Abstract
This study investigated the utility of periostin, a matricellular protein, as a prognostic biomarker in patients with idiopathic pulmonary fibrosis (IPF) who received nintedanib. Monomeric and total periostin levels were measured by enzyme-linked immunosorbent assay in 87 eligible patients who participated in a multicenter prospective study. Forty-three antifibrotic drug-naive patients with IPF described in previous studies were set as historical controls. Monomeric and total periostin levels were not significantly associated with the change in forced vital capacity (FVC) or diffusing capacity of the lungs for carbon monoxide (DLCO) during any follow-up period. Higher monomeric and total periostin levels were independent risk factors for overall survival in the Cox proportional hazard model. In the analysis of nintedanib effectiveness, higher binarized monomeric periostin levels were associated with more favorable suppressive effects on decreased vital capacity (VC) and DLCO in the treatment group compared with historical controls. Higher binarized levels of total periostin were associated with more favorable suppressive effects on decreased DLCO but not VC. In conclusion, higher periostin levels were independently associated with survival and better therapeutic effectiveness in patients with IPF treated with nintedanib. Periostin assessments may contribute to determining therapeutic strategies for patients with IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF), pathologically usual interstitial pneumonia (UIP), is the most common idiopathic interstitial pneumonia (IIP) with unknown etiology1,2,3. Patients with IPF have a grave prognosis, with a median survival of approximately 3 years1,2,3,4. The decrease in forced vital capacity (FVC) or diffusing capacity of the lung for carbon monoxide (DLCO) and acute exacerbation have been reported as significant risk factors for mortality in IPF patients3,4,5,6,7,8. Recently, two fibrotic drugs for IPF, nintedanib and pirfenidone, have been shown for safety and efficacy9,10. Treatment with nintedanib reduced the decrease in FVC, the incidence of acute exacerbation of IPF, and the worsening health-related quality of life in two replicate randomized, double-blind, phase 3 trials (INPULSIS-1 and INPULSIS-2)9. Moreover, a meta-analysis of 12,956 patients with IPF across 8 randomized controlled trials (RCT) and 18 cohort studies showed that treatment with antifibrotic drugs including nintedanib and pirfenidone reduced the risk of all-cause mortality and acute exacerbation11. Therefore, nintedanib and pirfenidone therapy for IPF is recommended in the global and Japanese guidelines1,2,12. However, determining the appropriate timing of therapy initiation for IPF is difficult because the rate of disease progression varies among individuals13. Thus, a prognostic factor is required for the decision-making of therapeutic strategies for IPF13. Nevertheless, there is no established biomarker to predict mortality, progression, or therapeutic response in IPF patients. In previous reports, the gender-age-pulmonary physiology (GAP) stage based on clinical, e.g., gender or age, and physiological, e.g., FVC and DLCO, variables can predict overall survival in IPF patients14,15. Moreover, some blood biomarkers such as monocyte count, circulating protein fragments degraded by matrix metalloproteases (MMPs) MMP1 and MMP7, C–C motif chemokine ligand 18 (CCL18), Krebs von den Lungen-6 (KL-6), surfactant protein D (SP-D), and surfactant protein A (SP-A) were reported to be associated with decreased pulmonary function, incidence of acute exacerbation, and/or mortality in IPF patients16,17,18,19,20,21,22,23,24.
Periostin is an extracellular matrix and matricellular protein that modulates cell–matrix interactions via αvβ1, αvβ3, or αvβ5 integrin receptor25. Periostin is secreted from fibroblasts, epithelial cells, and endothelial cells via stimulation by interleukin (IL)-4, IL-13, and transforming growth factor (TGF)-β, and contributes to the pathophysiology of fibrosis during systemic sclerosis, allergic rhinitis, chronic rhinosinusitis, and atopic dermatitis26,27,28. In addition, we previously reported upregulation of periostin in the lung tissue of mice with bleomycin-induced lung injury and its increase expression in the lungs and serum of human IIP29,30. We and other groups have reported that higher serum periostin levels were associated with decreased pulmonary function and shortened overall survival and time-to-event, including more than a 5% decrease in FVC, acute exacerbation or death, and increases in the extent of abnormal findings on high-resolution computed tomography (HRCT) in patients with IPF30,31,32. Unfortunately, periostin is not a specific biomarker for IPF because it is upregulated in various other diseases26,27,28. Izuhara et al. established a new enzyme-linked immunosorbent assay (ELISA) kit that specifically detects the monomeric form (SS20A × SS19D, capture and detection antibody). The level of monomeric periostin is more specific for IPF compared with that measured by conventional ELISA kits that detect both the monomeric and oligomeric forms (SS18A × SS17B, total periostin)33. We showed that both serum monomeric and total periostin were associated with decreases in FVC and DLCO in a multicenter prospective analysis33. Moreover, serum periostin was reported to be associated with mortality in patients with acute exacerbation of fibrotic interstitial lung disease (ILD) and fibrotic hypersensitivity pneumonia34,35.
