, Best, The Netherlands). Short- and long-axis cine images were acquired during breath holds of 10–15 s with a standard balanced steady-state free precession sequence. Main PA cross-sectional images were obtained for blood flow quantification by using a 2D phase-contrast acquisition with through-plane velocity encoding perpendicular to the pulmonary artery trunk, with imaging parameters as follows: 35 phases per cardiac cycle; flip angle 15 degrees, slice thickness 8 mm, field of view 340 × 400 mm, temporal resolution 25 ms, velocity encoding 200 cm/s (cross-sectional image of the PA using phase-contrast through-plane sequences illustrated in supplementary material S1).
CMR image analysis
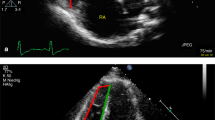
CMR images were analyzed offline at our CMR-core lab by a single observer, who was blinded to the clinical data, using specialized software (Medis Suite, version 3.1, Leiden, The Netherlands). LV contours were outlined manually on the cardiac cine images using QMass 8.1. The contours of the PA were semiautomatically traced and, where needed, manually adjusted for each phase using QFlow 8.1. The PA maximum cross-sectional area (Amax) and minimum area (Amin) were acquired from the end-systolic and end-diastolic (either magnitude or phase) images, respectively (Figs. 1, 2).
PA cross-sectional MR images. (A–D) PA cross-sectional phase-contrast MR image acquisition perpendicular to the pulmonary trunk. Cross-sectional PA areas were measured by (A) magnitude and (B) velocity images in end-systole and (C) magnitude and (D) velocity images in end-diastole. Vessel contours (red circle) were traced throughout the entire cardiac cycle. PA pulmonary artery.
Method for PA PWV, AT and ET measurements. (A) The plot shows the flow area of the PA of eight cardiac phases during early systole. The slope of a fitted line to the early systole gives Δflow/Δarea = PWV. (B) The plot shows the time course of the PA blood flow. The left dotted vertical line is the time of peak systolic flow. The right dotted vertical line is the time of the end of ejection. AT is the time interval between the onset of the PA blood flow and the peak flow (short red double arrow). ET is the time interval between the onset and the end of systolic PA blood flow (long red double arrow). PA pulmonary artery, AT acceleration time, ET ejection time.
PA stiffness indices were assessed in two categories: (a) compliance and (b) flow velocities, primarily using PA relative area change (RAC) and pulse wave velocity (PWV), both of which have been shown to predict outcomes in patients with pre-capillary PH10,11,24. The main compliance parameter analyzed was the RAC based on the PA area change (AC). The AC was defined as AC = Amax − Amin, RAC was defined respectively as RAC = (Amax − Amin)/Amin. A smaller area change (both AC and RAC) indicates a stiffer vessel.
PWV was evaluated as the ratio between flow variation (Δflow) and area variation (Δarea) by the flow area (QA) method24,25,26. The PWV values were derived from the slope of a line fitted to the early systole (Fig. 2). Other hemodynamic parameters assessed included acceleration time (AT), ejection time (ET), flow per min (FPM), mean pressure gradient (MPG), net flow volume (NFV), peak flow velocity (PV), and peak pressure gradient (PPG).
AT and ET were determined as the time interval between the onset of blood flow and peak flow and the time interval between the onset and end of systolic blood flow, respectively (Fig. 2).
To assess the intra- and interobserver reproducibility, 10 random cases (5 healthy volunteers and 5 HF patients) were analyzed by 2 observers (X.H and P.B.) blinded to the clinical data at 2 separate times (median 5 days apart from eachother).
Statistical analysis
Data for categorical variables are expressed as percentages and were compared by chi-square test. Continuous data were tested for normality with the Shapiro–Wilk test. Normally distributed continuous variables are presented as mean ± standard deviation, and differences between groups were compared using independent t-tests or analysis of variance. Non-normally distributed continuous variables are presented as medians (interquartile ranges), and different groups were compared with the nonparametric Mann–Whitney U test or the Kruskal–Wallis test as appropriate. Multivariate linear regression was performed to adjust for age differences in baseline characteristics. Correlations between variables were explored using the Pearson or Spearman correlation coefficients, and further evaluation of relationships between parameters was performed with linear regression analysis. Intra- and interobserver variability were tested by Bland–Altman agreement analysis, intraclass correlation coefficient, and coefficient of variation. The analyses were performed with dedicated statistical packages (SPSS 25.0, Chicago, USA; GraphPad Prism 8.0, San Diego, USA; MedCalc 19.0, Belgium).
