Abstract
In-utero exposure to fine particulate matter (PM2.5) and specific sources and components of PM2.5 have been linked with lower birthweight. However, previous results have been mixed, likely due to heterogeneity in sources impacting PM2.5 and due to measurement error from using ambient data. Therefore, we investigated the effect of PM2.5 sources and their high-loading components on birthweight using data from 198 women in the 3rd trimester from the MADRES cohort 48-h personal PM2.5 exposure monitoring sub-study. The mass contributions of six major sources of personal PM2.5 exposure were estimated for 198 pregnant women in the 3rd trimester using the EPA Positive Matrix Factorization v5.0 model, along with their 17 high-loading chemical components using optical carbon and X-ray fluorescence approaches. Single- and multi-pollutant linear regressions evaluated the association between personal PM2.5 sources/components and birthweight, adjusting for gestational age, maternal age, race, infant sex, parity, diabetes status, temperature, maternal education, and smoking history. Participants were predominately Hispanic (81%), with a mean (SD) gestational age of 39.1 (1.5) weeks and age of 28.2 (6.0) years. Mean birthweight was 3295.8 g (484.1) and mean PM2.5 exposure was 21.3 (14.4) µg/m3. A 1 SD increase in the mass contribution of the fresh sea salt source was associated with a 99.2 g decrease in birthweight (95% CI − 197.7, − 0.6), and aged sea salt was associated with a 70.1 g decrease in birthweight (95% CI − 141.7, 1.4). Magnesium, sodium, and chlorine were associated with lower birthweight, which remained after adjusting for PM2.5 mass. This study found evidence that major sources of personal PM2.5 including fresh and aged sea salt were negatively associated with birthweight, with the strongest effect on birthweight from Na and Mg. The effect of crustal and fuel oil sources differed by infant sex with negative associations seen in boys compared to positive associations in girls.
Similar content being viewed by others
Introduction
Low birthweight (LBW) is an endemic negative health outcome, with an estimated 8.3% of newborns born in the United States (U.S.) having a birthweight below 2500 grams (g)1. LBW is known to be associated with several negative health outcomes, including infant mortality2,3, later-life obesity4, diabetes5, cardiovascular disease6,7, and impaired-cognitive development8,9. Many of these negative health outcomes often disproportionately affect race/ethnicity groups; for example, obesity and type-2 diabetes prevalence are highest in Hispanic and Black communities10,11.
Combined with the greater burden of some negative health outcomes faced by Hispanic and Black communities, they also experience the greatest cumulative burden of air pollution exposure12,13. Various epidemiological studies, including several meta-analyses across the world, have found a modest association between ambient air pollution exposure during the in-utero period and birthweight, including LBW14,15,16,17. Of these ubiquitous air pollutants, a moderate association between particulate matter (PM) with an aerodynamic diameter less than 2.5 µm (PM2.5) and lower birthweight has been found using both ambient18,19,20 and personal monitoring approaches for PM2.5 exposure assessment21,22. Exposure to PM2.5 likely creates a hostile intrauterine environment which is hypothesized to explain its toxic effects, and while the biological mechanisms behind this effect are still emerging, studies suggest that oxidative stress, DNA methylation, and endocrine disruption may be at play and may lead to placental inflammation and growth restriction23,24. A recent meta-analysis highlighted the 3rd trimester to be an important window of vulnerability for the effect of ambient PM2.5 on birthweight15, possibly due to this being the period where most fetal growth occurs and therefore, particularly susceptible to biological disruption25. This may be due to effects on the maternal inflammatory pathways in late pregnancy, which contribute to the risk of adverse pregnancy outcomes26.
However, while reviews have concluded that there is a relationship between exposure to ambient PM2.5 and decreases in birthweight15,17,27, there is a great deal of heterogeneity in the findings, possibly due to measurement error from estimating personal exposure to PM2.5 of outdoor origin from ambient data28,29. Additionally, this may also be explained in part by the fact that PM2.5 is actually a mixture of organic and inorganic chemicals, with specific sources and/or components of PM2.5 being shown to differ in their toxicity with respect to birthweight12,30,31.
In our prior study using personal monitoring in the 3rd trimester, there was not a pronounced association between total personal exposure to PM2.5 and birthweight; however, specific indoor sources (such as candle and incense smoke) and exposure to PM2.5 more impacted by sources of outdoor origin appeared to be more strongly associated with lower birthweight32. These earlier findings are in line with the literature, with studies showing exposure to traffic-related sources including on-road gasoline and diesel traffic were negatively associated with birthweight30,33,34. Also, secondhand smoke (SHS) exposure is associated with reduced birthweight and increased risk of LBW35,36. To date, it is unclear whether the risk associated with these sources may be driven by exposure to the source itself as a unique mixture of pollutants or by any of its specific marker or signature chemical components. Therefore, in this analysis, we propose to investigate the effects of both major contributing sources of personal PM2.5 and their high-loading or signature components on birthweight.
This is an important question since the chemical composition of PM2.5 in conjunction with other properties like size distribution determines particles’ toxicity. The chemical composition of PM2.5 exposures may also differ by race/ethnicity with researchers finding that Hispanic individuals are exposed to elevated levels of 13 out of 14 PM2.5 components they investigated compared to non-Hispanic Whites in California33. Of these components, several were linked to increased risk of LBW and reduced birthweight27,33. For example, Basu et al.33 found significant reductions in birthweight per 1 IQR increase in exposure to outdoor vanadium (\(\beta :\) − 32; 95% CI − 38, − 27), titanium (\(\beta\): − 15; 95% CI − 17, − 13), zinc (\(\beta\):-10; 95% CI − 12, − 7), and elemental carbon (\(\beta\):-16; 95% CI − 19, − 14)33. Furthermore, a meta-analysis from Sun et al.27, corroborated these findings, but they also found that other components including silicon and nickel were elevated in Hispanic neighborhoods compared to Non-Hispanic White neighborhoods and were also negatively associated with birthweight.
However, as with the existing health literature on the effects of PM2.5 mass, there is a great deal of heterogeneity of results when looking at the mixture of sources and components that compose it12,27,37. One possibility is that individual exposure is assigned using estimates of outdoor concentrations at the residential level38, which fail to account for time-activity patterns and infiltration of outdoor pollutants indoors29,39, which introduces exposure measurement error. However, this error might be exacerbated when investigating the effects of chemical components of PM2.5 because of their greater spatial variability relative to PM2.5 mass concentration as a whole40. Additionally, total personal exposure to major contributing sources and components of PM2.5 also includes contributions from indoor sources and personal activity or behavior-related sources and not just outdoor sources.