However, the patients who participated our previous studies included many antifibrotic drug therapy-naive individuals. The specific objectives in the present study were clarifying the association of monomeric and total periostin levels with decreased pulmonary function and survival in IPF patients treated with nintedanib and nintedanib effectiveness.
Patients and methods
Study participants
The present study was a multicenter prospective cohort study. Study samples were obtained from consecutive patients with IPF who received care at 19 collaborative facilities from 2015 to 2021. The main inclusion criteria for participation were as follows: patients diagnosed with IPF based on global guidelines and scheduled to start nintedanib therapy within 2 months after enrollment; and aged ≥ 40 years. Similarly, the main exclusion criteria were as follows: patients who developed acute exacerbation within 3 months prior to enrollment; pregnancy; corticosteroid therapy of more than 10 mg per day of prednisolone equivalent; immunosuppressants; and pirfenidone, N-acetylcysteine, and any investigational treatments for IPF. Patients who could not continue nintedanib therapy or be observed for more than 3 months from baseline were dropped out and the data of these patients were excluded from analyses. Eligible patients with IPF were selected according to multidisciplinary discussion diagnosis following global criteria3 by two each of board-certified clinical, radiological investigators who had considerable experience in thoracic diagnosis. Histological diagnoses of cases that underwent lung biopsies were performed individually at collaborating facilities. Diagnosis of acute exacerbation of IPF was defined in accordance with the criteria detailed in a previous report36. As historical control data for evaluating nintedanib effectiveness, we used serum biomarker levels measured in 43 antifibrotic drug-naive patients with IPF who were selected from 60 patients who participated in the CoDD-PF or Kurume study30,33.
Study protocol
The date of serum collection before the start of nintedanib therapy was set as the baseline (day 0). Sera were collected for measuring the level of periostin every 6 months from baseline. We evaluated pulmonary function, serum level of lactate dehydrogenase (LDH), KL-6, and SP-D as well as the modified Medical Research Council (mMRC) dyspnea scale every 6 months for up to 12 months after baseline. Similarly, chronic obstructive pulmonary disease assessment test (CAT), distance and minimum SpO2 on a 6-min walk test at baseline and 12 months later, was performed. Chest HRCT examinations without contrast medium were performed at baseline using a variety of scanners. Beyond 1 year after baseline, only outcomes including death and acute exacerbation were observed up to completion of the study.
The treatment protocol with nintedanib was based on recommendations similar to those provided in the INPULSIS trials9. The recommended starting dose of nintedanib is 150 mg twice a day. Dose reductions of up to 100 mg twice a day and treatment interruptions are recommended to manage adverse events. After improvement of adverse events, nintedanib can be reintroduced upon the decision of the investigator.
The protocol of HRCT and diagnosis with radiological pattern based on global guidelines were performed as reported previously3,30,32,33. Two board-certificated radiologists with 35 and 33 years of experience, respectively, who specialized in diffuse lung diseases with experience in chest CT interpretation, independently evaluated the HRCT findings. After assessing the interobserver agreement, the final decision was made by consensus. The radiologists were blinded to clinical information.
Measurement of periostin by ELISA
Duplicated serum samples were obtained from study participants and then stored at − 80 °C until human periostin ELISA assay, which we previously established33.
Statistical analysis
Data are expressed as the median (25th–75th percentiles of the interquartile range) or least squares mean ± standard error. Differences between two groups were analyzed as appropriate using the Wilcoxon rank-sum test or Fisher’s exact test. Associations between two groups were analyzed using the Spearman’s rank correlation coefficient. The agreement between two independent observers was assessed using Cohen’s kappa statistics when classifying HRCT patterns according to the IPF guidelines. Progression of IPF was defined as a greater than 5% decrease in the FVC over 6 or 12 months based on a previous report6. Using the Kaplan–Meier method, the overall survival was compared by log-rank test and cut-off levels were set based on previous studies as follows: DLCO at baseline, 39%; monomeric and periostin level, 15 ng/mL and 100 ng/mL, respectively; and decrease in FVC and DLCO over 6 or 12 months, 5% and 15%, respectively6,7,8,33. In Cox proportional hazards model analysis, we detected variables significantly (P < 0.05) associated with overall survival by univariate analyses. Cox analysis adjusted for age, gender, FVC, and DLCO at baseline as confounding factors was performed to assess whether baseline changes in serum monomeric and total periostin levels were associated with overall survival by multivariate analysis. For evaluating the association between biomarker level and nintedanib effectiveness, relative changes in VC and DLCO during the 6-month period after baseline were compared between the study participants (treatment group) and historical controls according to biomarker-high and -low groups. The median levels of each biomarker in the study participants were set as the cut-off levels. Data of the treatment group and historical controls were compared by multiple regression analysis adjusted for age, VC, and DLCO at baseline. P < 0.05 was taken to represent statistical significance. All statistical analyses were performed using R Statistical Software (version R 4.2.0; R Foundation for Statistical Computing, Vienna, Austria) and JMP 14.0 (SAS Institute Japan, Tokyo, Japan).
