Abstract
This study identifies fungi associated with Euwallacea fornicatus and determines whether these fungal species play the role of primary symbiont. E. fornicatus adults that emerged from the branches of infested trees in Okinawa main island, Japan, were collected and used to isolate fungi. Fusarium kuroshium and Penicillium citrinum were the most dominant fungal associates of females and males, respectively. F. kuroshium was much more frequently isolated from the head, including mycangia (fungus-carrying organs), of females than any other body parts. We inoculated healthy mango saplings with F. kuroshium or F. decemcellulare, both of which were symbionts of E. fornicatus females infesting mango trees. F. kuroshium decreased leaf stomatal conductance and rate of xylem sap-conduction area and increased length and area of xylem discoloration of the saplings, thereby weakening and killing some. These results suggest that F. kuroshium, a mycangial fungus of E. fornicatus, inhibits water flow in mango trees. This study is the first to report that F. kuroshium causes wilt disease in mango trees and that it is a primary fungal symbiont of E. fornicatus.
Similar content being viewed by others
Introduction
The ambrosia beetle, a group of wood-boring mycetophagous insects belonging to subfamilies Scolytinae and Platypodinae in the Curculionidae family, provides a prime example of fungus-insect symbiosis1,2. The adult female of these species possesses mycangia (fungus-carrying structures)3 which carry fungal spores that can be stored in natal galleries of host trees as food for larvae4,5. However, some fungal symbionts are phytopathogenic and cause harm to the trees6,7,8,9. These pathogens tend to occupy new habitats established by their vector beetles and are often lethal to host trees10,11. International trade is one of the main factors responsible for globally spreading many ambrosia beetles12,13,14.
The shot hole borer (SHB) of Euwallacea ambrosia beetles (Scolytinae)-Fusarium dieback (FDB) is a pest-disease combination affecting various tree species in many countries and regions8,15,16. SHB taxa include species such as (i) tea shot hole borer (TSHB), (ii) polyphagous shot hole borer (PSHB), and (iii) kuroshio shot hole borer (KSHB), their corresponding scientific name are: E. perbrevis Schedl, E. fornicatior Eggers, E. fornicatus Eichhoff, and E. kuroshio Gomez et Hulcr, respectively16 (Table 1). These four Euwallacea species, which are termed the E. fornicatus species complex, when unidentifiable, exhibit a broad host range involving a total of 412 plant species in 75 families17, including important crops, such as tea (Camellia sinensis L.)18, avocado (Persea americana Mill.)8,15,19,20, box-elder (Acer negundo L.), castor bean (Ricinus communis L.), English oak (Quercus robur L.)15,20, and London plane (Platanus acerifolia Willd.)21 (Table 1). All four Euwallacea species possess oral mycangia3 and act as vectors of members of the Ambrosia Fusarium Clade22,23.
Fusarium kuroshium Na et al., obtained from E. kuroshio24 is related to, but distinct from F. euwallaceae Freeman et al., which is associated with E. fornicatus15 (Table 1). F. kuroshium acts as a causal agent of FDB in several tree species due to its accelerated growth in wood tissue, which blocks xylem vessels and obstructs water flow in trees24,25,26. Internal symptoms characteristic of FDB manifest as reddish-to-dark brown lesions (and its variations) in the xylem8 and reduced stomatal conductance and net photosynthetic rates of leaves and plant biomass27.
In Japan, E. fornicatus (the name formerly used before being categorized as a species complex) was first recorded on Leucaena glauca (Benth) on Chichi-jima Island in 197328. In 2000, E. fornicatus was declared as a pest of mango (Mangifera indica L.) trees in Tokuno-shima Island29. Since 2007, this pest has been causing severe damage to mango orchards in Okinawa, the main island30. However, despite the threats posed to the mango industry, the causal relationship between E. fornicatus infestations and the decline in mango trees remain unclear. This is mainly attributed to the lack of clarity regarding the role played by the fungus as a link between the borer and mango trees.