Therefore, in this analysis, we aimed to investigate the relationship between exposure to six chemically derived major sources of personal PM2.5 in the 3rd trimester of pregnancy with infant birthweight. To accomplish this goal, we leveraged personal measurements of exposure to PM2.5 mass and its components and source apportionment models. We fit single and multi-pollutant models for the sources and also as a secondary aim, we investigated the independent effects of their high-loading “signature” chemical components to determine whether particular elements were driving the adverse health effects of the source mixtures.
Materials and methods
Study population
This work takes place in a 214-participant personal PM2.5 exposure monitoring sub-study nested within the Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) study, an ongoing prospective cohort study of just over 1000 pregnant, primarily Hispanic, low-income pregnant women in Los Angeles County41. MADRES aims to investigate the cumulative impact of environmental pollutants and psychosocial, behavioral, and built environmental risk factors on maternal and infant health outcomes as described in more detail elsewhere41. Briefly, pregnant women were enrolled in the cohort through partnerships with four prenatal care clinics in Los Angeles, CA from November 2015. Eligibility for this study included: (1) at least 18 years old, (2) a singleton birth, (3) less than 30 weeks gestation at recruitment, (4) HIV negative, (5) having no physical, mental, or cognitive disability that would prevent the participant from providing informed consent, and (6) not currently incarcerated.
Of the 214 participants in the personal exposure monitoring study, nine were removed due to incomplete or erroneous personal PM2.5 mass exposure data or birth outcome data. Four participants did not have PM2.5 source data and were removed from the analysis. A multivariate k-nearest neighbor outlier detection analysis revealed three outliers in terms of personal exposure to the six sources. These were excluded from further analysis. However, given these points were very influential in the models, results including and excluding them are presented side-by-side for completeness in this analysis. This resulted in a sample of 198 mother-infant dyads used in the final models (201 in the outlier-included models).
Participants were recruited by trained, bilingual MADRES staff members during a 3rd trimester visit to the University of Southern California (USC) clinic, where consenting women were asked to participate in the in-utero personal exposure monitoring sub-study for a 48-h monitoring period. This sample was comparable to the larger MADRES cohort on key demographics, birth outcomes, and ambient air pollution metrics.
Personal PM2.5 exposure monitoring
Total personal PM2.5 exposure was measured over an integrated 48-h monitoring period in the 3rd trimester using a custom-designed sampling protocol between October 2016 and February 2020. The 3rd trimester was chosen because most fetal weight gain occurs in this trimester25. Participants were shown and provided with instructions by trained staff members on the correct use of the personal exposure monitoring device, which was housed in a crossbody purse. Instructions included a demonstration of how to wear the purse, making sure to keep the sampling inlet located on the purse shoulder strap free from obstructions and in the breathing zone. Additionally, participants were requested to wear the device as much as possible during normal daily activities, with a limited number of exceptions, including driving, showering, sleeping, etc. Participants were asked to keep the sampling device safe and away from water, high humidity (such as showering), heat, children, and pets, and when unable to wear the device, place it as near as possible, such as on the passenger seat if driving, and a side-table while sleeping.
The purse contained a Gilian Plus Datalogging Pump (Sensidyne Inc.) connected to a Harvard PM2.5 Personal Environmental Monitor (PEM) with a pre-weighed 37 mm Pall Teflo filter. The device was programmed to start at midnight the day after enrollment into the sub-study, and actively sampled at a 50% cycle and a 1.8 L per minute (LPM) flow rate. The sampling device was programmed to shut off after the 48-h sampling period and collected by staff members the following day when a brief exit survey was conducted. The devices were then transferred to the USC Exposure Analytics lab for analysis. Pump data was downloaded, evaluated for errors, and stored securely. Filters were equilibrated within a dedicated chamber and gravimetrically weighed in temperature and relative humidity-controlled glove box using an MT-5 microbalance (Mettler Toledo, Inc.) to obtain PM2.5 mass concentration reported in μg/m3. The methodology of this personal monitoring study has been described in greater detail elsewhere42.
Elemental speciation analysis using X-ray fluorescence
Elemental data was obtained via an X-ray fluorescence analysis43 that determined the elemental composition of PM2.5 collected on personal sampling filters. Concentrations of elements (reported in ng/m3) identified in the source apportionment analysis44 as markers or high-loading species in the source profiles were used in this current analysis. These included: aluminum (Al), barium (Ba), bromine (Br), calcium (Ca), chlorine (Cl), copper (Cu), magnesium (Mg), nickel (Ni), silicon (Si), sodium (Na), sulfur (S), titanium (Ti), vanadium (V), and zinc (Zn).
Optical carbon fractions analysis
A multiwavelength optical absorption approach was used to measure concentrations of several carbon fractions (reported in μg/m3) in the personal PM2.5 samples, including: (1) Black Carbon (BC), (2) Brown Carbon (BrC), and (3) Environmental Tobacco Smoke (ETS). This method is described in more detail elsewhere45, and its performance is consistent with other carbon apportionment approaches45. Briefly, this method uses an integrating sphere radiometer which measures the difference in absorption of transmitted light passed through a mass-loaded Teflo filter. Each of the three carbon components measured with this approach has a different optical density at varying wavelengths, which allows for the identification and quantification of their respective concentration from their optical properties. For the purposes of this study, ETS refers to the carbon fraction concentration obtained via this multiwavelength optical analysis, while the secondhand smoke (SHS) source (explained below) refers to one of the six major contributing sources of personal PM2.5 identified in the PMF analysis. This source had high loadings of several different but highly correlated components, namely ETS and BrC.
Personal PM2.5 sources
Six major contributing sources of personal PM2.5 were used in this analysis, obtained from an earlier source apportionment analysis of these personal exposure filter samples using the EPA Positive Matrix Factorization model (EPA PMF v5.0)44. The PMF analysis used PM2.5 mass and the concentrations of 36 components (33 elements and 3 optical carbon fractions) as inputs to derive the six sources and their predicted mass contributions. The elements and carbon species were obtained from X-ray fluorescence (XRF) and multiwavelength optical absorption carbon speciation analyses, respectively, at the Research Triangle Institute International, Inc (described in more detail below). For this current study, only 17 (14 elements and 3 optical carbon fractions) high-loading components or signature tracers of the six sources (noted in parentheses) were investigated with birthweight including:
(1) Traffic (BC, Zn, Ba), (2) Secondhand Smoke (BrC, ETS, Br), (3) Aged Sea Salt (S, Na, Mg), (4) Fresh Sea Salt (Cl, Na, Mg), Fuel oil (Cu, Ni, V), and Crustal (Si, Ca, Ti, Al).
Birthweight outcome
Infant birthweight (grams) was abstracted from participants’ electronic medical records (EMR). Given that birthweight and gestational age are closely linked, birthweight-for-gestational age z-scores that were either sex or parity specific were also assessed, as described in46. However, the results were not materially different from continuous birth; therefore, only the continuous birthweight models are presented.
