Abstract
This study aimed to investigate the relationship between serum phosphate levels, changes in serum phosphate levels, and 28-day mortality in patients with septic shock. In this retrospective study, data were collected from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database between 2008 and 2019. Patients were divided into three groups according to the tertiles of serum phosphate levels. Kaplan–Meier curves and log-rank test analyses were used for survival analysis. Multivariate logistic regression, and restricted cubic spline (RCS) curve were used to explore the association between serum phosphate, delta serum phosphate levels and 28-day mortality. In total, 3296 patients with septic shock were included in the study, and the 28-day mortality was 30.0%. Serum phosphate levels were significantly higher in the non-survivor group than in the survivor group. The Kaplan–Meier curves showed significant differences among the three groups. Multivariate logistic regression analysis and the RCS curve showed that serum phosphate levels were independently and positively associated with the 28-day mortality of septic shock. Non-survivors had higher delta serum phosphate levels than survivors. Survival analysis showed that patients with higher delta serum phosphate levels had higher 28-day mortality. A non-linear relationship was detected between delta serum phosphate and 28-day mortality with a point of inflection at − 0.3 mg/dL. Serum phosphate levels were positively and independently associated with 28-day mortality in septic shock. Delta serum phosphate level was a high-risk factor for patients with septic shock.
Similar content being viewed by others
Introduction
Sepsis is a life-threatening organ dysfunction caused by a non-homeostatic host response to infection. Septic shock is a lethal complication of sepsis characterized by persistent tissue hypoperfusion after adequate fluid resuscitation1. Numerous studies have reported that septic shock affects 10%–30% of patients admitted to the intensive care unit (ICU) and causes an increased mortality of approximately 45–63%2,3,4. Despite the adoption of multiple measures, septic shock is still associated with high mortality, prolonged hospitalization, and increased hospital costs, and has become a major public health issue worldwide2,5,6. Early identification of high-risk factors in patients with septic shock might help guide clinical practice and reduce mortality rates7.
Phosphorus plays a crucial role in the maintenance of cellular integrity and organ function8,9,10. Phosphate refers to the inorganic phosphorus that exerts multiple physiological functions, such as membrane transport, energy metabolism, skeletal mineralization, and muscle contraction9,11. Numerous studies have revealed that higher serum phosphate levels are associated with adverse outcomes in various diseases, including chronic kidney disease (CKD), acute ischemic stroke, blunt trauma, and chronic obstructive pulmonary disease (COPD)11,12,13,14. Several recent studies have indicated that serum phosphate disturbances contribute to worse sepsis outcomes8,11,15,16. However, the association between serum phosphate levels and 28-day mortality in patients with septic shock remains unclear. Therefore, in this retrospective study, we aimed to explore the relationship between serum phosphate levels and 28-day mortality in patients with septic shock using the Medical Information Mart for Intensive Care IV (MIMIC-IV) database.
Materials and methods
Data source
All the data analyzed in our study were extracted from the MIMIC-IV database. The MIMIC-IV database is a large and publicly accessible critical care database that consists of more than 60,000 patients admitted to the Beth Israel Deaconess Medical Center (BIDMC) between 2008 and 2019. We were permitted to access the database after completing online training in the Collaborative Institutional Training Initiative (CITI) program (Record ID: 46,785,473 for Zhenyu Peng). Our study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Study population
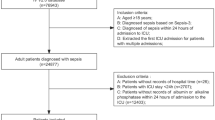
Adult patients diagnosed with septic shock were enrolled in this study according to the ICD-9 diagnostic code 78,552 and ICD-10 diagnostic code R6521 in the MIMIC-IV database (n = 7546). The following patients were excluded: (1) those with more than one ICU admission (n = 3338); (2) length of ICU stay less than 24 h (n = 465); (3) those without serum phosphate measurement in the first 24 h of ICU admission (n = 42); and (4) those without two or more detections of serum phosphate (n = 405); Finally, as shown in Fig. 1, 3296 patients diagnosed with septic shock were included in this study.