Therefore, in this study, we identify the pest species and clarify the associated fungal flora to determine the primary symbiont, with particular reference to mycangial fungi. Moreover, to demonstrate a causal relationship, we assessed the pathogenicity of the symbiont in relation to mango trees.
Results
Beetle collection and identification
A total of 130 beetle specimens (♀ = 85; ♂ = 45) were collected from July 31 to October 12, 2018. A literature survey23 confirmed that the morphological characteristics of all examined beetles (Supplementary Fig. S1b; ♀) were consistent with those of E. fornicatus (PSHB).
The phylogenetic analysis returned 36 most parsimonious trees which differed only in the placement of some conspecific individuals (Fig. 1). Species and the three major lineages of PSHB were monophyletic with bootstrap values > 96. The individual sampled from this study (SAX551) was confirmed as E. fornicatus and as a member of the PSHB1 clade (as in Wang et al.31) which supports the morphological identification.
Phylogenetic placement of the sampled female beetle (SAX551) based on COI and CAD DNA sequences confirming its identity as Euwallacea fornicatus. One of 36 most parsimonious trees demonstrates the relationship of SAX551 among other Euwallacea species and its placement in the PSHB1 clade. Numbers after species names correspond to the last two digits of Genbank numbers. Numbers at nodes are bootstrap values.
Fungal flora
A total of 512 isolates were purified: 97, 118, and 122 isolates were obtained from the head, thorax, and abdomen of females, respectively, while 56, 60, and 59 isolates were from head, thorax, and abdomen of males, respectively (Table 2). Following morphological categorization, 66 selected isolates were sequenced. Finally, 23 fungal isolates with varying compositions were identified as 20 species from females and 15 species from males, including two identical species in both sexes. The sequences of eight isolates failed to amplify via PCR, and these were treated as unknown species (Table 2).
Phylogenetic analysis for Fusarium fungi
The DNA sequencing data consisted of a total of 1,745 positions. Fusarium pseudensiforme (NRRL 46517) and Fusarium sp. [AF-9] (NRRL22643) were used as the outgroup32,33. According to the phylogenetic tree, the Fusarium species isolated from the head, including oral mycangia of E. fornicatus, was placed together with F. kuroshium in an independent clade within the ambrosia fusaria (Fig. 2). Therefore, we identified the ambrosia fusaria associated with E. fornicatus infesting mango tree in this study as F. kuroshium.
Phylogenetic placement of Fusarium species isolated from the head and oral mycangia of female adults of Euwallacea fornicatus. Fungal isolates obtained in this study are in a bold and red background. The phylogeny tree was constructed using the maximum likelihood method based on the Kimura 2-parameter model with MEGA7. The tree involved 70 nucleotide sequences. All positions with less than 95% site coverage were eliminated; fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. A total of 1745 positions were present in the final dataset.
Relative dominance and frequency of occurrence
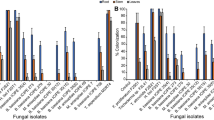
In females, most isolates belonging to F. kuroshium showed the highest relative dominance (RD) (78%) in the head, with RD in the thorax and abdomen being 14.4% and 16.4%, respectively (Table 2). The frequency of occurrence (FO) of F. kuroshium in the head (89.4%) was much higher than that of other fungi in the head (1.2–43.5%), while its FO in the thorax and the abdomen were 20.0% and 23.5%, respectively (Table 2); these differences were statistically significant (Fig. 3). Another Fusarium fungus, F. decemcellulare, was found in thorax and abdomen of females, but with a lower RD and a lower FO (Table 2).
Frequency of occurrence of fungal species isolated from the head, thorax, and abdomen of female adults of Euwallacea fornicatus. Figures at A and B compare the frequencies of fungal species and the frequencies of Fusarium kuroshium among various body parts, respectively. The figure in parentheses indicates the number of beetles tested. Mean frequencies with different letters are significantly different among fungal species or body parts at the 1% level, using Fisher’s exact test with Bonferroni correction. SD, standard deviation.