Covariate data
Possible covariates were determined a priori from the air pollution and birth outcomes literature. Factors assessed included maternal demographics, pregnancy and birth outcomes, study design characteristics (such as hospital of birth), and meteorology. Self-report data were collected during the MADRES cohort follow-up through a sequence of staff administered in-person and telephone-based questionnaires. All questionnaires were available in either English or Spanish. Anthropometric assessments were conducted via regular clinic visits. Due to the timing of this personal monitoring study in the 3rd trimester of pregnancy, data primarily came from the 3rd trimester visit, the personal monitoring study exit survey, and birth-related datasets and variables, with additional variables assessed at the baseline visit including race/ethnicity and pre-pregnancy body mass index (BMI; kg/m2).
Additional pregnancy and birth-related covariates were also evaluated for confounding. Gestational age at birth (GA; weeks) was estimated with a hierarchical approach of differing methods from the preferred ultrasound measurement of crown-rump length at < 14 weeks gestation (60%), ultrasound measurement of fetal biparietal diameter at < 28 weeks’ gestation (30%), and from physicians’ clinical estimate from EMR (10%). Parity was defined as 1 or more previous births and included a missing category with 6 participants so as not to lose sample size. Infant sex was obtained through EMR.
Maternal demographics included the following: Age at baseline (continuous; years), education (completed < 12th grade, completed high school, at least some college), household income (less than $15,000, $15,000–29,999, $30,000+, don’t know), and diabetes status [no diabetes, glucose intolerant, diabetes (chronic and gestational)]. Race/ethnicity was categorized as Hispanic, non-Hispanic Black, and non-Hispanic Other. Pre-pregnancy BMI (continuous; kg/m2) was calculated from self-reported pre-pregnancy weight and standing height measured by MADRES staff at participants’ first visit by either a stadiometer (Perspectives model PE-AIM-101) or EMR. Self-report weight was chosen because participants entered the study at differing weeks of gestation.
Meteorological factors evaluated in this study included ambient air temperature (Celsius) (calculated as the average of minimum and maximum air temperature) and relative humidity (%), averaged over the 3rd trimester and estimated at the residential location based on a high-resolution (4 km × 4 km) gridded surface meteorological dataset 47, and seasonality using the following levels, “Cool (Winter), Warm (Summer), and Transition (Spring and Autumn)”.
Statistical analysis
Descriptive statistics
Descriptive statistics of key sample demographics and mean and standard deviations were calculated for concentrations of personal PM2.5 mass, six PMF-derived sources of personal PM2.5, and 17 high-loading components. The distribution of birthweight, personal PM2.5 mass concentration, and each source and component were investigated to identify any issues with normality and potential influential points. Bivariate analyses using Kruskal–Wallis one-way analysis of variance tests and Spearman’s correlation coefficients were conducted between personal PM2.5 mass, its major contributing sources, and birthweight by various sample characteristics to elicit any additional potential confounders for our regression analysis, in addition to those identified in previous literature27.
Linear regression models
Single- and multi-pollutant linear regression models were used to investigate the primary aim of this study, that is, the association between in-utero exposure to major personal PM2.5 sources and birthweight, adjusting for gestational age at birth, maternal age, race/ethnicity, infant sex, parity, diabetes status, temperature, maternal education, and personal smoking history. Even though this study assessed SHS as a source of PM2.5, it did not correlate strongly with our smoking covariate (never/ever smoker). However, this smoking covariate did seem to be a confounder and impact our main effects, therefore, it was kept within the model. Meteorological variables, such as season, were excluded from the models after statistical confounding checks showed no material differences in the main effects. This was done to conserve statistical power. The effect of total personal PM2.5 on birthweight, previously reported by this group32, was included in relevant tables for comparison purposes. PM2.5 sources that were not highly correlated with one another, as determined by a bivariate Spearman correlation analysis and/or a variance inflation factor (VIF) below 10 in the regression, were used to evaluate the effect of each source on birthweight, adjusting for one or more other PM2.5 sources. Multi-pollutant models were conducted with up to four personal PM2.5 sources included in each model; however, three- and four-pollutant model results did not materially differ. Therefore, only single- and two-pollutant models are reported. Additionally, the association between PM2.5 and birthweight has been shown to differ by the sex of the infant, therefore, this study evaluated whether the effect of each PM2.5 source exposure on birthweight was modified by sex. Non-linear effects were evaluated by modeling each PM2.5 source on birthweight using generalized additive models (GAMs) and assessing Akaike information criterion (AICs) vs. linear regression models. As a sensitivity analysis, the association of each PM2.5 source on birthweight was examined only among full-term births (37 weeks or older gestation) due to apprehensions about potential bias from factoring in gestational age as a confounder, given the possibility that it might act as a mediator48. This was conducted with and without the inclusion of gestational age as a covariate.
For the secondary aim of this study, this study attempted to determine whether it was the source itself (a particular mix of components) driving any adverse health effects or an individual element or component. To evaluate whether it is the PM2.5 source (the mixture) or any of its high-loading components that are driving the observed association between sources and birthweight, the effect of the 17 high-loading PM2.5 components on birthweight was investigated. Additionally, because PM2.5 mass concentration may be related to both birthweight and the concentration of the PM2.5 components (especially more abundant ones), further analyses adjusting component models for PM2.5 mass were performed via two approaches, which have different interpretations, while similarly attempting to account for PM2.5 mass itself. The first was by adjusting for PM2.5 mass in the individual component models by directly including it as a simple covariate or potential confounder. According to Mostofsky et al.49, this parameter “represents the impact of higher levels of the constituent (and its correlates), holding the other constituents constant”49. The second approach was to create component residuals by regressing the mass concentration of each PM2.5 component (dependent) on the total PM2.5 mass concentration (independent). The component coefficient “represents the increase in risk associated with higher levels of the constituent while holding PM2.5 constant”49.
Due to concerns with outliers being influential as determined by model diagnostics in 3 out of 6 main source models, a multivariate K-nearest neighbor outlier detection analysis was conducted in JMP Pro 16 (SAS Institute, Inc., Cary, NC, USA). This was used to identify outliers up to a distance of 8 nearest neighbors along the concentrations of all six personal PM2.5 sources. This analysis allowed us to objectively identify data points that were materially different from the overall sample across six dimensions. All effect estimates and 95% confidence intervals were scaled and reported per 1 SD increase in the main exposure of interest. An alpha of 0.05 was selected as a priori significance level for our main exposure/outcome analyses, while 0.10 was used for the infant sex interaction analyses to allow more leniency for statistical interactions due to the increased power requirement. Model diagnostics were conducted to ensure models were not affected by multicollinearity or influential points. The analysis was conducted using SAS v9.4 (SAS Institute, Inc., Cary, NC, USA).