Data extraction
The following data were extracted by pgAdmin4 PostgreSQL from the MIMIC-IV database: (1) demographic characteristics: age, gender and weight; (2) vital signs: temperature, mean arterial pressure (MAP), saturation of peripheral oxygen (SpO2), heart rate, and respiratory rate; (3) laboratory tests: white blood cells (WBC), creatinine, sodium, calcium, serum phosphate, and lactate; (4) infection site: respiratory system, urinary system, digestive system and other sites; (5) comorbidities: congestive heart failure, chronic pulmonary disease, diabetes, renal disease, liver disease, malignant tumor, cerebrovascular disease, and peripheral vascular disease; (6) interventions: renal replacement therapy (RRT), ventilation and vasopressor use; (7) severity score: simplified acute physiology score (SAPS II) and sequential organ failure assessment (SOFA); (8) outcomes: ICU stay, in-hospital stay, ICU mortality, in-hospital mortality and 28-day mortality.
Statistical analysis
The sample size for our study was determined using conventional parameters—80% statistical power and a 5% significance level. Initially estimated at 133–198 patients based on prior research17,18,19, we increased the sample size to 3296 patients to enhance robustness. Continuous variables that were normally distributed or skewed were expressed as mean ± standard deviation (SD) or median with the first and third quartiles, respectively. Categorical variables were presented as numbers and percentages. Statistical differences were analyzed using Student’s t-test, Kruskal Wallis H test, Chi-squared test, or one way ANOVA, as appropriate. Patients with septic shock were divided into three groups based on the tertiles of serum phosphate values: T1 group (serum phosphate < 3.2 mg/dL, n = 1115), T2 group (3.2 mg/dL ≤ serum phosphate < 4.5 mg/dL, n = 1108) and T3 group (serum phosphate ≥ 4.5 mg/dL, n = 1073). Univariate and multivariate logistic regression analyses were performed to evaluate the hazard ratio (HR) of the covariates for 28-day mortality. We constructed Kaplan–Meier curves to illustrate the survival of patients in the different groups. Three models were used to minimize the effects of confounding factors. The crude model was not adjusted for the covariates. Model I was adjusted for age, gender, and weight. Model II was adjusted for all covariates in this study. A restricted cubic spline (RCS) curve was performed to reveal the dose–response relationship between serum phosphate levels and 28-day mortality. Moreover, the delta serum phosphate level was calculated as the difference between the initial serum phosphate level and the last serum phosphate level measured in the ICU. Kaplan–Meier curves were used to assess the 28-day survival probabilities of the high and low-delta serum phosphate groups. We performed RCS curve to determine the association between delta serum phosphate level and 28-day mortality. Stata version 15.0 (College Station, Texas, USA) and R software version 4.2.0 (R Foundation, Vienna, Austria) were used to perform the statistical analyses in this study. Statistical significance was defined as a two-sided P < 0.05.
Institutional review board statement
The MIMIC-IV database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and informed consent was obtained for the original data collection. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Baseline characteristics
As shown in Supplementary Table 1 and Supplementary Table 2, 3296 eligible patients with septic shock were enrolled in the study. Of these patients, 989 (30.0%) died within 28 days of ICU admission. Serum phosphate levels were significantly higher in the non-survivor group than in the survivor group. All patients were categorized into the T1 (n = 1115), T2 (n = 1108) and T3 (n = 1073) groups according to the tertiles of serum phosphate values. As displayed in Table 1, the patients in the T3 group had a higher proportion of males, and higher weight, WBC, creatinine, lactate, SAPS II, and SOFA as well as a higher prevalence of congestive heart failure, diabetes, renal disease, liver disease, and peripheral vascular disease. However, temperature, MAP, and sodium levels were lower in the T3 group. Interventions such as ventilation, RRT, and vasopressor use were frequently required in the T3 group. Patients with higher serum phosphate levels had longer ICU stay, and higher ICU mortality, in-hospital mortality, and 28-day mortality.
Univariate and multivariate analyses
Univariate and multivariate analyses were performed to assess the HR of the covariates for 28-day mortality in patients with septic shock as shown in the Supplementary Table 3. Univariate analysis showed that age, temperature, MAP, SpO2, heart rate, respiratory rate, creatinine, lactate, urinary system infection, digestive system infection, congestive heart failure, renal disease, liver disease, malignant tumor, peripheral vascular disease, RRT, ventilation, vasopressor use, SAPSII, SOFA and serum phosphate levels were associated with 28-day mortality. Multivariate analysis demonstrated that age, temperature, MAP, SpO2, heart rate, respiratory rate, creatinine, calcium, urinary system infection, digestive system infection, liver disease, malignant tumor, ventilation, SAPS II, SOFA, and serum phosphate levels were associated with 28-day mortality after adjusting for confounding factors.