Ethics approval and consent to participate
As described above, the study complied with the Declaration of Helsinki, the protocols were approved by the responsible ethics committees, and all patients gave written informed consent. The study was registered at the German Clinical Trials Register (DRKS, registration number: DRKS00015615).
Results
Population characteristics
A total of 74 subjects were prospectively enrolled in this study: control subjects (n = 19) and HF patients (n = 55), who were further divided into 3 HF subgroups: HFpEF group (n = 20), HFmrEF group (n = 18), and HFrEF group (n = 17). In line with previously published analyses, baseline characteristics are presented in Table 1 for completeness19–23.
PA stiffness and flow parameters
In in the pooled HF group as well as in all three HF groups separately, PA proved to be stiffer than in the control group (Tables 2, 3, Figs. 3, 4). All HF patient groups showed an increased PA stiffness with reduced RAC and increased PWV as compared to control subjects.
Comparison of PA stiffness and flow hemodynamics across all groups. Graphs with mean values and standard deviations for pulmonary artery Amin (A), AC (B), RAC (C), PWV (D), AT (E), NFV (F), FPM (G), and PV (H) in the controls group and HF subgroups. Amin minimum pulmonary artery across-sectional area, AC area change, AT acceleration time, FPM flow per min, HFpEF heart failure with preserved ejection fraction, HFmrEF heart failure with mid-range ejection fraction, HFrEF heart failure with reduced ejection fraction heart failure, HV healthy volunteer, i.e. control subject, NFV net flow volume, PV peak flow velocity, PWV pulse wave velocity, RAC relative area change.
Correlation between NT-proBNP level or functional capacity (NYHA class and 6MWD) and PA RAC. The graphs show the results of the linear regression analysis in the entire study between NT-proBNP and RAC (A), NYHA class and RAC (B), and 6 MWD and RAC (C). Green solid circles represent the control group, whereas purple squares, light blue triangles and red triangles represent the HFpEF group, HFmrEF group and HFrEF group, respectively. Black solid lines are the fitted lines of regression analysis for all datasets. 6MWD 6 min walk distance, NYHA class New York Heart Association functional class, HFpEF heart failure with preserved ejection fraction, HFmrEF heart failure with mid-range ejection fraction, HFrEF heart failure with reduced ejection fraction heart failure, HV healthy volunteer, i.e. control subject, NT-proBNP N-terminal pro brain natriuretic peptide, RAC relative area change.
The remaining hemodynamic parameters assessed, including NFV, FPM, PV, PPG, and MPG, were also altered in the HF groups compared to the controls (S2 and S3). The measurements of both PA stiffness and flow parameters are consistent, e.g. the higher the PA stiffness was, the higher was the pulse wave velocity. A summary of the correlations is given in the supplementary information (S4).
Comparison between groups
All HF groups showed an increase in PA stiffness compared to control subjects (Table 3), e.g. RAC was highest in controls, while the HF group RAC values were similar (Table 3).
Correlation with symptom burden
Both clinical and laboratory measures of disease severity were associated with PA stiffness and altered flow parameters. Patients with a higher clinical symptom burden, assessed by both NYHA functional class and 6MWD, had stiffer PA parameters and altered flow parameters (Fig. 4, Fig. S6). This association was also confirmed by a laboratory surrogate of disease burden (NT-proBNP), i.e. a lower RAC was associated with a higher NT-proBNP level (r = − 0.31, p < 0.01) and a higher NYHA class (r = − 0.59, p ≤ 0.01).
Reproducibility of measurements
Intra- and interobserver variability was low for all CMR measurements, supporting a high reliability of the measurements (S6 and S7).
Discussion
In this study, we quantitatively assessed for the first time PA stiffness and blood flow dynamics using advanced CMR in HF patient including all three HF entities and in healthy subjects without HF. We demonstrate that (a) PA stiffness is higher in all three HF groups compared to control subjects and PA hemodynamic parameters are consistently altered. Furthermore, we (b) detected significant correlations between the presence and degree of PA stiffness and the disease burden with regards to both clinical and laboratory HF parameters. Comparisons between the different HF entities revealed (c) that PA stiffness is similar between the three HF entities.
Acknowledging the similarity across HF entities indicates that PA stiffness may be independent from the systemic vascular changes due to the RAAS system and RAAS inhibiting therapeutic effects.