Ethical approval and consent to participate
Study procedures were approved by the USC Institutional Review Board (IRB) and all participants completed written informed consent at the first study visit (IRB: HS-16-00530). This study was performed according to the ethical guidelines expressed in the Strengthening of the Reporting of Observational Studies in Epidemiology (STORB) guideline and the Declaration of Helsinki.
Results
Descriptive statistics
Sample characteristics for the full sample are presented in Table 1 The participants of this study were predominantly Hispanic (81%), lower income (43% had income below $30,000), with a mean age of 28 years, and 63% had a previous pregnancy. Around 70% of the women were overweight or obese, and 34% had glucose intolerance or diabetes (chronic or gestational). Participants’ infants were 51% female and had a mean (SD) birthweight of 3295.8 (484.1) grams and gestational age of 39.1 (1.5) weeks at the time of birth.
Total personal PM2.5 exposure had a mean (SD) of 21.3 (14.4) μg/m3. The average estimated mass contributions of the six personal PM2.5 sources were as follows: SHS 12.0 (9.2) μg/m3, crustal 2.1 (3.5) μg/m3, fuel oil 2.1 (1.6) μg/m3, aged sea salt 0.8 (0.8) μg/m3, fresh sea salt 0.9 (2.1) μg/m3, and traffic 0.5 (0.6) μg/m3 (Table 2). Carbon fractions concentrations were generally similar; however, ETS was the highest with a mean of 1.4 μg/m3 but also had the highest variability with a SD of 5.5 μg/m3. Sulfur, sodium, silicon, and chlorine were the elements measured at the highest concentrations, with a mean (SD) of 397.7 (283.9), 311.1 (305.7), 165.7 (200.0), and 129.5 (259.4) ng/m3, respectively (Table 2).
The relationships between personal PM2.5 mass and its six sources along with key demographics are presented in Table S.1 of the supplement. There was no noticeable difference in personal PM2.5 mass exposure by sociodemographic and other covariates. However, personal PM2.5 mass concentration was highest in Hispanic and non-Hispanic Black participants compared to non-Hispanic Others, and was roughly 2 and 4 μg/m3 greater in participants with diabetes (chronic and gestational) than those with glucose intolerance and without diabetes. Traffic, SHS, and Aged and Fresh Sea Salt were highest in Hispanic participants, crustal was highest in participants that did not complete high school, and SHS was highest in participants with glucose intolerance and those reporting < $15,000 maternal income.
There were modest associations between several of the PM2.5 sources (Table 3). Traffic was positively correlated with crustal (ρ = 0.35; p < 0.001) and negatively correlated with fresh sea salt (ρ = − 0.22; p = 0.002) and fuel oil (ρ = − 0.17; p = 0.017). SHS was negatively correlated with aged sea salt (ρ = − 0.22; p = 0.002), and fresh sea salt (ρ = − 0.26; p < 0.001). A Spearman correlation matrix for the components is presented in Table S.2 Spearman correlations varied between − 0.58 for ETS and BC (p < 0.001) to 0.86 for Na and Mg (p < 0.001). The majority of correlations were positive.
Associations of personal PM2.5 and components with birthweight
Overall, in the fully adjusted final models, there was a small negative association between personal PM2.5 exposure and birthweight (β = − 33.5; 95% CI − 103.2, 36.1) per 1 SD increase pollutant. There was an average decrease of 99.2 g (95% CI − 197.7, − 0.6) in birthweight for the fresh sea salt source. This result remained after adjusting individually for fuel oil and crustal sources but became marginally significant after adjustment for aged sea salt. Aged sea salt was associated with a 70.1 g (95% CI − 141.7, 1.4) decrease in birthweight. This remained after further adjustments for fresh sea salt, traffic, fuel oil, and crustal sources (Table 4).
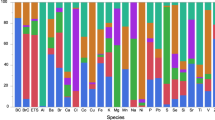
Two high-loading components of fresh sea salt and aged sea salt, Na (β = − 89.4; 95% CI − 163.0, − 15.7) and Mg (β = − 153.2; 95% CI − 248.7, − 57.7), were statistically associated with lower birthweight (Fig. 1). SHS as a source and two of the major contributing components had small negative associations with birthweight. The effect estimates did not materially change after adjustment for personal PM2.5 mass as a covariate or when looking at PM2.5 component residuals. In a sensitivity analysis of just full-term births, the results did not materially change when gestational age was removed as a covariate; however, there were slight differences when gestational age was also included in these models (Table S.3).
Associations Between High-Loading Components of the Six Personal PM2.5 Sources and Birthweight in Single-Pollutant Models, Adjusting for Personal PM2.5 Mass Concentration, and Using the Component Residuals as the Main Exposure, Respectively. Notes: PM2.5 = particulate matter with an aerodynamic diabetes less than 2.5 µm; significance < 0.05; β = change in birthweight per 1 SD increase in pollutant; adj = adjusting; all models were adjusted for gestational age at birth, maternal age, race/ethnicity, infant sex, parity, diabetes status, temperature, maternal education, and personal smoking history.
Effects of personal PM2.5 sources on birthweight by infant sex
While not meeting statistical significance, the effect of personal PM2.5 on birthweight was more negative in males (β = − 63.3 g; 95% CI − 169.4, 42.8) compared to females (β = − 11.6 g; 95% CI − 103.0, 79.9; Table 5). The effect of the crustal source on birthweight was modified by infant sex, with males observing a − 83.4 g (95% CI − 187.9, 21.0) decrease in birthweight per 1 SD increase in exposure to the crustal source compared to a 127.0 g (95% CI − 17.4, 271.4) increase for females (Interaction: p = 0.020). Additionally, the effect of the fuel oil source on birthweight was − 31.1 (95% CI − 114.8, 52.5) for males and 55.0 (95% CI − 21.0, 130.9) for females (Interaction: p = 0.135).
Discussion
This analysis investigated the effect of major contributing sources of personal PM2.5 exposure on birthweight in the 3rd trimester within the MADRES in-utero personal exposure monitoring study. To our knowledge, this is the first analysis relating chemically derived sources of personal PM2.5 exposure with birthweight in a largely lower income, Hispanic, health disparities population leveraging “gold standard” personal exposure monitoring data. Prior studies reported concerns about exposure misclassification due to spatial variability of PM2.5 components when using ambient monitoring data to assign individual exposure40. This present study used sources derived from personal exposure monitoring data, which may remove much of this concern by measuring PM2.5 components for each individual in their personal breathing zone39.