Penicillium citrinum was a commonly detected species, with FOs of 37.6% and 43.5% in the thorax and abdomen of females, respectively (Table 2). However, the FO of P. citrinum in the head of females was never detected, although in males, it was dominant in all body parts compared with the females (Table 2).
External symptom
The initial change observed was that several saplings subjected to the toothpicks inoculated with F. kuroshium (FK) treatment started to wilt rapidly around 3 days following inoculation, and 4 saplings (FK_3, 7, 8, 10; referred to as FK_D) finally died (Supplementary Figs. S1 and S4). The other 6 saplings (FK_1, 2, 4, 5, 6, 9; referred to as FK_L) survived until the end of monitoring (Supplementary Fig. S4). No wilting was observed on all saplings subjected to the toothpicks inoculated with F. decemcellulare (FD) and sterilized control toothpicks (CT) treatments (Supplementary Fig. S4). After inoculation, leaf stomatal conductance (LSC) in FK_D and FK_5 and 9 decreased markedly, with FK_5 and 9 showing slight recovery, whereas LSCs in the other FK, FD, and CT treatments maintained over 100 of its value throughout monitoring (Fig. 4).
Mean leaf stomatal conductance in each Mangifera indica sapling. Means were calculated from data obtained via measuring the same 5 leaves twice a week between 9:00 and 13:00 h using a leaf porometer. FK F. kuroshium, with four saplings (red line) that eventually perished. FD F. decemcellulare; CT sterilized control toothpick. The treatment information is shown in Table 3.
Internal symptoms
In contrast to FD and CT, no pink area above the inoculation site was observed in FK_D (Supplementary Fig. S5). The rates (percent ratios) of xylem sap-conduction area (XS) values (Fig. 5) of FK_D were 0% at 0–40 cm distance from the site, whereas those of FK_L, FD, and CT mostly ranged between 60% and 100%, except for the site representing 0 cm. In addition, a pink area was observed at a − 5 cm distance from the site, even in FK_D (Supplementary Fig. S5), while the two saplings of FK_D had over 60% XS, as did FD and CT (Fig. 5). These findings indicated that water had flowed from roots to the upper stems, passing through the narrow but still functional zones of xylem.
Rates of xylem sap-conduction and xylem discoloration in each Mangifera indica sapling. FK F. kuroshium, with four saplings (red line) that eventually perished. FD F. decemcellulare; CT sterilized control toothpick. The treatment information is shown in Table 3.
Brown areas were clearly observed in FK_D overall, as also in FD at the site (0 cm) (Supplementary Fig. S5). As evidence of this, rates (percent ratios) of xylem discoloration area (XD) in FK_D were 100%, except at − 5 cm distance from the site in three saplings; furthermore, at 0 cm, those in most of FK_L and two of the FDs were over 40% (Fig. 5).
Lesions caused by the fungal inoculum and drilling wounds spread from the inoculation site (Fig. 6). In FK_D, the lesion area could no longer be recognized due to it being masked by necrosis. Therefore, the lesion measurements of FK_L were compared with those of FD and CT. Average lesion lengths in the longitudinal direction, were significantly longer in FK_L (1.47 cm), FD (1.04 cm), and CT (0.63 cm), in that order (Fig. 6c). Moreover, in the tangential direction, the average of FK_L was significantly longer than that of FD and CT, both of which showed no significant difference (Fig. 6d).
Lesion lengths showing xylem discoloration caused by Fusarium kuroshium, Fusarium decemcellulare, and a sterilized toothpick (control). (a,b) show measurement directions (L: longitudinal; T: tangential) of lesion length. If lesions between inoculation sites were connected, the data were not used. (c,d) show box (25–75% data range) and whisker (values within 1.5 interquartile range) plots of the length in longitudinal and tangential directions, respectively. Mean lengths with different letters are significantly different among treatments at the 1% level; Kruskal–Wallis test with Bonferroni correction; SD standard deviation.
In re-isolation, F. kuroshium was certain to be found in stem tissues around inoculation sites of FK_D. By contrast, fungal inocula were not detected in any other saplings.