Ethical approval and consent to participate
We received specific approval for all procedures from the Institutional Review Board (IRB) of Kurume University School of Medicine and collaborative facility in accordance with the ethical standards of the Helsinki Declaration of 2013. The approval numbers (date of IRB approval) of our study are 08067 (August 2, 2008), 11216 (February 29, 2012), and 15096 (August 11, 2015). Written informed consent was obtained from all patients.
Results
Characteristics and outcomes
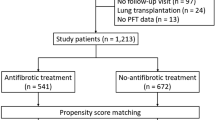
Study enrollment is shown in Fig. 1. Among 112 enrolled patients with IPF, 12 patients did not meet the study criteria, because of the failure to meet to HRCT criteria of UIP in 11 patients or serum collection in 1 patient. Among 100 eligible patients, 13 patients dropped out of the study because of discontinuation of nintedanib therapy in 12 patients and failure to visit the facility within 3 months after baseline in 1 patient. Eighty-seven patients completed the study, including 78 males and 75 ex-smokers and 1 current smoker, with a median age of 72.0 (68.0–76.0) years. The agreement between two independent observers in classifying HRCT patterns was good (kappa value, 0.687; P < 0.001). The patients’ characteristics and outcomes are shown in Table 1. Fifteen of 87 patients (17%) underwent surgical lung biopsy, among which histological classifications included definite and possible UIP pattern in 12 (80%) and 3 (20%) patients, respectively. Monomeric and total periostin levels in collected sera at baseline were 12.2 (9.6–15.9) ng /mL and 83.0 (66.0–106.0) ng /mL, respectively. Serum levels of LDH, KL-6, and SP-D and FVC, VC, and DLCO were 215.0 (192.0–236.0) IU/L, 802.0 (613.0–1160.0) IU/mL, 272.0 (161.0–358.0) ng/mL, 71.7 (60.9–80.1)%, 69.8 (60.7–80.1)%, and 57.1 (48.7–67.9)% at baseline. Among 87 study participants, corticosteroid therapies and long-term oxygen therapies were performed in 6 (7%) and 8 (9%) patients at baseline. During 1174.0 (928.0–1537.0) days of observation, 23 (26%) patients developed acute exacerbation and 36 (41%) died. Nintedanib therapy was discontinued in 30 (35%) of 87 patients during the observation period. The median duration of treatment with nintedanib was 634.0 (371.0–1239.0) days.
Correlations between clinical parameters and changes in pulmonary function
We analyzed whether serum periostin levels were associated with changes in pulmonary function (Table 2). None of the analyzed biomarkers comprising monomeric, total periostin, LDH, KL-6, or SP-D at baseline were significantly associated with relative changes in FVC or DLCO at any follow-up period (Table 2). Similarly, changes in these biomarkers over 6 or 12 months were not significantly associated with relative changes in FVC nor DLCO in any follow-up period (data not shown). In contrast, GAP score at baseline was significantly associated with relative changes in FVC over 12 months (R = − 0.23, P = 0.042), and age was significantly associated with relative change in DLCO over 6 and 12 months (R = − 0.26 and − 0.27, P = 0.0098 and 0.043).
Moreover, we compared serum biomarker levels between progressive and non-progressive patients after the start of treatment with nintedanib. The results of comparing monomeric and total periostin levels between patients with and without a decrease greater than 5% over 6 or 12 months is shown in Fig. 2. Among 80 patients who underwent a pulmonary function test 6 months after the start of nintedanib, 27 (34%) had a greater than 5% decrease in the FVC. Among 71 patients who underwent a pulmonary function test 12 months after the start of nintedanib, 38 (54%) had a greater than 5% decrease in the FVC. There were no significant differences in monomeric and total periostin levels between patients with and without progression in the analyses of 6- and 12-month changes as follows: monomeric periostin, 11.0 (8.4–14.5) ng/mL vs 12.5 (10.3–16.1) ng/mL (P = 0.084) and 12.9 (9.9–16.6) ng/mL vs 11.1 (9.2–14.7) ng/mL (P = 0.19); total periostin, 80.0 (58.0–102.0) ng/mL vs. 85.0 (69.0–106.0) ng/mL (P = 0.44) and 81.0 (66.0–109.0) ng/mL vs. 88.0 (66.0–97.0) ng/mL (P = 0.84). Similarly, there were no significant differences in other biomarker levels between patients with and without progression in the analyses of 6- and 12-month changes as follows: LDH, 217.0 (197.0–234.0) IU/L vs. 208.0 (189.5–236.0) IU/L (P = 0.51) and 216.5 (193.5–239.3) vs 214.0 (194.0–228.5) IU/L (P = 0.61); KL-6, 797.0 (691.0 vs 1286.0) IU/mL vs 855.0 (598.5–1160.0) IU/mL (P = 0.58) and 837.0 (611.0–1319.5) IU/mL vs 774.7 (594.0–1149.0) IU/mL (P = 0.54); SP-D, 275.0 (222.0 vs. 311.0) IU/mL vs. 272.0 (137.0–415.0) IU/mL (P = 0.85) and 273.5 (161.8–352.8) IU/mL vs. 275.0 (127.0–433.0) IU/mL (P = 0.88). Neither serum monomeric nor total periostin level was associated with changes in pulmonary function for up to 12 months.