Overall, this study found that total personal PM2.5 was marginally negatively associated with birthweight; however, some of its more specific sources, including fresh sea salt and aged sea salt were more strongly negatively associated with birthweight. Both sources contained Na and Mg as high-loading components, which were the elements that were most negatively associated with birthweight in this analysis. The findings in this present study differ from prior studies which found no association between sea or marine salt sources and reduced birthweight50,51. There are several possible explanations for the different findings. For example, Bell et al.50 was conducted in Connecticut and Massachusetts, while the present study was in California, likely resulting in regional differences in the mixture of marine or sea salt sources or other pollutants that track along with it. Additionally, differences in the timing of exposure assessment may also explain differences in results. Prior studies looking at the effects of outdoor Na concentrations on birthweight are limited; however, Basu et al.33 found that Na was associated with decreased birthweight in California. Similarly, the outdoor concentration of sodium ion (Na+) was associated with lower birthweight on the East Coast37. The relationship between Mg has been less studied, but a study in California found no association between Mg and birthweight52. It is not apparent why sea salt, particularly fresh sea salt, has the most adverse effect on birthweight in our study. It is possible fresh sea salt may be correlated with offshore marine shipping emissions given they both originate over the ocean and may be transported to receptor locations under similar meteorological and wind conditions. Components of marine shipping emissions (Ni, V, and EC) have been associated with lower birthweight12,53. However, in our study, the fuel oil personal exposure source (with loadings of Ni and V) was poorly correlated with fresh sea salt. Fresh sea salt as an outdoor source of PM2.5 is also correlated with being closer to the coast and the ports in Los Angeles, CA54, yet this spatial pattern was not apparent in our personal monitoring study for the fresh sea salt or the fuel oil sources. The fuel oil personal exposure source in our study may be capturing impacts of heavy-duty machinery and industrial equipment that burn heavier residual fuel oil, which is very common in Los Angeles, CA rather than picking up shipping emission signals from the ports55.
Fresh and aged sea salt did differ in an important way, with fresh sea salt containing higher loadings of Cl which is replaced with S as fresh sea salt undergoes photochemical reactions and becomes aged sea salt56,57. While the two sources share Na and Mg as high-loading components, they are not highly correlated. Differences in their impacts on birthweight may be due to other components or factors that correlate with them. For example, in our study, the effect of Cl on birthweight was stronger than S per 1SD of each pollutant. Previously, S was associated with decreased birthweight33,58, while results for Cl have been mixed with two studies finding reductions in birthweight33,37, while another found no association with LBW risk59. Interestingly, these studies found an association with Cl and decreased birthweight in New England and California, highlighting that this may not be a local phenomenon.
Other considerations for why aged sea salt might be negatively associated with birthweight are through secondary formation processes. Aged sea salt as a personal exposure source was highly correlated with outdoor ozone and temperature in our study. Both ozone and aged sea salt undergo chemical aging and transformation processes in the atmosphere under similar conditions of high temperature which could explain this correlation54,57,60. However, 8-h maximum ozone concentration was not associated with birthweight for the same 48 h sampling period or the whole third trimester within our sample (estimated at the residence using inverse distance weighted squared spatial interpolation, data not shown). This suggests there may be other processes, factors, or co-exposures associated with personal exposure to fresh and aged sea salt—both of clear outdoor origin—that may also be negatively associated with birthweight.
When components are more negatively associated than the source itself, it may imply that that particular compound is more toxic than the whole mixture. Efforts to identify sources or important components can lead to actionable interventions. For example, researchers found that stricter caps in Europe on sulfur content in marine fuel led to 22% reductions in sulfur dioxide gas and 6% reductions in PM2.5, which in turn resulted in a 7% reduced risk of LBW61. Similar policies have been implemented in California62. Additionally, in California, while there has been a general reduction in ambient PM2.5 concentrations over the past 20 + years due to regulatory interventions63, this study adds to the literature that specific sources or components of PM2.5 still place pregnant mothers at risk of adverse birth outcomes.
This study found that SHS and two high-loading components (ETS and BrC) were negatively associated with birthweight; however, the effect estimates for ETS and BrC were more negative than the SHS source itself. Overall, our results agree with prior studies that found a negative association between SHS and birthweight36,64. Additionally, a prior study investigating the effect of total personal PM2.5 on birthweight, found that self-reported prenatal SHS reduced birthweight22.
This current study did not find a particularly strong association between the personal traffic exposure source or its components. This differs from prior studies that have generally concluded that traffic-related exposures are related to lower birthweight65 and an increased risk of LBW51,52,53. Personal exposure to Zn was not associated with lower birthweight, which is inconsistent with the consensus of prior studies of ambient Zn27,33, although others have also found no association30. Zn is correlated with tire wear, one of the two dominant non-tailpipe emissions66. The personal traffic exposure source used in this study is characterized by high loadings of BC which is a marker of tailpipe combustion but also contains high loadings of Ba and Zn, markers of non-tailpipe or non-exhaust brake and tire wear emissions, respectively. As such, the traffic source may be capturing both fuel combustion and abrasive vehicular wear emissions combined54. Interestingly, while researchers in London found an increased risk of LBW from non-exhaust traffic ambient PM2.5 (did not hold up after adjustment for other air pollutants), the risk of LBW associated with non-exhaust traffic ambient PM2.5 was consistently lower across all models compared to traffic-exhaust related ambient PM2.567. Additionally, different spatial scales may explain the differences observed between studies as prior literature has used ambient monitoring compared to personal monitoring in this study. Similarly, personal exposure to the fuel oil source was not associated with reductions in birthweight in this study; however, there was evidence that infant sex may modify this relationship.
This study found a small negative association between crustal exposure and lower birthweight. Additionally, a statistical interaction was found by infant sex, with negative effects seen in males and positive effects in females. Together with fuel oil, this provides evidence for potential differences in the underlying biomechanisms of how air pollution affects health, which may interact with sex-based biological differences among fetuses. Notably, unlike other components we investigated, effect estimates for crustal components were the most altered when adjusted for personal PM2.5 mass. This suggests that the effect of these components may be confounded by the effect of PM2.5 mass because PM2.5 may be correlated with both the component and the health outcome49, possibly because crustal components are more abundant and therefore more correlated with PM2.5 mass.
This study has several strengths, including the use of chemical speciation data and source apportionment derived PM2.5 sources, from “gold standard” personal exposure monitoring data. Prior studies that assessed PM2.5 sources and components27,30 used outdoor measurements which do not account for exposures that occur indoors or in-transit due to time-activity patterns, indoor sources, and infiltration of outdoor sources into the home. MADRES is a well-characterized cohort, with a vast array of individual-level covariate data available, making this an excellent study for this research question to be conducted. Furthermore, this study provided evidence for the effect of PM2.5 sources on birthweight in a health disparities population, which may experience not just a greater burden of adverse health outcomes and environmental exposures, but also lower access to health care and resources to alleviate the impact of such burdens68,69.