Discussion
In this study, we define a ‘symbiotic’ relationship as a close and long-term biological interaction between ambrosia beetles and their fungal associates. These fungal associates must be stored in the beetles’ mycangia before being released into the galleries, and they should represent a significant proportion of the fungal flora collected from the mycangia. Fungi associated with ambrosia beetles were classified into primary ambrosia fungi (PAF) and auxiliary ambrosia fungi (AAF)34. The findings of this study indicated that, of the 23 species of fungi that were identified, F. kuroshium was most closely associated with E. fornicatus females and dominant in its head. Thus, the female, which has oral mycangia in its head3, serves as the vector for F. kuroshium (Table 2), which is a PAF. The nutritional role played by F. kuroshium, the spores and/or hyphae of which serves as a food source for E. fornicatus larvae, remains to be analyzed. The other fungal species found in the head of E. fornicatus showed far lower dominance and were isolated from only a few beetle samples, thus being regarded as AAF. By contrast, in E. fornicatus males, P. citrinum was more frequently isolated than other species (Table 2), suggesting that a lack of mycangia may strongly affect the abundance and dominance of the fungal flora.
Fusarium fungus-Euwallacea beetle symbiosis has been reported in many countries and regions (Table 1) but with high levels of variation between them23,35,36. F. kuroshium has been isolated from the heads of E. kuroshio, which attacks California sycamore (Platanus racemosa Nutt.) and avocado (P. americana) in California, United States23,24 (Table 1). However, our results indicated that F. kuroshium is associated with E. fornicatus in Okinawa main island, Japan. Previous studies have shown that, although a strict relationship exists between them in native areas36, the relationship becomes unstable in invasion areas, due to “host shifts”, which are attributed to Fusarium fungi being able to change host beetles over time16,35. Several exotic species have switched or gained fungal associates in areas they were newly introduced36,37. Co-phylogenetic analyses have been conducted to assess symbiont fidelity within these symbioses35. Thus, although F. kuroshium-E. fornicatus symbiosis in Okinawa may remain similar in origin, it may have possibly been switched during adaptation to a new habitat, namely mango orchards. Furthermore, recent studies have revealed that E. fornicatus and E. kuroshio can reproduce and survive on each other’s symbiotic fungi in their invasive range on artificial media36, although normally E. fornicatus does not reproduce well in avocado38. Our study indicates that these possibilities remain unresolved.
F. kuroshium is the causal agent of FDB in several tree species, in which the fungus grows into wood tissue, blocks xylem vessels, and obstructs water flow throughout the host plant8,24,39. To the best of our knowledge, this study is the first to demonstrate the pathogenicity of F. kuroshium in mango. Herein, 40% of the F. kuroshium-inoculated saplings died with symptoms similar to those seen in mango orchards, which indicated that xylem sap-conduction, as well as leaf stomatal conductance and xylem coloration, were seriously compromised. Our inoculation tests clarified that F. kuroshium caused the largest tangential and longitudinal lesions, which tend to destroy xylem parenchyma cells via mycelial invasion40. Thus, the novel FDB observed in mango in Okinawa, Japan following E. fornicatus migration may be attributed to a unique beetle-fungus-tree combination. This is similar to Fusarium spp.-E. fornicatus symbiosis in wilt syndrome and avocado trees seen in Israel41.
Conversely, F. decemcellulare appears to be a ‘by-chance’ species in the thorax and abdomen of E. fornicatus, being classified as AAF (Table 2). A pathogenicity study conducted in Puerto Rico42 found that saplings inoculated with F. decemcellulare showed no FDB and displayed lesions that were significantly larger than those of the control but smaller than those in saplings inoculated with F. kuroshium. These results suggested that F. decemcellulare may have weak pathogenic potential pertaining to mango. However, according to a study conducted by Qi et al.43 in China, F. decemcellulare did cause dieback in M. indica ‘Keitt’, which is a variety that is different from the one (M. indica ‘Irwin’) used in this study. Therefore, more detailed studies to evaluate the susceptibility of different varieties to fungal pathogenicity and beetle-linked boring may be warranted.