Comparisons of periostin levels between progressive and non-progressive patients after start of nintedanib. Serum monomeric and total periostin levels were compared between progressive and non-progressive participants after start of nintedanib. Monomeric (A) and total (B) periostin levels were compared between participants with and without a decrease in FVC by more than 5% over a 6 months period. Moreover, monomeric (C) and total (D) periostin levels were compared between participants with and without a decrease in FVC by more than 5% over a 12 months period. FVC, forced vital capacity. *P < 0.05 was considered to represent statistical significance.
Analysis of risk factors associated with outcome using Cox proportional hazards model
The results of the Cox proportional hazards model are shown in Table 3. We attempted to clarify whether baseline or changes in serum periostin levels were associated with overall survival. Among clinical data at baseline, higher monomeric (relative risk (RR) 6.1; 95% confidential interval (CI) 1.0–36.0; P = 0.047) and total periostin (RR 6.6; 95%CI 1.2–36.3; P = 0.031) and lower DLCO (RR 0.012; 95%CI 0.00074–0.19; P = 0.0018) were significantly associated with shortened overall survival in the univariate analysis (Table 3). While adjusting for some combinations of confounding factors that are potentially associated with shortened overall survival including age, gender, FVC, and DLCO at baseline in the multivariate analysis, higher monomeric and total periostin levels at baseline remained significant or tended to be risk factors of shortened overall survival (Table 4A,B). In the univariate analysis of changes in clinical data over 6 months, only lower changes in DLCO were significantly associated with shortened overall survival (RR 0.027; 95%CI 0.0032–0.23; P = 0.0010), with lower changes in FVC tending to be associated (RR 0.059; 95%CI 0.0035–1.0; P = 0.050) (Table 3). Similarly, higher changes in total periostin (RR 57.0; 95%CI 4.0–801.0; P = 0.0027) and lower changes in FVC (RR 0.027; 95%CI 0.0029–0.25; P = 0.0014) and DLCO (RR 0.014; 95%CI 0.0012–0.15; P = 0.00050) over 12 months were significantly associated with shortened overall survival (Table 3). While adjusting for some combinations of changes in FVC and/or DLCO, risk factors of mortality in the multivariate analysis, neither change in monomeric and total periostin levels was significantly associated with overall survival (Table 4C,D). Baseline levels and changes in biomarkers other than periostin, including LDH, KL-6, and SP-D, were not associated with overall survival (Table 3). Variables that were significantly associated or tended to be associated with the overall survival in the univariate analysis of the Cox proportional hazards model including monomeric periostin, total periostin, DLCO, decrease in FVC and DLCO over 6 months, and decrease in FVC and DLCO over 12 months were analyzed using the Kaplan–Meier method (Fig. 3). Among these variables, a high decrease in the FVC over 12 months (P = 0.0031) and DLCO over 6 and 12 months (P = 0.0047 and 0.0065) were significantly associated with a shortened overall survival. Moreover, neither serum levels of monomeric nor total periostin, LDH, KL-6, nor SP-D were associated with incidence of acute exacerbation of IPF (data not shown). In the present study, monomeric and total periostin levels at baseline were independent risk factors for mortality but not other biomarkers including LDH, KL-6, and SP-D.
Kaplan–Meier curves for overall survival. Survival curves for overall survival using the Kaplan–Meier Method. Variables significantly associated or tended to be associated with overall survival in univariate analysis of the Cox proportional hazards model including monomeric periostin (A), total periostin (B), DLCO (C), decreases in FVC (D) and DLCO (E) over 6 months period, and decreases in FVC (F) and DLCO (G) over 12 months period were analyzed using log-rank test. FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide. *P < 0.05 was considered to represent statistical significance.