The sample size of this study is a potential limitation with a final working sample of 198 (201 in the model that included the outliers), which while small for population-based health studies, is actually fairly large for personal exposure monitoring studies70,71,72. However, even with this potential limitation, we were able to detect several associations between major sources of PM2.5 and their respective high-loading components. Another possibility is participation bias due to differences in the type of expecting mothers that chose to participate in the personal exposure monitoring study component of the MADRES cohort study. However, we did not observe any material differences between participants who chose to participate in the personal exposure monitoring study compared to the larger cohort, except that they were slightly more likely to have had a prior child (data not shown).
A potential limitation of our study pertains to the techniques employed to analyze the mixtures of PM2.5 components and sources. While alternative methodologies were considered, we opted for the methods detailed in this paper for two main reasons. Firstly, given the constraints in sample size, we believed that venturing into other methodologies might not yield robust results. Secondly, our chosen source apportionment approach inherently serves as a form of mixture modeling, aligning with our research objectives, which primarily was to investigate the impact of PM2.5 sources, with the secondary aim of evaluating whether it was the source or specific components that drove the adverse health effect.
Finally, the 48-h sampling period in the 3rd trimester is a limitation as this may not be representative of typical or longer duration. The enhanced personal monitoring methods over shorter durations offer a more accurate estimation of genuine or “true” personal exposure compared to conventional modeled ambient techniques. However, there is a trade-off as they struggle to capture temporal variations due to short measurement times, which is especially concerning when representing extended periods like a pregnancy trimester. The shorter measurement period is unfortunately a limitation of the technology, with battery life and the burden to participants prohibitive for much longer time periods. Yet, the correlation between personal PM2.5 and ambient PM2.5 for the same 48-h sampling period was very similar to that with the 3rd trimester average ambient PM2.532, suggesting the ambient segment of total PM2.5 exposure was likely consistent across the period. Also, we found reasonable concordance between time-activity patterns between two different questionnaire sources, including an exit survey after the 48-h sampling period, and a 3rd trimester questionnaire (data not shown), suggesting these exposure factors may not drastically differ. Together, these increase confidence in the representativeness of the measurements in terms of what participants are truly experiencing considering time-activity patterns and outdoor exposures are major contributing factors to personal PM2.5.
Conclusion
Overall, this study found evidence that major outdoor sources of personal PM2.5 including fresh sea salt, aged sea salt, and to a lesser extent, SHS and crustal sources, were negatively associated with birthweight in a health disparities population in Los Angeles, CA. Mg, Na, Cl components were most strongly associated with negative birthweight. Additionally, the effect of crustal and fuel oil sources differed by infant sex with negative associations seen in boys compared to positive associations in girls.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Martin, J. A., Hamilton, B. E., Osterman, M. J. K., & Driscoll, A. K. National Vital Statistics Reports Vol. 68, No. 13. Births: Final Data for 2018 47 (2019).
Vilanova, C. S. et al. The relationship between the different low birth weight strata of newborns with infant mortality and the influence of the main health determinants in the extreme south of Brazil. Popul. Health Metr. 17, 15. https://doi.org/10.1186/s12963-019-0195-7 (2019).
Watkins, W. J., Kotecha, S. J. & Kotecha, S. All-cause mortality of low birthweight infants in infancy, childhood, and adolescence: Population study of England and Wales. PLoS Med. https://doi.org/10.1371/journal.pmed.1002018 (2016).
Jornayvaz, F. R. et al. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovasc. Diabetol. 15, 73. https://doi.org/10.1186/s12933-016-0389-2 (2016).
Mi, D., Fang, H., Zhao, Y. & Zhong, L. Birth weight and type 2 diabetes: A meta-analysis. Exp. Ther. Med. 14, 5313–5320. https://doi.org/10.3892/etm.2017.5234 (2017).
Huxley, R. et al. Is birth weight a risk factor for ischemic heart disease in later life?. Am. J. Clin. Nutr. 85(5), 1244–1250. https://doi.org/10.1093/ajcn/85.5.1244 (2007).
Umer, A. et al. Association between birth weight and childhood cardiovascular disease risk factors in West Virginia. J. Dev. Orig. Health Dis. 11, 86–95. https://doi.org/10.1017/S204017441900045X (2020).
Upadhyay, R. P. et al. Cognitive and motor outcomes in children born low birth weight: A systematic review and meta-analysis of studies from South Asia. BMC Pediatr. 19, 35. https://doi.org/10.1186/s12887-019-1408-8 (2019).
Whitaker, A. H. et al. Motor and cognitive outcomes in nondisabled low-birth-weight adolescents: Early determinants. Arch. Pediatr. Adolesc. Med. 160, 1040. https://doi.org/10.1001/archpedi.160.10.1040 (2006).
Petersen, R. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev. Chronic Dis. https://doi.org/10.5888/pcd16.180579 (2019).
Rossen, L. M. Neighbourhood economic deprivation explains racial/ethnic disparities in overweight and obesity among children and adolescents in the USA. J. Epidemiol. Community Health 68, 123–129. https://doi.org/10.1136/jech-2012-202245 (2014).
Bell, M. L. & Ebisu, K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect. 120, 1699–1704. https://doi.org/10.1289/ehp.1205201 (2012).
Mikati, I., Benson, A. F., Luben, T. J., Sacks, J. D. & Richmond-Bryant, J. Disparities in distribution of particulate matter emission sources by race and poverty status. Am. J. Public Health 108, 480–485. https://doi.org/10.2105/AJPH.2017.304297 (2018).
Lamichhane, D. K., Leem, J.-H., Lee, J.-Y. & Kim, H.-C. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ. Health Toxicol. https://doi.org/10.5620/eht.e2015011 (2015).
Li, X. et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ. Pollut. 227, 596–605. https://doi.org/10.1016/j.envpol.2017.03.055 (2017).
Pedersen, M. et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir. Med. 1, 695–704. https://doi.org/10.1016/S2213-2600(13)70192-9 (2013).
Stieb, D. M. et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999–2008. Environ. Res. 148, 513–526. https://doi.org/10.1016/j.envres.2016.04.025 (2016).
Huang, C. et al. Ambient air pollution and adverse birth outcomes: A natural experiment study. Popul. Health Metr. https://doi.org/10.1186/s12963-015-0050-4 (2015).
Nyadanu, S. D. et al. Prenatal exposure to ambient air pollution and adverse birth outcomes: An umbrella review of 36 systematic reviews and meta-analyses. Environ Pollut. 306, 119465. https://doi.org/10.1016/j.envpol.2022.119465 (2022).
Schembari, A. et al. Ambient air pollution and newborn size and adiposity at birth: Differences by maternal ethnicity (the Born in Bradford Study Cohort). Environ. Health Perspect. 123, 1208–1215. https://doi.org/10.1289/ehp.1408675 (2015).