Our study also revealed that Lasiodiplodia theobromae (Pat.) Griffon & Maubl., classified as AAF, consistently appeared in the females of all three body parts (Table 2). Previous studies have reported that L. theobromae caused serious disease in mango trees in Sindh, Pakistan44. This discovery suggests that this fungal pathogen may potentially have risk for wilt disease in mango trees also in Japan. To address this question, further inoculation experiments involving this fungus on mango trees or saplings are required.
Conclusions
This study found that F. kuroshium, a mycangial fungus of E. fornicatus, may be a pathogen of mango, whereas F. decemcellulare only showed weak pathogenic potential with respect to mango. Our findings provide strong evidence that F. kuroshium-E. fornicatus symbiosis causes mango wilt disease at least in Okinawa main island, Japan, and may thereby contribute to target detection for wilt disease management.
Materials and methods
Sample collection
In the summer of 2018, M. indica (variety; Irwin) trees showing wilting and discoloration of leaves were found in a mango orchard in Nago city, Okinawa main island, Japan. The trees displayed numerous small holes on the surfaces of their trunks and branches, and these holes were found extruding wood particles (Supplementary Fig. S1a). This infestation pattern was typical of boring by ambrosia beetles.
A representative 50-cm long log (Supplementary Fig. S1a) was cut from an infested branch on August 30, 2018 and rapidly transported to the laboratory of Nagoya University. The log was placed in a container, which was then sealed, to capture emerging adult beetles. The adults were collected every few days and immediately placed in 1.5-mL sterile tubes using sterilized forceps for fungal isolation at a later stage.
All collected adults were observed under stereo microscopes (OLYMPUS SZ6045-TRPT and SZX16) (Olympus Optical Co., Ltd., Tokyo, Japan) and identified using morphological features23. Some of them were forwarded to Michigan State University for confirmation by both morphology and DNA analysis. Images were obtained using a high-resolution microscope camera (s) (HRMC) (OLYMPUS DP12 and DP20) with 3D software (OLYMPUS Cellsens Standard).
Molecular confirmation of beetle identity
In addition, DNA from one female specimen (voucher SAX551) was extracted and amplified via PCR and sequenced partial cytochrome oxidase I (COI) (675 bp) and carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD) (501 bp) following the protocol in Cognato et al.45. Sequences (COI: OR822285; CAD: OR827366) were subject to a Blast search in GenBank which returned similar Euwallacea sequences generated by Wang et al.31. These sequences (COI: MN619931, MN619937, MT026213, MT623419-MT62341951, MT623453-MT623457; CAD: MN620205, MN620212, MT634154-MT634186, MT634188-MT634192) were assembled into a Nexus file for phylogenetic analysis. In PAUP*46, a heuristic search with 100 stepwise random additions was used to find the most parsimonious trees. Bootstrap values were calculated with 300 pseudoreplicates using heuristic searches with simple addition.
Fungal isolation from the adults and culturing
Potato dextrose agar (PDA: 4 g potato starch, 20 g dextrose, 15 g agar, distilled water up to 1 L) supplemented with streptomycin sulfate (100 mg/L), and synthetic low-nutrient agar (SNA: 1 g KH2PO4; 1 g KNO3; 0.5 g MgSO4·7H2O; 0.5 g KCl; 0.2 g Glucose; 20 g Agar; 1 L distilled water) were autoclaved at 121 °C for 15 min. Sterile 9-cm Petri dishes (INA-OPTIKA Co., Ltd., Osaka, Japan) containing 10-ml PDA or SNA culture medium were prepared and kept in a sterile laminar flow chamber under UV light until culture medium solidification. Fungal cultures on PDA were used to characterize colony and odor, whereas those on SNA were used to examine microscopic characters.
Whole beetles were surface-washed by vortexing for 15 s in 1.5-mL sterile tubes containing 1 mL sterile distilled water and one small drop of Tween 20 (< 10 μL). The surface-washed beetles were dried on sterile filter paper after rinsing with sterile distilled water. Each beetle sample was separated into head, thorax, and abdomen under a dissection microscope with two sterilized pins. Afterward, the three body parts without being ground up were singly inoculated on PDA plates at 25 °C for 7–10 days. The total number of fungal colonies formed on each plate was recorded, and the colonies were transferred to other PDA plates for purification.