Analyses of the associations between serum periostin levels and nintedanib effectiveness
A comparison of clinical data at baseline (the time of first measurement of biomarkers) and relative changes in VC and DLCO over 6 months in 80 patients in the present study (treatment group) and 43 in a historical control group (antifibrotic drug-naive patients) are shown in Table 5 and Fig. 4. Seven patients in the treatment group whose pulmonary function at 6 months from baseline could not be measured were excluded. Patients in the treatment group were significantly older and had significantly lower VC than the historical control group (Table 5). There is no significant difference of ILD pattern on HRCT, gender, and rate of smokers and patients treated corticosteroid (Table 5). Decreases in both VC and DLCO in the treatment group were significantly milder than in the historical controls (Fig. 4A,B). A comparison of changes in VC and DLCO according to biomarker-high and -low groups are shown in Fig. 4C–L. In the high monomeric periostin group, decrease in VC in the treatment group was significantly milder than in the historical controls, whereas there was no difference between the two groups in the low expression group (Fig. 4C). In contrast, decrease in VC was significantly milder in the treatment group than in the historical control group, regardless of biomarker levels other than monomeric periostin (Fig. 4E,G,I,K). Among patients with high monomeric and total periostin, KL-6, and LDH, decrease in DLCO in the treatment group was significantly milder than in the historical controls, whereas there was no difference between the two in the low expression group of these biomarkers (Fig. 4D,F,H,L). In contrast, decreased DLCO was significantly milder in the treatment group than in the historical controls, regardless of SP-D level (Fig. 4J). This suggested that some biomarkers were associated with short-term suppressive effects on decrease in pulmonary function following treatment with nintedanib. Among the biomarkers analyzed in the present study, only monomeric periostin was associated with the suppressive effects on decreases in both VC and DLCO.
Analysis of the associations between biomarker levels and nintedanib efficacy. As the historical control data, we used serum biomarker levels measured in 43 antifibrotic drug-naive patients with IPF who were selected from 60 patients who participated in previously published studies28,31. For evaluating the association between biomarker levels and efficacy of nintedanib, relative changes in VC and DLCO from baseline over 6 months were compared between the study participants (treatment group) and historical controls according to biomarker-high and -low groups. The median of each biomarker in the present study was set as the cut-off level. Statistical analysis for comparing two groups was performed by multiple regression analysis adjusted for age, VC, and DLCO at baseline. Changes in VC, and DLCO in the treatment group are shown by white spots on the black background bar, and those in the historical control group are shown by black spots on the white background bar. Comparisons of VC (A) and DLCO (B) in all patients in the treatment group and in historical controls are shown. The results of the comparison of decrease in VC are shown in (C) (monomeric periostin), (E) (total periostin), (G) (KL-6), (I) (SP-D), and (K) (LDH), and those of decrease in DLCO are shown in (D) (monomeric periostin), (F) (total periostin), (H) (KL-6), (J) (SP-D), and L (LDH). Data are expressed as the least squares mean ± standard error unless otherwise stated. LDH, lactate dehydrogenase; KL-6, Krebs von den Lungen-6; SP-D, surfactant protein D; VC, vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide. *P < 0.05 was considered to represent statistical significance.
Discussion
In the present study, serum monomeric and total periostin were independent risk factors of shortened overall survival but not other biomarkers such as LDH, KL-6, or SP-D in patients treated with nintedanib. Neither monomeric nor total periostin levels were significantly associated with relative changes in FVC or DLCO for up to 1 year. In the analysis of the association between serum biomarker levels and effectiveness of nintedanib therapy, some biomarker levels, particularly those of monomeric periostin, were associated with a suppressive effect on decrease in pulmonary function.
Participants in many previous studies of prognostic factors for ILD, such as GAP stage, were not limited to those treated with antifibrotic drugs14,15. In the INMARK trial, a phase 3 RCT of nintedanib therapy for IPF patients with a diagnosis made within the previous 3 years and more than 80% of FVC, the product of C-reactive protein degraded by matrix metalloprotease was associated with changes in FVC for 52 weeks but not the reducing rate of decrease in FVC by nintedanib17. The post-hoc analysis of a phase 3 RCT of pirfenidone therapy (ASCEND and CAPACITY 1 and 2 trials) for IPF suggested that evaluations of a combination of CCL18, C-X-C motif chemokine ligand 14, and total periostin were better at predicting the prognosis and therapeutic effect compared with any single biomarker21. Adegunsoye et al. performed a pooled, multicenter, propensity-matched analysis of IPF patients with and without antifibrotic drug exposure. In this study, CA-125, CXCL13, MMP7, chitinase-3-like protein-1, and osteopontin predicted differential transplant-free survival in antifibrotic drug-exposed patients but at higher thresholds than in antifibrotic drug-non-exposed individuals37. Yoshikawa et al. showed that increased serum levels of SP-A was an independent predictor of disease progression in 43 IPF patients who were treated with antifibrotic drugs38.