PA wall stiffness is considered an important determinant of cardiovascular risk at an early stage of pulmonary disease11,27. Changes in vascular mechanics often precede gross remodeling; thus, an assessment of PA stiffness can be a useful tool in the early identification of pathological changes in the pulmonary vasculature—which has already been proven in PAH, but never in PH in the context of HF10,11,24,28,29.
We detected important differences in the indices of PA stiffness between control group and HF patients. There was a significant reduction in PA absolute AC, RAC and AT, as well as a significant elevation in PA PWV in patients with HF. The differences in PA stiffness measurements observed in our study are consistent with findings from previous studies that employed both CMR and right heart catheterization as diagnostic tools. Specifically, 2 studies primarily focused on PA stiffness and its predictive value in pulmonary arterial hypertension9,11. Three complementary studies concentrated more on right heart/pulmonary artery pressures and their associated CMR-determined RV changes16,17,18. Therefore, our results add to the growing body of literature supporting the clinical relevance of PA stiffness measurements. A previous report demonstrated that PH in patients with HF is common30. Consistently, we showed for the first time mild pulmonary hypertension in HF patients by CMR. In the HF group, especially in the HFmrEF and HFrEF subgroups, we found significant PA lumen dilatation (Amin, Table 3), which suggests long-term volume overload due to left-sided heart disease and/or abnormal changes in pulmonary vessel walls due to pathological factors.
Recent data show that abnormal PA hemodynamic status occurs in the early stages of HF and is strongly associated with unfavorable outcomes in HF5,6,31. Prior studies showed that PA flow hemodynamic parameters were significantly decreased in PH and chronic thromboembolic pulmonary hypertension (CTEPH) compared to healthy subjects11,16,17,32. Similar alterations in PA flow hemodynamics were detected for NFV, FPM, and PV in our study.
Previous studies have shown that increasing PA stiffness is associated with worsening functional parameters in patients with PH and in those with HF13,33,34.
Our measurements appear to be reliably assessed as indicated by our intra-observer and inter-observer variability assessment with a high reproducibility of measurements. These results are in line with previous studies that tested observer variability for PA imaging by CMR in other cohorts33.
Our observation requires a prospective, follow-up analysis or a confirmation in a cohort followed longitudinally to assess the prognostic relevance of PA stiffness and PA hemodynamics. PA stiffness may present a central characteristic of HF patients with the potential to serve as a relevant parameter to better characterize and group HF patients.
Limitations
This study was a single-center study. Thus, a center-specific bias could not be excluded. However, single-center data collection bears several advantages: inclusion of a homogenous patient population; a standard work-up routine; and consistent quality of the CMR examination. In addition, there was a small sample size of the various HF subgroups. Therefore, it is difficult to extrapolate these results to a general population. Although we divided our subjects into HF subgroups according to the most recent guidelines, HF is a complex clinical syndrome with various etiologies that could impact the changes in PA hemodynamics. The absence of long-term follow-up data for this patient cohort restricts our ability to evaluate the long-term clinical significance of our findings.
Conclusions
CMR is a reproducible noninvasive technique to assess PA stiffness and flow hemodynamics. As we could demonstrate for the first time in the context of HF patients an post-capillary PH, CMR is able to detect significant differences in stiffness and blood flow hemodynamics between subjects without HF and HF patients. HF patients show higher values for PA stiffness compared to controls subjects.
Data availability
The data can be accessed upon request with a research proposal submitted to the investigator via the corresponding author.
Abbreviations
- AC:
-
Area change
- Amax:
-
Maximum pulmonary artery cross-sectional area
- Amin:
-
Minimum pulmonary artery across-sectional area
- AT:
-
Acceleration time
- CMR :
-
Cardiovascular magnetic resonance imaging
- CTEPH:
-
Chronic thromboembolic pulmonary hypertension
- CV :
-
Cardiovascular
- ET :
-
Ejection time
- FPM :
-
Flow per min
- HF:
-
Heart failure
- HFmrEF:
-
Heart failure with preserved ejection fraction
- HFpEF:
-
Heart failure with mildly-reduced ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LVEF:
-
Left ventricular ejection fraction
- MPG :
-
Mean pressure gradient
- NFV :
-
Net flow volume
- PA:
-
Pulmonary artery
- PAH:
-
Pulmonary arterial hypertension
- PCMR:
-
Phase contrast magnetic resonance
- PPG:
-
Peak pressure gradient
- PV:
-
Peak flow velocity
- PWV:
-
Pulse wave velocity
- RAC:
-
Relative area change
References
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726. https://doi.org/10.1093/eurheartj/ehab368 (2021).