Fungal identification and quantification
Using the method described by Jiang et al.47,48, the purified isolates from each body part were initially sub-identified at the morphotype level, based on colony properties (e.g., color, thickness, transparency, texture, and growth speed) and fungal micro-structures. They were observed and photographed using a compound microscope (OLYMPUS BX41) equipped with HRMC (OLYMPUS DP23). Five isolates of each morphotype were stored on PDA slants at 25 ℃.
At least one isolate from each morphotype was selected for DNA extraction. Final isolate identification was based on the sequencing of internal transcribed spacer (ITS) rDNA, adding three genes for Fusarium fungi: translational elongation factor 1-α (TEF1), DNA-directed RNA polymerase II largest (RPB1), and second largest subunit (RPB2) (Fig. 2). These sequences were amplified using primer pairs, ITS1F and ITS4 for ITS sequences49, EF1 and EF2 for TEF150, AF-RPB1F and AF-RPB1R for RPB147, and AF-RPB2F and AF-RB2R for RPB247. Amplicons were purified and sequenced as described by Jiang et al.47,48. A homology search was performed with each obtained sequence on the web site of NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and used together with morphological characters to identify isolated fungi (Supplementary Table S1). For the identification of ambrosia fusaria, phylogenetic analysis was conducted with related Fusarium spp. (Fig. 2, Supplementary Table S2). The phylogeny tree was constructed using the maximum likelihood method based on the Kimura 2-parameter model with MEGA751. The tree involved 70 nucleotide sequences. All positions with < 95% site coverage were eliminated; fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. A total of 1,745 positions were present in the final dataset.
RD and FO of fungal species isolated from each body part were calculated using the equations below:
Pathogenicity tests
A total of 30 mango saplings (1-year-old) were used for this test (Table 3). The variety ‘Irwin’ of M. indica was selected because the cultivated trees of this variety commonly show severe dieback symptoms in mango orchards of Japan. All experimental research and field studies involving plants, whether cultivated or wild, including the collection of plant material, adhered to relevant institutional, national, and international guidelines and legislation. The methods employed were in accordance with the appropriate guidelines, regulations, and legislation. The plant material was sourced as follows:
Nature of Biological resource: Plant.
Common Name: Mango.
Scientific Name (Genus and Species): Mangifera indica.
Exact part used: Stem.
Source of access (Wild/Culture/Trader): Trader.
Exact place (village, Taluk, District, State) of access of Biological source: Nursery tree growers (members of Japan Agricultural Cooperatives).
All saplings were acquired from a nursery stock of the growers and transferred to the greenhouse of Nagoya University (Nagoya, Japan) on May 21, 2021. After two weeks, the saplings were transferred to plastic bags filled with potting media (Super soil, Akimoto Tensanbutsu Co. Ltd., Mie, Japan), and each pot was covered with a fine nylon net to prevent the entry of root feeders (scarab beetle). None showed disease symptoms until the beginning of fungal inoculation.
To resolve issues associated with high air temperatures during the growth period of saplings, a black sunshade net was installed inside the greenhouse. A data logger (Thermochron G type, KN laboratories Inc., Osaka, Japan) was set 1.2 m above the floor to monitor the air temperature within.
F. kuroshium (EF-1-H) and F. decemcellulare (EF-44-A), which were isolated from E. fornicatus, were designated as fungal inocula in this test (Table 3). They were grown on PDA media in a 9-cm Petri dish for 3 weeks (25 ℃, dark). At the same time, ten sterilized toothpicks (L = 7 cm, d = 2.2 mm) were added to each dish to adhere to their hyphae. Sterilized toothpicks were used as the control inoculum. Before inoculation, four sets of holes (4 mm diameter) were made on the stem (1.15 ± 0.12 cm, diameter; mean ± SD) (Supplementary Table S3) of each sapling by vertically drilling through the center of the stem with an electric drill, starting at 5 cm above soil surface with 2 cm intervals between holes (Supplementary Fig. S2).