Jedrychowski, W. et al. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: An epidemiologic prospective cohort study in Poland. Environ. Health Perspect. 112(13), 1398–1402. https://doi.org/10.1289/ehp.7065 (2004).
Jedrychowski, W. et al. Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter. Environ. Res. 109(4), 447–456. https://doi.org/10.1016/j.envres.2009.01.009 (2009).
Clemente, D. B. P. et al. Prenatal ambient air pollution, placental mitochondrial DNA content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth cohorts. Environ. Health Perspect. 124, 659–665. https://doi.org/10.1289/ehp.1408981 (2016).
Li, Z. et al. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 169, 248–254. https://doi.org/10.1016/j.ecoenv.2018.10.109 (2019).
Kiserud, T. et al. The world health organization fetal growth charts: Concept, findings, interpretation, and application. Am. J. Obstet. Gynecol. 218, S619–S629. https://doi.org/10.1016/j.ajog.2017.12.010 (2018).
Gogna, P. et al. Ambient air pollution and inflammatory effects in a Canadian pregnancy cohort. Environ. Epidemiol. 5(5), e168 (2021).
Sun, X. et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: A meta-analysis. Environ. Pollut. 211, 38–47. https://doi.org/10.1016/j.envpol.2015.12.022 (2016).
Carroll, R. J. Measurement error in epidemiologic studies. In Encyclopedia of Biostatistics. American Cancer Society (2005). https://doi.org/10.1002/0470011815.b2a03082
Kioumourtzoglou, M.-A. et al. Exposure measurement error in PM2.5 health effects studies: A pooled analysis of eight personal exposure validation studies. Environ. Health 13, 2. https://doi.org/10.1186/1476-069X-13-2 (2014).
Laurent, O. et al. Low birth weight and air pollution in California: Which sources and components drive the risk?. Environ. Int. 92–93, 471–477. https://doi.org/10.1016/j.envint.2016.04.034 (2016).
Ng, C. et al. Source apportionment of fine particulate matter and risk of term low birth weight in California: Exploring modification by region and maternal characteristics. Sci. Total Environ. 605–606, 647–654. https://doi.org/10.1016/j.scitotenv.2017.06.053 (2017).
O’Sharkey, K. et al. In-utero personal exposure to PM2.5 impacted by indoor and outdoor sources and birthweight in the MADRES cohort. Environ. Adv. 9, 100257. https://doi.org/10.1016/j.envadv.2022.100257 (2022).
Basu, R. et al. Effects of fine particulate matter and its constituents on low birth weight among full-term infants in California. Environ. Res. 128, 42–51. https://doi.org/10.1016/j.envres.2013.10.008 (2014).
Wilhelm, M. & Ritz, B. Residential proximity to traffic and adverse birth outcomes in Los Angeles County, California, 1994–1996. Environ. Health Perspect. 111, 207–216 (2003).
Khader, Y. S., Al-Akour, N., Alzubi, I. M. & Lataifeh, I. The association between second hand smoke and low birth weight and preterm delivery. Matern. Child Health J. 15(4), 453–459. https://doi.org/10.1007/s10995-010-0599-2 (2011).
Wahabi, H. A. et al. Effects of secondhand smoke on the birth weight of term infants and the demographic profile of Saudi exposed women. BMC Public Health. 13, 341. https://doi.org/10.1186/1471-2458-13-341 (2013).
Ebisu, K., Belanger, K. & Bell, M. L. The association between airborne PM2.5 chemical constituents and birth weight-implication of buffer exposure assignment. Environ. Res. Lett. https://doi.org/10.1088/1748-9326/9/8/084007 (2014).
Harris, G., Thompson, W. D., Fitzgerald, E. & Wartenberg, D. The association of PM2.5 with full term low birth weight at different spatial scales. Environ. Res. 134, 427–434. https://doi.org/10.1016/j.envres.2014.05.034 (2014).
Gray, S. C., Edwards, S. E. & Miranda, M. L. Assessing exposure metrics for PM and birthweight models. J. Expo Sci. Environ. Epidemiol. 20(5), 469–477. https://doi.org/10.1038/jes.2009.52 (2010).
Bell, M. L., Ebisu, K. & Peng, R. D. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J. Expo Sci. Environ. Epidemiol. 21, 372–384. https://doi.org/10.1038/jes.2010.24 (2011).
Bastain, T. M. et al. Study design, protocol and profile of the maternal and developmental risks from environmental and social stressors (MADRES) pregnancy cohort: A prospective cohort study in predominantly low-income Hispanic Women in Urban Los Angeles. BMC Pregnancy Childbirth 19(1), 189. https://doi.org/10.1186/s12884-019-2330-7 (2019).
Xu, Y. et al. The impact of GPS-derived activity spaces on personal PM2.5 exposures in the MADRES cohort. Environ. Res. 214, 114029. https://doi.org/10.1016/j.envres.2022.114029 (2022).
Gill, R. Modern Analytical Geochemistry: An Introduction to Quantitative Chemical Analysis Techniques for Earth, Environmental and Materials Scientists (Routledge, 2014). https://doi.org/10.4324/9781315844381.
Xu, Y., Yi, L., Cabison, J., Rosales, M., O’Sharkey, K., Chavez, T. A., et al. Sources of personal PM2.5 exposure during pregnancy in the MADRES cohort. J. Exp. Sci. (2023).
Lawless, P. Multiwavelength absorbance of filter deposits for determination of environmental tobacco smoke and black carbon. Atmos. Environ. 38, 3373–3383. https://doi.org/10.1016/j.atmosenv.2004.03.038 (2004).
Aris, I. M., Kleinman, K. P., Belfort, M. B., Kaimal, A. & Oken, E. A 2017 US reference for singleton birth weight percentiles using obstetric estimates of gestation. Pediatrics 144, e20190076. https://doi.org/10.1542/peds.2019-0076 (2019).
Abatzoglou, J. T. Development of gridded surface meteorological data for ecological applications and modelling. Int. J. Climatol. 33(1), 121–131. https://doi.org/10.1002/joc.3413 (2011).
Wilcox, A. J., Weinberg, C. R. & Basso, O. On the pitfalls of adjusting for gestational age at birth. Am. J. Epidemiol. 174(9), 1062–1068 (2011).
Mostofsky, E. et al. Modeling the association between particle constituents of air pollution and health outcomes. Am. J. Epidemiol. 176, 317–326. https://doi.org/10.1093/aje/kws018 (2012).
Bell, M. L. et al. Prenatal exposure to fine particulate matter and birth weight. Epidemiology 21, 884–891. https://doi.org/10.1097/EDE.0b013e3181f2f405 (2010).