Savarese, G., Stolfo, D., Sinagra, G. & Lund, L. H. Heart failure with mid-range or mildly reduced ejection fraction. Nat. Rev. Cardiol. 19, 100–116. https://doi.org/10.1038/s41569-021-00605-5 (2022).
Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385, 1451–1461. https://doi.org/10.1056/NEJMoa2107038 (2021).
Hashemi, D. et al. Evaluation of the HFA-PEFF Score: Results from the prospective DIAST-CHF cohort. ESC Heart Fail. https://doi.org/10.1002/ehf2.14131 (2022).
Lam, C. S. et al. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J. Am. Coll. Cardiol. 53, 1119–1126. https://doi.org/10.1016/j.jacc.2008.11.051 (2009).
Ghio, S. et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 37, 183–188. https://doi.org/10.1016/s0735-1097(00)01102-5 (2001).
Miller, W. L., Grill, D. E. & Borlaug, B. A. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: Pulmonary hypertension and heart failure. JACC Heart Fail. 1, 290–299. https://doi.org/10.1016/j.jchf.2013.05.001 (2013).
Duran, A. & Mandras, S. Pulmonary hypertension in heart failure. Curr. Opin. Cardiol. 36, 205–210. https://doi.org/10.1097/HCO.0000000000000834 (2021).
Ray, J. C. et al. Pulmonary arterial stiffness assessed by cardiovascular magnetic resonance imaging is a predictor of mild pulmonary arterial hypertension. Int. J. Cardiovasc. Imaging 35, 1881–1892. https://doi.org/10.1007/s10554-018-1397-y (2019).
Forouzan, O. et al. Exercise-induced changes in pulmonary artery stiffness in pulmonary hypertension. Front. Physiol. 10, 269. https://doi.org/10.3389/fphys.2019.00269 (2019).
Gan, C. T. et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132, 1906–1912. https://doi.org/10.1378/chest.07-1246 (2007).
Chung, H. J., Hwang, H. B. & Lee, N. Y. The association between primary open-angle glaucoma and blood pressure: Two aspects of hypertension and hypotension. Biomed. Res. Int. 2015, 827516. https://doi.org/10.1155/2015/827516 (2015).
Dalos, D. et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 68, 189–199. https://doi.org/10.1016/j.jacc.2016.04.052 (2016).
Dragu, R. et al. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur. J. Heart Fail. 17, 74–80. https://doi.org/10.1002/ejhf.192 (2015).
Stevens, G. R. et al. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc. Imaging 5, 378–387. https://doi.org/10.1016/j.jcmg.2011.11.020 (2012).
Weir-McCall, J. R., Liu-Shiu-Cheong, P. S., Struthers, A. D., Lipworth, B. J. & Houston, J. G. Pulmonary arterial stiffening in COPD and its implications for right ventricular remodelling. Eur. Radiol. 28, 3464–3472. https://doi.org/10.1007/s00330-018-5346-x (2018).
Liu, C. Y. et al. Pulmonary artery stiffness in chronic obstructive pulmonary disease (COPD) and emphysema: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. J. Magn. Reson. Imaging 47, 262–271. https://doi.org/10.1002/jmri.25753 (2018).
Sanz, J. et al. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc. Imaging 2, 286–295. https://doi.org/10.1016/j.jcmg.2008.08.007 (2009).
Hashemi, D. et al. Myocardial deformation assessed among heart failure entities by cardiovascular magnetic resonance imaging. ESC Heart Fail. https://doi.org/10.1002/ehf2.13193 (2021).
Blum, M. et al. Variability of myocardial strain during isometric exercise in subjects with and without heart failure. Front. Cardiovasc. Med. https://doi.org/10.3389/fcvm.2020.00111 (2020).
Doeblin, P. et al. CMR tissue characterization in patients with HFmrEF. J. Clin. Med. https://doi.org/10.3390/jcm8111877 (2019).
Tanacli, R. et al. Range variability in CMR feature tracking multilayer strain across different stages of heart failure. Sci. Rep. 9, 16478. https://doi.org/10.1038/s41598-019-52683-8 (2019).
Tanacli, R. et al. Multilayer myocardial strain improves the diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail. https://doi.org/10.1002/ehf2.12826 (2020).