Decrease in pulmonary function is the most important prognostic factor and the endpoint of prognostic biomarker studies39. The result showing no significant association between periostin levels and decrease in pulmonary function in the present study was inconsistent with those in previous studies performed by our and other study groups30,31,33. In these previous studies, many study participants were not treated with antifibrotic drugs, whereas all present study participants were treated with nintedanib. The lack of association between periostin levels and decrease in pulmonary function may have been caused by suppressing effect by nintedanib therapy. To resolve this issue, association between serum periostin levels and changes in pulmonary function over 1 year from baseline should be analyzed in a further study.
Treatment with antifibrotic drugs can cause some adverse events such as gastrointestinal involvement, resulting in high medical expenses9,10,12. Thus, one of the significant clinical questions of treatment for IPF is whether early intervention with antifibrotic drug therapy for patients with mild disease is appropriate40,41. A quantitative, point-in-time data surveillance study of IPF showed that antifibrotic drug use was 60% for all patients, compared with only 37% for those with milder disease with FVC > 75% in Japan at 201940. The results of the present study suggest that early intervention with nintedanib therapy is recommended for patients with high serum periostin levels because they have high mortality and therapeutic responses to nintedanib. Precision medicine in IPF clinical practice remains an unmet need when using biomarkers to predict prognosis and therapeutic effectiveness. Because tailored therapies based on precision medicine can improve treatment outcomes by the early intervention for patients who can benefit from treatment, the development of new biomarkers is needed42. Establishment of such biomarkers predicting outcomes of IPF could contribute to decision-making in therapeutic management.
The present study has some limitations. First, the sample size was small. We will plan further studies in larger populations. Second, in the analysis of the association between biomarker levels and changes in pulmonary function, the presence of cases in which pulmonary function could not be measured over time because of worsening conditions should be considered as omitted variable bias. Third, the analysis of associations between biomarker levels and therapeutic effectiveness was limited to comparisons with historical controls. The use of historical controls might cause selection and/or temporal bias due to the patient characteristics or standard of care. However, we think that the results in the present study are reliable because we performed multiple regression analysis using the treatment group and historical controls, adjusting for the VC, DLCO, and age. Fourth, in the present study, we set the endpoint as FVC for analyzing the association between biomarker levels and outcome, and VC for comparisons between the treatment group and historical controls, because the endpoint was VC but not FVC in our previous study.
Conclusions
We showed that serum levels of monomeric and total periostin but not other biomarkers were associated with overall survival in IPF patients who received nintedanib. Moreover, some biomarkers, particularly monomeric periostin, were associated with a suppressive effect on decrease in pulmonary function by nintedanib. Periostin assessments may contribute to determining therapeutic strategies for patients with IPF.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Raghu, G. et al. Idiopathic pulmonary fibrosis (an Update) and progressive pulmonary fibrosis in adults: An Official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 205, e18–e47 (2022).
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, 44–68 (2018).
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824 (2011).
Natsuizaka, M. et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 190, 773–779 (2014).
Flaherty, K. R. et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am. J. Respir. Crit. Care Med. 168, 543–548 (2003).
Zappala, C. J. et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur. Respir. J. 35, 830–836 (2010).
Latsi, P. I. et al. Fibrotic idiopathic interstitial pneumonia: The prognostic value of longitudinal functional trends. Am. J. Respir. Crit. Care Med. 168, 531–537 (2003).
Mogulkoc, N. et al. Greater Manchester Pulmonary Fibrosis Consortium. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am. J. Respir. Crit. Care Med. 164, 103–108 (2001).
Richeldi, L. et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082 (2014).
King, T. E. Jr. et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2083–2092 (2014).
Petnak, T., Lertjitbanjong, P., Thongprayoon, C. & Moua, T. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Chest 160, 1751–1763 (2021).
Homma, S. et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig. 56, 268–291 (2018).
Ley, B. et al. Predictors of mortality poorly predict common measures of disease progression in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 194, 711–718 (2016).
Ryerson, C. J. et al. Predicting survival across chronic interstitial lung disease: The ILD-GAP model. Chest 145, 723–728 (2014).
Kondoh, S. et al. Validation of the Japanese disease severity classification and the GAP model in Japanese patients with idiopathic pulmonary fibrosis. Respir. Investig. 54, 327–333 (2016).
Kreuter, M. et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 204, 74–81 (2021).
Maher, T. M. et al. Biomarkers of extracellular matrix turnover in patients with idiopathic pulmonary fibrosis given nintedanib (INMARK study): A randomised, placebo-controlled study. Lancet Respir. Med. 7, 771–779 (2019).