Association between serum phosphate and 28-day mortality
Kaplan–Meier curves were constructed to illustrate the survival of patients with septic shock in the different groups. As shown in Fig. 2, the 28-day mortality rate was significantly higher in the T3 group than in the T2 and T1 groups (log-rank p < 0.001). The crude model, model I, and model II were used to explore the association between serum phosphate levels and 28-day mortality in septic shock. As shown in Table 2, serum phosphate levels were positively correlated with increased risk of 28-day mortality in the crude model (HR = 1.20, 95%CI:1.17–1.24, P < 0.001), model I (HR = 1.22, 95%CI:1.18–1.26, P < 0.001) and model II (HR = 1.07, 95% CI:1.02–1.12, P = 0.003). Serum phosphate levels were converted from continuous variables to categorical variables. Patients in the T3 group demonstrated a higher risk of 28-day mortality than those in the T1 group in all three models (T3 in crude model: HR = 2.34, 95%CI: 2.00–2.75, P < 0.001, P for trend < 0.001; T3 in model I: HR = 2.35, 95%CI: 2.00–2.75, P < 0.001, P for trend < 0.001; T3 in model II: HR = 1.40, 95%CI: 1.16–1.69, P = 0.001, P for trend = 0.001). The RCS curve was used to assess the dose–response relationship between serum phosphate levels and 28-day mortality. As shown in Fig. 3, a linear association was discovered between serum phosphate levels and 28-day mortality after adjusting for all confounders (P for non-linearity = 0.2).
Association between delta serum phosphate and 28-day mortality
As shown in Supplementary Figure 1, non-survivors had higher delta serum phosphate level than survivors. As shown in Supplementary Figure 2, Kaplan–Meier survival analysis demonstrated that patients with high delta serum phosphate level had higher 28-day mortality than those with low delta serum phosphate. Furthermore, as shown in Supplementary Figure 3, the RCS curve demonstrated a non-linear relationship between delta serum phosphate and 28-day mortality after adjusting for all confounders (P for non-linearity < 0.01). We used a linear regression model and a two-piecewise linear regression model to explore the association between delta serum phosphate level and 28-day mortality. As shown in Table 3, the two-piecewise linear regression model was superior for fitting the association because the p value for the log-likelihood ratio test was < 0.05. The inflection point of delta serum phosphate was − 0.3 mg/dL by threshold effect analysis. There was a positive association between them at delta serum phosphate level ≤ − 0.3 mg/dL (HR 1.07, 95% CI 1.01–1.04, P = 0.0331). The risk of 28-day mortality was increased significantly at delta serum phosphate level > − 0.3 mg/dL (HR 1.30, 95% CI 1.25–1.36, P < 0.0001).
Discussion
Septic shock is characterized by profound circulatory, cellular, and metabolic abnormalities and is the leading cause of death in hospitals1,2,3,20. Numerous studies have revealed that abnormalities in serum phosphate levels are associated with worse outcomes in various diseases21,22,23,24. However, the relationship between serum phosphate levels and 28-day mortality in patients with septic shock remains unclear. In this retrospective study, we analyzed 3296 patients with septic shock from the MIMIC-IV database and found that the non-survival group had significantly higher serum phosphate levels than the survival group. Serum phosphate levels were independently positively associated with and 28-day mortality in patients with septic shock. Delta serum phosphate level was significantly higher in non-survivors of septic shock. Patients with higher serum delta serum phosphate level had worse outcomes in patients with septic shock. The relationship between delta serum phosphate level and 28-day mortality was non-linear with a point of inflection at − 0.3 mg/dL.