Swift, A. J. et al. Pulmonary artery relative area change detects mild elevations in pulmonary vascular resistance and predicts adverse outcome in pulmonary hypertension. Invest. Radiol. 47, 571–577. https://doi.org/10.1097/RLI.0b013e31826c4341 (2012).
Peng, H. H., Chung, H. W., Yu, H. Y. & Tseng, W. Y. Estimation of pulse wave velocity in main pulmonary artery with phase contrast MRI: Preliminary investigation. J. Magn. Reson. Imaging 24, 1303–1310. https://doi.org/10.1002/jmri.20782 (2006).
Vulliemoz, S., Stergiopulos, N. & Meuli, R. Estimation of local aortic elastic properties with MRI. Magn. Reson. Med. 47, 649–654. https://doi.org/10.1002/mrm.10100 (2002).
Colebatch, H. J. & Ng, C. K. Rate of increase in pulmonary distensibility in a longitudinal study of smokers. Thorax 43, 175–182. https://doi.org/10.1136/thx.43.3.175 (1988).
Ben-Yehuda, O. & Barnett, C. Magnetic resonance assessment of pulmonary artery compliance: A promising diagnostic and prognostic tool in pulmonary hypertension?. JACC Cardiovasc. Imaging 2, 296–298. https://doi.org/10.1016/j.jcmg.2008.10.014 (2009).
Berger, R. M., Cromme-Dijkhuis, A. H., Hop, W. C., Kruit, M. N. & Hess, J. Pulmonary arterial wall distensibility assessed by intravascular ultrasound in children with congenital heart disease: An indicator for pulmonary vascular disease?. Chest 122, 549–557. https://doi.org/10.1378/chest.122.2.549 (2002).
Guazzi, M. & Naeije, R. Pulmonary hypertension in heart failure: Pathophysiology, pathobiology, and emerging clinical perspectives. J. Am. Coll. Cardiol. 69, 1718–1734. https://doi.org/10.1016/j.jacc.2017.01.051 (2017).
Leung, C. C., Moondra, V., Catherwood, E. & Andrus, B. W. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am. J. Cardiol. 106, 284–286. https://doi.org/10.1016/j.amjcard.2010.02.039 (2010).
Kawakubo, M. et al. Three-dimensional phase contrast magnetic resonance imaging validated to assess pulmonary artery flow in patients with chronic thromboembolic pulmonary hypertension. Radiol. Phys. Technol. 10, 249–255. https://doi.org/10.1007/s12194-016-0383-0 (2017).
Nethononda, R. M. et al. Gender specific patterns of age-related decline in aortic stiffness: A cardiovascular magnetic resonance study including normal ranges. J. Cardiovasc. Magn. Reson. 17, 20. https://doi.org/10.1186/s12968-015-0126-0 (2015).
Yildirim, E. et al. Relationship between pulmonary artery stiffness and functional capacity in patients with heart failure with reduced ejection fraction. Korean Circ. J. 47, 929–938. https://doi.org/10.4070/kcj.2017.0081 (2017).
Acknowledgements
The authors thank the technicians of the German Heart Center Berlin for the performance of high-quality CMR.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Centre for Cardiovascular Research (DZHK), funded by the German Federal Ministry of Education and Research, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)-CRC-1470-B06 and the Charité—Universitätsmedizin Berlin, Germany, as well as the German Heart Institute Berlin, Germany, and Myocardial Solutions. Our research was also supported by an unrestricted research Grant from Philips Healthcare. D.H. received a CMR specific research Grant from the DZHK (Grant Number: 81X3100220).
Author information
Authors and Affiliations
Contributions
D.H., X.H. and S.K. conceived and designed the study. T.K., M.K., D.H., L.M., M.B., F.E., W.M.K., B.P., H.-D.D., and A.S. acquired clinical data. D.H., X.H., M.N., S.Z., R.T., and S.K. acquired and analyzed imaging data. X.H. performed the statistical analysis. D.H. and X.H. drafted the manuscript. J.E., P.B., L.S., A.S. and S.K. revised and amended critical parts of the manuscript. All authors contributed to the interpretation of the data and approved the final version of this manuscript. Subjects consented for publication as part of the consent to participate in the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, X., Hashemi, D., Erley, J. et al. Noninvasive evaluation of pulmonary artery stiffness in heart failure patients via cardiovascular magnetic resonance. Sci Rep 13, 22656 (2023). https://doi.org/10.1038/s41598-023-49325-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49325-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.