Organ, L. A. et al. Biomarkers of collagen synthesis predict progression in the PROFILE idiopathic pulmonary fibrosis cohort. Respir. Res. 20, 148 (2019).
Rosas, I. O. et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 5, e93 (2008).
Prasse, A. et al. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 179, 717–723 (2009).
Neighbors, M. et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: Post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir. Med. 6, 615–626 (2018).
Yokoyama, A. et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 11, 164–168 (2006).
Takahashi, H. et al. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am. J. Respir. Crit. Care Med. 162, 1109–1114 (2000).
Ishikawa, N., Hattori, N., Yokoyama, A. & Kohno, N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir. Investig. 50, 3–13 (2012).
Conway, S. J. et al. The role of periostin in tissue remodeling across health and disease. Cell Mol. Life Sci. 71, 1279–1288 (2014).
Yamaguchi, Y. et al. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br. J. Dermatol. 168, 717–725 (2013).
Takayama, G. et al. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 118, 98–104 (2006).
Okamoto, M., Izuhara, K., Ohta, S., Ono, J. & Hoshino, T. Ability of periostin as a new biomarker of idiopathic pulmonary fibrosis. Adv. Exp. Med. Biol. 1132, 79–87 (2019).
Uchida, M. et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 46, 677–686 (2012).
Okamoto, M. et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur. Respir. J. 37, 1119–1127 (2011).
Naik, P. K. et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am. J. Physiol .Lung Cell Mol. Physiol. 303, L1046-1056 (2012).
Tajiri, M. et al. Serum level of periostin can predict long-term outcome of idiopathic pulmonary fibrosis. Respir. Investig. 53, 73–81 (2015).
Ohta, S. et al. The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PLoS ONE 12, e0174547 (2017).
Shimizu, H. et al. Association of serum monomeric periostin level with outcomes of acute exacerbation of idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. Ann. Transl. Med. 9, 739 (2021).
Nukui, Y. et al. Periostin as a predictor of prognosis in chronic bird-related hypersensitivity pneumonitis. Allergol. Int. 68, 363–369 (2019).
Collard, H. R. et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176, 636–643 (2007).
Adegunsoye, A. et al. Circulating plasma biomarkers of survival in antifibrotic-treated patients with idiopathic pulmonary fibrosis. Chest 158, 1526–1534 (2020).
Yoshikawa, T. et al. Surfactant protein A as a biomarker of outcomes of anti-fibrotic drug therapy in patients with idiopathic pulmonary fibrosis. BMC Pulm Med. 20, 27 (2020).
Inoue, Y. et al. Diagnostic and prognostic biomarkers for chronic fibrosing interstitial lung diseases with a progressive phenotype. Chest 158, 646–659 (2020).
Lancaster, L. et al. Idiopathic pulmonary fibrosis: Physician and patient perspectives on the pathway to care from symptom recognition to diagnosis and disease burden. Respirology 27, 66–75 (2022).
Salisbury, M. L. et al. Antifibrotic drug use in patients with idiopathic pulmonary fibrosis. Data from the IPF-PRO registry. Ann. Am. Thorac. Soc. 17, 1413–1423 (2020).
Karampitsakos, T., Juan-Guardela, B. M., Tzouvelekis, A. & Herazo-Maya, J. D. Precision medicine advances in idiopathic pulmonary fibrosis. EBioMedicine. 95, 104766 (2023).
Acknowledgements
We thank Mr. Yoshinori Azuma (Shino-Test Corporation, Kanagawa, Japan, M.S.) for measuring monomeric and total periostin by ELISA, and Yoshinori Kawabata (Saitama Prefectural Cardiovascular and Respiratory Center, Japan, M.D., Ph.D.) and Junya Fukuoka (Nagasaki University, Nagasaki, Japan, M.D., Ph.D.) for advising on the histological diagnoses.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 19K08637, 22K08248: TH) from the Ministry of Education, Culture, Science, Sports and Technology, Japan and Kakihara Science Technology Foundation (March 2022, TH). The study expenses were paid to the Division of Respirology, Rheumatology, and Neurology, Department of Internal Medicine, Kurume University School of Medicine. The enzyme-linked immunosorbent assay of periostin in the present study was performed free of charge by Shino-Test Co., Ltd.