In recent years, a series of risk factors have been identified as predictors of death from septic shock, including old age, serum lactate level, red blood cell distribution width, blood urea nitrogen level, creatinine level, and SOFA score25,26,27,28,29,30. Despite the development of therapeutic agents and strategies for treating septic shock, the mortality rate remains consistently high among critically ill patients4,31,32,33,34. Therefore, there is an urgent need to identify more effective indicators for evaluating outcomes of septic shock. Serum phosphate level is an easily accessible parameter in clinical setting35,36,37. Several studies have demonstrated that abnormal serum phosphate levels contribute to adverse outcomes in patients with various diseases. In the Chronic Renal Insufficiency Standards Implementation Study (CRISIS), Eddington et al. demonstrated that higher phosphate levels were associated with increased mortality in non-dialysis patients with CKD stages 3 and 412. Similarly, Campos-Obando et al. reported that hyperphosphatemia was related to increased all-cause mortality and COPD mortality in men based on the Rotterdam Study14. An observational study by Kim et al. showed that hyperphosphatemia was a strong predictor of 30-day mortality in patient with blunt trauma 11. Zhong et al. observed a U-shaped association between serum phosphate levels and all-cause mortality in a retrospective cohort study of 2944 patients with acute ischemic stroke13. Accumulating evidence has indicated that higher serum phosphate levels are significantly associated with worse outcomes in patients with sepsis16,38,39. However, no relevant studies have focused on the relationship between serum phosphate levels and the prognosis of septic shock. In the present study, we found that the serum phosphate level was positively and independently associated with the 28-day mortality of patients with septic shock after adjusting for potential confounders. Therefore, the serum phosphate level is a high-risk factor for death due to septic shock.
Phosphate is dynamically changing in the body8,15. Several studies have identified that changes in serum phosphate levels are predictive factors for adverse outcomes in critically ill patients21,22,23,24. Dekker et al. found that changes in serum phosphate levels during high-flux hemodialysis or hemodiafiltration are strongly related to the calcification propensity in dialysis patients40. Kim et al. showed that an increase in phosphate level at 48 h (delta phosphate > 0) was associated with an 8.62-fold increased risk of all-cause mortality in patients with acute kidney injury (AKI) undergoing continuous venovenous hemodiafiltration41. Wang et al. reported that delta phosphate level was associated with 28-day mortality in patients with septic AKI in a retrospective cohort42. However, the association between changes in serum phosphate levels and the 28-day mortality due to septic shock remains unclear. In this study, we found that higher delta serum phosphate level was associated with a higher risk of 28-day mortality in patients with septic shock. 28-day mortality increased dramatically when delta serum phosphate was ≥ -0.3 mg/dL. Therefore, delta serum phosphate level is a high-risk factor for patients with septic shock.
Hyperphosphatemia commonly occurs in patients with increased catabolism, tissue destruction, crush injuries, rhabdomyolysis, or hyperthermia15,43. Recent numerous studies have demonstrated that hyperphosphatemia is observed in various diseases. Manghat et al. reported that systemic infections caused cellular breakdown and release phosphate from the cells into the extracellular fluid, contributing to hyperphosphatemia44. Opie et al. demonstrated the increased coronary venous inorganic phosphate concentration caused by ATP utilization in hypoxic cardiomyocytes45. Tranquada et al. found that lactic acidosis transferred intracellular phosphate into the circulation, resulting in hyperphosphatemia during shock46. Therefore, systemic infections, tissue hypoperfusion, and lactic acidosis might contribute to hyperphosphatemia in septic shock. The mechanisms underlying the relationship between serum phosphate levels and mortality in patients with septic shock have not yet been fully elucidated. Several mechanisms may explain these observations. Hyperphosphatemia causes endothelial dysfunction and vascular calcification, resulting in impaired microcirculatory blood flow and organ dysfunction39,47. Accumulating evidences demonstrates that hyperphosphatemia contributes to inflammation, oxidative stress, and mitochondrial dysfunction, all of which are involved in the pathogenesis of septic shock16,35. Further studies are required to elucidate these mechanisms.
The strengths of this study are as follows: Firstly, it was a large cohort study with high-quality data from the MIMIC-IV database. Secondly, we adjusted potential confounders and reached representative and reliable conclusions. Thirdly, this is the first study to investigate the relationship between dynamic changes in serum phosphate levels and the 28-day mortality in patients with septic shock. However, this study has several limitations. Firstly, bias could not be avoided due to missing data and unmeasured variables in this retrospective study. Secondly, the diagnosis of septic shock was based on ICD-9 and ICD-10 codes and was different from sepsis 3.0, which might have limited generalizability. Thirdly, although we adjusted for creatinine and renal disease as covariates, it would have been better to exclude patients with renal dysfunction from further studies. Finally, data related to the consumption of foods containing inorganic phosphorus additives were unavailable in the MIMIC IV database. Therefore, multicenter prospective studies are required to confirm our findings.