Fungal inoculation was conducted on September 9, 2021, as previously described by Jiang et al.52. Toothpicks contained with F. kuroshium (FK) and F. decemcellulare (FD) were inserted into the holes of each of 10 mango saplings (Table 1). CT were also inserted into the holes of 10 saplings. Immediately after inoculation, each inoculation site was sealed with paraffin tape (Parafilm) to prevent dehydration.
Monitoring after inoculation
All inoculated saplings were examined daily for external symptoms until October 21, 2021. The maximum air temperature in the greenhouse during this test period was 31.42 ± 4.29 °C.
Classification of the wilting process was defined as follows: i) no symptoms (NS): no apparent difference compared to non-inoculated saplings; ii) leaf wilting (LW): some leaves began to droop and wilt; iii) browning of leaves and discoloration of branches (BD): almost all leaves became brown and branch discolored; iv) sprouting of shoot (SS): some shoots sprouted below inoculation part (Supplementary Fig. S3).
LSC was measured in mmol/m2/s units during 9:00–13:00 h on sunny days (on days when rain was forecast, measurement was moved to an earlier or later time) twice a week, using a leaf porometer (SC1-Leaf Porometer, Decagon Devices Inc., Pullman, WA, USA). Each sapling was watered using 400–500 mL of water between 16:00–17:00 h of the previous day. Five leaves in each sapling were selected randomly and numbered with tags in ascending order, to fix the measurement order of all leaves. The five data points for each sapling were averaged.
Xylem sap-conduction test
After monitoring external symptoms in the inoculated saplings, some were evaluated for water-flow in their main stems (FK = 10, FD = 10, CT = 6 saplings) (Supplementary Fig. S4). Immediately after being cut at the base of the main stems, the cut ends were immersed in 1% (w/v) aqueous acid fuchsin for 5 h in the greenhouse. Next, the main stems were cut into 5-cm long segments. The xylem sap-conduction area (pink area, dyed with acid fuchsin; functional xylem), the xylem discoloration area (brown area, reacted to drilling injury and mycelial infestation; non-functional xylem), and the whole cross-section area (excluding the pith) (Supplementary Fig. S5) of the cut ends of these segments were measured. Images of the cut ends were obtained via a digital camera (Olympus Tg-3), and each area was estimated using ImageJ (Win64, version 1.53f51, National Institutes of Health, USA).
XS or XD in each segment was calculated as follows:
Lesion length measurement and re-isolation of inoculated fungi
After obtaining images of cut ends for the xylem sap-conduction test, the segments were used to measure lesion (brown-colored wood tissues) lengths in longitudinal and tangential directions (Fig. 6a,b) and for the re-isolation of inoculated fungi, including additional segments of CT-01 sapling (Supplementary Fig. S4).
An upper 1 cm section was cut off from each 5-cm long segment and longitudinally divided into two parts using a tree pruner. The two parts were immediately surface-sterilized for 1 min in 70% (v/v) ethyl alcohol solution. A second sterilization was performed using 1% (v/v) antiformin solution for 1 min. A rinse wash was performed using sterile distilled water for 1 min followed by drying using sterile filter paper for 1 min.
The sterilized section parts were placed on PDA (25 ℃, dark), and re-isolated fungi were confirmed via colony morphology and DNA sequencing methods described above.
The remaining 4-cm long segment was also divided longitudinally into two parts using a tree pruner. The cross-sections were photographed for the purpose of detecting lesions spreading around the inoculation site (Fig. 6a,b). ImageJ was used to measure the lesion lengths in the photographs.
Statistical analyses
Differences in the median FOs among adult body parts or the fungal species were compared using Fisher’s exact test. Lesion lengths in inoculation treatments were compared using the Kruskal–Wallis test. Multiple comparisons among them were conducted using the post-hoc tests with Bonferroni correction. Analyses were performed using SPSS version 19.0 software (IBM Corporation, Armonk, NY, USA, 2010).