Ethics declarations
Competing interests
Masaki Okamoto, Hiroshi Mukae, Yasunari Miyazaki, Tomohiro Handa, Naoki Hamada, and Tomoaki Hoshino received more than 1,000,000 JPY as research funding from Nippon Boehringer-Ingelheim, co., ltd. Kiminori Fujimoto received more than 1,000,000 JPY as research funding from Micron KK. Satoshi Ikeda received more than 1,000,000 JPY as research funding from Chugai pharmaceutical co., ltd. and AstraZeneca pharmaceuticals co., ltd. Kazuhiro Yatera received more than 1,000,000 JPY as research funding from FUJIFILM, co., ltd., Taiho pharmaceuticals co., ltd., GlaxoSmithKline K.K., Insmed Inc., Bayer pharmaceuticals co., ltd. Hirotaka Nishikiori received more than 1,000,000 JPY as research funding from Nippon Nippon Boehringer-Ingelheim, co., ltd. and M3, Inc., Tokyo. Yoshinori Tanino received more than 1,000,000 JPY as research funding from Nippon Boehringer-Ingelheim, co., ltd. and StemRIM Inc. Hironao Hozumi received more than 1,000,000 JPY as research funding from TERUMO LIFE SCIENCE FOUNDATION. Tomohiro Handa received more than 1,000,000 JPY as research funding from FUJIFILM, co., ltd. Hiroshi Ishii received more than 1,000,000 JPY as research funding from Shionogi pharmaceuticals co., ltd. Kenji Izuhara received more than 1,000,000 JPY as research funding from Shino test co., ltd., AstraZeneca pharmaceuticals co., ltd., and Torii Pharmaceutical co., ltd. Masaki Okamoto, Hiroshi Mukae, Noriho Sakamoto, Takashi Ogura, Yoshikazu Inoue, Yasunari Miyazaki, Motoyasu Kato, Hiroshi Ishii, Satoshi Konno, Arata Azuma and Takafumi Suda received more than 500,000 JPY as honoraria from Nippon Boehringer-Ingelheim, co., ltd. Takeshi Johkoh received more than 500,000 JPY as honoraria from Nippon Boehringer-Ingelheim, co., ltd., Kyorin Pharmaceutical co., ltd., and AstraZeneca pharmaceuticals co., ltd. Satoshi Ikeda received more than 500,000 JPY as honoraria from Ono Pharmaceutical co., ltd., Chugai pharmaceutical co., ltd., Pfizer Japan inc., AstraZeneca pharmaceuticals co., ltd., Bristol-Myers Squibb Company. Yasuhiro Kondoh and Yasuhiko Yamano received more than 500,000 JPY as honoraria from Nippon Boehringer-Ingelheim, co., ltd. and Bristol-Myers Squibb, co., ltd. Kosaku Komiya received more than 500,000 JPY as honoraria from Nippon Boehringer-Ingelheim, co., ltd. and Kyorin Pharmaceutical, co., ltd. Kazuhiro Yatera received more than 500,000 JPY as honoraria from Nippon Boehringer-Ingelheim, co., ltd., AstraZeneca pharmaceuticals co., ltd., GlaxoSmithKline K.K., Novartis Pharma, co., ltd., Kyorin Pharmaceutical, co., ltd. Toru Tsuda received more than 500,000 JPY as honoraria from AstraZeneca pharmaceuticals co., ltd. Hiroshi Ohnishi received more than 500,000 JPY as honoraria from Novartis Pharma, co., ltd. Noboru Hattori received more than 500,000 JPY as honoraria from Nippon Boehringer-Ingelheim, co., ltd., and AstraZeneca pharmaceuticals co., ltd. Takafumi Suda received more than 500,000 JPY as honoraria from Nippon Boehringer-Ingelheim, co., ltd. and AstraZeneca pharmaceuticals co., ltd. Takashi Ogura and Toru Tsuda received more than 500,000 JPY as manuscript fees from Nippon Boehringer-Ingelheim, co., ltd. Noriho Sakamoto, Yasunari Miyazaki, and Satoshi Konno received more than 1,000,000 JPY as donations from Nippon Boehringer-Ingelheim m, co., ltd. Kazuhiro Yatera received more than 1,000,000 JPY as donations from TEIJIN PHARMA co., ltd. and Taiho pharmaceuticals co., ltd. Kenji Izuhara received more than 1,000,000 JPY as donations from Shino test co., ltd. Tomohiro Handa belongs to an endowed department sponsored by TEIJIN PHARMA., co., ltd. Satoshi Konno belongs to an endowed department sponsored by Nippon Boehringer-Ingelheim m, co., ltd. Yoshikazu Inoue is a clinical trial advisor of Boehringer Ingelheim, Inc, Taiho Pharma, Kyorin Pharmaceutical co.ltd., Roche/Promedior co.ltd., Galapagos, CSL Behring, Vicore Pharma AB, Savara Inc. The enzyme-linked immunosorbent assay of periostin in the present study was performed free of charge by Shino-Test Co., Ltd. Other co-authors have no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okamoto, M., Fujimoto, K., Johkoh, T. et al. A prospective cohort study of periostin as a serum biomarker in patients with idiopathic pulmonary fibrosis treated with nintedanib. Sci Rep 13, 22977 (2023). https://doi.org/10.1038/s41598-023-49180-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49180-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.