Conclusion
Serum phosphate levels were positively and independently associated with 28-day mortality in septic shock. Delta serum phosphate level was a high-risk factor for patients with septic shock.
Data availability
Publicly available datasets were analyzed in this study. This data can be found on the MIMIC-IV database (https://mimic.physionet.org/).
References
Mervyn, S. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315(8), 287. https://doi.org/10.1001/jama.2016.0287 (2016).
Aguirre, U. & Urrechaga, E. Diagnostic performance of machine learning models using cell population data for the detection of sepsis: A comparative study. Clin. Chem. Lab. Med. 61(2), 356–365. https://doi.org/10.1515/cclm-2022-0713 (2023).
Font, M. D., Thyagarajan, B. & Khanna, A. K. Sepsis and Septic Shock—Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. N. Am. 104(4), 573–585. https://doi.org/10.1016/j.mcna.2020.02.011 (2020).
Kalimouttou, A., Lerner, I., Cheurfa, C., Jannot, A.-S. & Pirracchio, R. Machine-learning-derived sepsis bundle of care. Intensiv. Care Med. 49(1), 26–36. https://doi.org/10.1007/s00134-022-06928-2 (2023).
Al-Ashry, H., Abuzaid, A., Asim, M. & El-Menyar, A. Microcirculation alteration and biomarker dilemma in early septic shock diagnosis and treatment. Curr. Vasc. Pharmacol. 14(4), 330–344. https://doi.org/10.2174/1570161114666160226145732 (2016).
Jin, W. W. et al. Analysis of risk factors affecting the prognosis of septic shock and clinical intervention. J. Biol. Regul. Homeost. Agents. 35(1), 295–301. https://doi.org/10.23812/20-676-L (2021).
Zhao, Q.-Y. et al. A machine-learning approach for dynamic prediction of sepsis-induced coagulopathy in critically Ill patients with sepsis. Front. Med. 7, 637434. https://doi.org/10.3389/fmed.2020.637434 (2020).
Al Harbi, S. A. et al. Association between phosphate disturbances and mortality among critically ill patients with sepsis or septic shock. BMC Pharmacol. Toxicol. 22(1), 30. https://doi.org/10.1186/s40360-021-00487-w (2021).
Thongprayoon, C. et al. Hospital-acquired serum phosphate derangements and their associated in-hospital mortality. Postgrad. Med. J. 98(1155), 43–47. https://doi.org/10.1136/postgradmedj-2020-138872 (2022).
Tiong, M. K. et al. Serum phosphate and mortality in incident dialysis patients in Australia and New Zealand. Nephrol. Carlton Vic. 26(10), 814–823. https://doi.org/10.1111/nep.13904 (2021).
Kim, D. W., Jung, W. J., Lee, D. K., Lee, K. J. & Choi, H. J. Association between the initial serum phosphate level and 30-day mortality in blunt trauma patients. J Trauma Acute Care Surg. 91(3), 507–513. https://doi.org/10.1097/TA.0000000000003271 (2021).
Eddington, H. et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN. 5(12), 2251–2257. https://doi.org/10.2215/CJN.00810110 (2010).
Zhong, C. et al. Serum alkaline phosphatase, phosphate, and in-hospital mortality in acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 27(1), 257–266. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.041 (2018).
Campos-Obando, N. et al. Serum phosphate levels are related to all-cause, cardiovascular and COPD mortality in men. Eur. J. Epidemiol. 33(9), 859–871. https://doi.org/10.1007/s10654-018-0407-7 (2018).
Seers, T. & Davenport, R. Phosphate metabolism and respiratory alkalosis: A forgotten lesson in COVID-19. Age Ageing. 49(6), 927. https://doi.org/10.1093/ageing/afaa176 (2020).
Miller, C. J. et al. Impact of serum phosphate in mechanically ventilated patients with severe sepsis and septic shock. J. Intensiv. Care Med. 35(5), 485–493. https://doi.org/10.1177/0885066618762753 (2020).