Wilhelm, M. et al. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ. Health Perspect. 120, 132–138. https://doi.org/10.1289/ehp.1103408 (2012).
Laurent, O. et al. A statewide nested case-control study of preterm birth and air pollution by source and composition: California, 2001–2008. Environ. Health Perspect. 124, 1479–1486. https://doi.org/10.1289/ehp.1510133 (2016).
Laurent, O. et al. Sources and contents of air pollution affecting term low birth weight in Los Angeles County, California, 2001–2008. Environ. Res. 134, 488–495. https://doi.org/10.1016/j.envres.2014.05.003 (2014).
Habre, R. et al. Contribution of tailpipe and non-tailpipe traffic sources to quasi-ultrafine, fine and coarse particulate matter in southern California. J. Air Waste Manag. Assoc. 71(2), 209–230. https://doi.org/10.1080/10962247.2020.1826366 (2021).
Arhami, M. et al. Size-segregated inorganic and organic components of PM in the communities of the Los Angeles Harbor. Aerosol. Sci. Technol. 43(2), 145–160. https://doi.org/10.1080/02786820802534757 (2009).
Habre, R. et al. Contribution of tailpipe and non-tailpipe traffic sources to quasi-ultrafine, fine and coarse particulate matter in Southern California. J. Air Waste Manag. Assoc. https://doi.org/10.1080/10962247.2020.1826366 (2020).
Hasheminassab, S. et al. Spatial and temporal variability of sources of ambient fine particulate matter (PM2.5) in California. Atmos. Chem. Phys. 14, 12085–12097. https://doi.org/10.5194/acp-14-12085-2014 (2014).
Pedersen, M. et al. Elemental constituents of particulate matter and newborn’s size in Eight European Cohorts. Environ. Health Perspect. 124, 141–150. https://doi.org/10.1289/ehp.1409546 (2016).
Ebisu, K. & Bell, M. L. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environ. Health Perspect. 120, 1746–1752. https://doi.org/10.1289/ehp.1104763 (2012).
Gard, E. E. et al. Direct observation of heterogeneous chemistry in the atmosphere. Science 279(5354), 1184–1187. https://doi.org/10.1126/science.279.5354.1184 (1998).
Lindgren, S. The coast is clear: Shipping emission standards, air quality and infant health. Transp. Res. Part D Transp. Environ. 100, 103067. https://doi.org/10.1016/j.trd.2021.103067 (2021).
Tao, L., Fairley, D., Kleeman, M. J. & Harley, R. A. Effects of switching to lower sulfur marine fuel oil on air quality in the San Francisco Bay area. Environ. Sci. Technol. 47, 10171–10178. https://doi.org/10.1021/es401049x (2013).
Lurmann, F., Avol, E. & Gilliland, F. Emissions reduction policies and recent trends in Southern California’s ambient air quality. J. Air Waste Manag. Assoc. 65, 324–335. https://doi.org/10.1080/10962247.2014.991856 (2015).
Hawsawi, A. M., Bryant, L. O. & Goodfellow, L. T. Association between exposure to secondhand smoke during pregnancy and low birthweight: A Narrative review. Respir. Care. 60(2), 135–140. https://doi.org/10.4187/respcare.02798 (2015).
Dong, S. et al. Maternal exposure to black carbon and nitrogen dioxide during pregnancy and birth weight: Using machine-learning methods to achieve balance in inverse-probability weights. Environ. Res. 211, 112978. https://doi.org/10.1016/j.envres.2022.112978 (2022).
Klöckner, P. et al. Tire and road wear particles in road environment—Quantification and assessment of particle dynamics by Zn determination after density separation. Chemosphere 222, 714–721. https://doi.org/10.1016/j.chemosphere.2019.01.176 (2019).
Smith, R. B. et al. Impact of London’s road traffic air and noise pollution on birth weight: Retrospective population based cohort study. BMJ https://doi.org/10.1136/bmj.j5299 (2017).
Mahajan, S. et al. Trends in differences in health status and health care access and affordability by race and ethnicity in the United States, 1999–2018. JAMA 326, 637–648. https://doi.org/10.1001/jama.2021.9907 (2021).
Wang, T. F., Shi, L., Nie, X. & Zhu, J. Race/ethnicity, insurance, income and access to care: The influence of health status. Int. J. Equity Health 12, 29. https://doi.org/10.1186/1475-9276-12-29 (2013).
Dadvand, P. et al. Surrounding greenness and exposure to air pollution during pregnancy: An analysis of personal monitoring data. Environ. Health Perspect. 120, 1286–1290. https://doi.org/10.1289/ehp.1104609 (2012).
Sarnat, J. A., Koutrakis, P. & Suh, H. H. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore, MD. J. Air Waste Manag. Assoc. 50, 1184–1198. https://doi.org/10.1080/10473289.2000.10464165 (2000).
Suh, H. H. & Zanobetti, A. Exposure error masks the relationship between traffic-related air pollution and heart rate variability (HRV). J. Occup. Environ. Med. 52, 685–692. https://doi.org/10.1097/JOM.0b013e3181e8071f (2010).
Acknowledgements
The authors gratefully acknowledge the contributions of Ms. Lisa Valencia and the larger MADRES team. We also thank the MADRES participants, their families, and our clinic partners for their time and effort.
Funding
The study was supported by NIEHS R01ES027409, NIEHS P30ES007048 pilot funding, and the MADRES Center (NIEHS/NIMHD P50ES026086, EPA 83615801, NIMHD P50MD015705).
Author information
Authors and Affiliations
Contributions
K.O.: Formal analysis; Investigation; Methodology; Visualization; Roles/Writing—original draft; Writing—review & editing. Y.X.: Data curation; Writing—review & editing. J.C.: Data curation; Investigation; Methodology; Project administration; Writing—review & editing. M.R.: Data curation; Investigation; Project administration; Writing—review & editing. T.C.: Data curation; Writing—review & editing. M.J.: Data curation; Writing—review & editing. T.Y.: Data curation; Writing—review & editing. D.L.: Data curation; Writing—review & editing. N.L.: Data curation; Writing—review & editing. C.T.C.: Investigation; Writing—review & editing. S.F.: Conceptualization; Data curation; Investigation; Writing—review & editing. T.B.: Conceptualization; Data curation; Funding acquisition; Investigation; Project administration; Resources; Writing—review & editing. C.B.: Conceptualization; Data curation; Funding acquisition; Investigation; Project administration; Resources; Writing—review & editing. R.H.: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Sharkey, K., Xu, Y., Cabison, J. et al. Effects of in-utero personal exposure to PM2.5 sources and components on birthweight. Sci Rep 13, 21987 (2023). https://doi.org/10.1038/s41598-023-48920-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48920-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.