Aguirre, U. & Urrechaga, E. Diagnostic performance of machine learning models using cell population data for the detection of sepsis: A comparative study. Clin. Chem. Lab. Med. CCLM. 61(2), 356–365. https://doi.org/10.1515/cclm-2022-0713 (2023).
Kalimouttou, A., Lerner, I., Cheurfa, C., Jannot, A.-S. & Pirracchio, R. Machine-learning-derived sepsis bundle of care. Intensiv. Care Med. 49(1), 26–36. https://doi.org/10.1007/s00134-022-06928-2 (2023).
Font, M. D., Thyagarajan, B. & Khanna, A. K. Sepsis and Septic Shock—Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. N. Am. 104(4), 573–585. https://doi.org/10.1016/j.mcna.2020.02.011 (2020).
Sirvent, J. M., Ferri, C., Baró, A., Murcia, C. & Lorencio, C. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am. J. Emerg. Med. 33(2), 186–189. https://doi.org/10.1016/j.ajem.2014.11.016 (2015).
Wen, T. et al. Association between admission serum phosphate and risk of acute kidney injury in critically ill patients with rhabdomyolysis: A retrospective study based on MIMIC-III. Injury. 54(1), 56456. https://doi.org/10.1016/j.injury.2022.10.024 (2023).
Yang, J., Cheng, Y., Wang, R. & Wang, Bo. Association between early elevated phosphate and mortality among critically ill elderly patients: a retrospective cohort study. BMC Geriatr. 22(1), 208. https://doi.org/10.1186/s12877-022-02920-z (2022).
Li, Z., Shen, T. & Han, Yi. Effect of serum phosphate on the prognosis of septic patients: A retrospective study based on MIMIC-IV database. Front. Med. 9, 728887. https://doi.org/10.3389/fmed.2022.728887 (2022).
Erritzøe-Jervild, M. et al. Hypophosphataemia is common in patients with aneurysmal subarachnoid haemorrhage. Acta Anaesthesiol. Scand. 65(10), 1431–1438. https://doi.org/10.1111/aas.13973 (2021).
Bruno, R. R. et al. ICU-mortality in old and very old patients suffering from sepsis and septic shock. Front. Med. 12, 1034 (2021).
Chen, H., Gong, S.-R. & Rong-Guo, Yu. Association between normalized lactate load and mortality in patients with septic shock: an analysis of the MIMIC-III database. BMC Anesthesiol. 21(1), 16. https://doi.org/10.1186/s12871-021-01239-3 (2021).
Ding, Q., Yingjie, Su., Li, C. & Ding, N. Red cell distribution width and in-hospital mortality in septic shock: A public database research. Int. J. Lab. Hematol. 44(5), 861–867. https://doi.org/10.1111/ijlh.13925 (2022).
Han, D. et al. Prognostic value of blood urea nitrogen/creatinine ratio for septic shock: An analysis of the MIMIC-III clinical database. BioMed Res. Int. 2021, 1–16 (2021).
Yue, S. et al. Construction and validation of a risk prediction model for acute kidney injury in patients suffering from septic shock. Dis Markers. 2022, 9367873. https://doi.org/10.1155/2022/9367873 (2022).
Liu, Z. et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand. J. Trauma Resusc. Emerg. Med. 27(1), 51. https://doi.org/10.1186/s13049-019-0609-3 (2019).
Lan, P. et al. Utilization of echocardiography during septic shock was associated with a decreased 28-day mortality: A propensity score-matched analysis of the MIMIC-III database. Ann. Transl. Med. 7(22), 662. https://doi.org/10.21037/atm.2019.10.79 (2019).
Tianyang, Hu., Qiao, Z. & Mei, Y. Urine output is associated with in-hospital mortality in intensive care patients with septic shock: A propensity score matching analysis. Front. Med. 8, 737654. https://doi.org/10.3389/fmed.2021.737654 (2021).
Maximiliano, M., Stefano, R., Giulio, M., Li-Wei, L., Riccardo, B. Prediction of septic shock onset in ICU by instantaneous monitoring of vital signs. Annual International Conf. of the IEEE Engineering in Medicine and Biology Society. 2768–2771 (2020). doi:https://doi.org/10.1109/EMBC44109.2020.9176276
Li, Q. et al. Origin of sepsis associated with the short-term mortality of patients: a retrospective study using the eICU collaborative research database. Int. J. General Med. 14, 50500. https://doi.org/10.2147/IJGM.S345050 (2021).
Jung, Y. H. et al. Prognostic value of serum phosphate level in adult patients resuscitated from cardiac arrest. Resuscitation 128, 56–62. https://doi.org/10.1016/j.resuscitation.2018.04.026 (2018).
Sternbach, G. L. & Varon, J. Severe hyperphosphatemia associated with hemorrhagic shock. Am. J. Emerg. Med. 10(4), 331–332. https://doi.org/10.1016/0735-6757(92)90013-n (1992).
Plantinga, L. C. et al. Serum phosphate levels and risk of infection in incident dialysis patients. Clin. J. Am. Soc. Nephrol. CJASN. 3(5), 1398–1406. https://doi.org/10.2215/CJN.00420108 (2008).
Wang, H. et al. Hyperphosphatemia rather than hypophosphatemia indicates a poor prognosis in patients with sepsis. Clin. Biochem. 91, 9–15. https://doi.org/10.1016/j.clinbiochem.2021.01.016 (2021).
Guo, C., Su, Y., He, L., Zeng, Z. & Ding, N. A non-linear positive relationship between serum phosphate and clinical outcomes in sepsis. Heliyon 8(12), e12619. https://doi.org/10.1016/j.heliyon.2022.e12619 (2022).
Dekker, M. et al. High-flux hemodialysis and high-volume hemodiafiltration improve serum calcification propensity. PloS One. 11(4), e0151508. https://doi.org/10.1371/journal.pone.0151508 (2016).
Kim, D. W. et al. Effect of Phoxilium on prognostic predictors in patients undergoing continuous venovenous hemodiafiltration. Kidney Res. Clin. Pract. 40(3), 457–471. https://doi.org/10.23876/j.krcp.20.217 (2021).
Wang, H. et al. The relationship and threshold of serum phosphate with regard to the 28-day mortality risk in sepsis patients undergoing continuous renal replacement therapy. J. Int. Med. Res. 48(1), 300060519831896. https://doi.org/10.1177/0300060519831896 (2020).
O’Connor, L. R., Klein, K. L. & Bethune, J. E. Hyperphosphatemia in lactic acidosis. N. Engl. J. Med. 297(13), 707–709. https://doi.org/10.1056/NEJM197709292971307 (1977).
Manghat, P., Sodi, R. & Swaminathan, R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 51(Pt 6), 631–656. https://doi.org/10.1177/0004563214521399 (2014).
Opie, L. H., Thomas, M., Owen, P. & Shulman, G. Increased coronary venous inorganic phosphate concentrations during experimental myocardial ischemia. Am. J. Cardiol. 30(5), 503–513. https://doi.org/10.1016/0002-9149(72)90041-0 (1972).
Tranquada, R. E., Grant, W. J. & Peterson, C. R. Lactic acidosis. Arch. Intern. Med. 117(2), 192–202 (1966).
Raikou, V. D. Serum phosphate and chronic kidney and cardiovascular disease: Phosphorus potential implications in general population. World J. Nephrol. 10(5), 76–87. https://doi.org/10.5527/wjn.v10.i5.76 (2021).
Funding
This work was supported by National Natural Science Foundation of China (81100221), Research Fund Project of Hunan Provincial Health Commission (B2014-023), Changsha Natural Science Foundation (kq2007055), Degree & Undergraduate Education Reform Project of Central South University (2022JGB076), and Hunan Provincial Natural Science Foundation (2022JJ30832).
Author information
Authors and Affiliations
Contributions
Writing manuscript: Q.H.; statistical analysis: W.N.; reviewing and editing: J.W.; conceptualization, data extraction, supervision: Z.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nan, W., Huang, Q., Wan, J. et al. Association of serum phosphate and changes in serum phosphate with 28-day mortality in septic shock from MIMIC-IV database. Sci Rep 13, 21869 (2023). https://doi.org/10.1038/s41598-023-49170-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49170-6
This article is cited by
-
Effect of early serum phosphate disorder on in-hospital and 28-day mortality in sepsis patients: a retrospective study based on MIMIC-IV database
BMC Medical Informatics and Decision Making (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.