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Jordal, B. H., Sequeira, A. S. & Cognato, A. I. The age and phylogeny of wood boring weevils and the origin of subsociality. Mol. Phylogenet. Evol. 59, 708–724 (2011).
Hulcr, J., Atkinson, T. H., Cognato, A. I., Jordal, B. H. & McKenna, D. D. Morphology, taxonomy, and phylogenetics of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species (eds Vega, F. E. & Hofstetter, R. W.) 41–84 (Elsevier, 2015).
Francke-Grosmann, H. Ectosymbiosis in wood-inhabiting insects. In Symbiosis: Associations of invertebrates, birds, ruminants and other biota (ed. Henry, S. M.) 141–206 (Elsevier, 1967).
Hulcr, J. et al. A Jungle in there: Bacteria in belly buttons are highly diverse, but predictable. PLoS ONE 7, e47712 (2012).
Beaver, R. A., Sittichaya, W. & Liu, L. Y. A synopsis of the scolytine ambrosia beetles of Thailand (Coleoptera: Curculionidae: Scolytinae). Zootaxa 3875, 1–82 (2014).
Hulcr, J. & Dunn, R. R. The sudden emergence of pathogenicity in insect–fungus symbioses threatens naive forest ecosystems. Proc. R. Soc. B 278, 2866–2873 (2011).
Mendel, Z. et al. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 40, 235–238 (2012).
Eskalen, A. et al. Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Dis. 97, 938–951 (2013).
Ploetz, R. C., Hulcr, J., Wingfield, M. J. & de Beer, Z. W. Destructive tree diseases associated with ambrosia and bark beetles: Black swan events in tree pathology?. Plant Dis. 97, 856–872 (2013).
Hughes, M. A. et al. No rest for the laurels: Symbiotic invaders cause unprecedented damage to southern USA forests. Biol. Invasions 19, 2143–2157 (2017).
Schuler, H. et al. Recent invasion and eradication of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae) from tropical greenhouses in Europe. Biol. Invasions 25, 299–307 (2023).
Roy, B. A. et al. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 12, 457–465 (2014).
Rabaglia, R. J. et al. Early detection and rapid response: A 10-year summary of the USDA Forest Service program of surveillance for non-native bark and ambrosia beetles. Am. Entomol. 65, 29–42 (2019).
Bierman, A., Roets, F. & Terblanche, J. S. Population structure of the invasive ambrosia beetle, Euwallacea fornicatus, indicates multiple introductions into South Africa. Biol. Invasions 24, 2301–2312 (2022).
Freeman, S. et al. Fusarium euwallaceae sp. nov.—a symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 105, 1595–1606 (2013).
Stouthamer, R. et al. Tracing the origin of a cryptic invader: Phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agric. For. Entomol. 19, 366–375 (2017).
Gomez, D. F., Lin, W., Gao, L. & Li, Y. New host plant records for the Euwallacea fornicatus (Eichhoff) species complex (Coleoptera: Curculionidae: Scolytinae) across its natural and introduced distribution. J. Asia-Pac. Entomol. 22, 338–340 (2019).
Danthanarayana, W. The distribution and host-range of the shot-hole borer (Xyleborus fornicatus Eich.) of tea. Tea Q. 39, 61–69 (1968).
Carrillo, D. et al. Distribution, pest status and fungal associates of Euwallacea nr. fornicatus in Florida avocado groves. Insects 7, 55 (2016).
Freeman, S. et al. Symbiotic association of three fungal species throughout the life cycle of the ambrosia beetle Euwallacea nr. fornicatus. Symbiosis 68, 115–128 (2016).
Paap, T., de Beer, Z. W., Migliorini, D., Nel, W. J. & Wingfield, M. J. The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: A new invasion in South Africa. Australas. Plant Pathol. 47, 231–237 (2018).
Kasson, M. T. et al. An inordinate fondness for Fusarium: Phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet. Biol. 56, 147–157 (2013).
Gomez, D. F. et al. Species delineation within the Euwallacea fornicatus (Coleoptera: Curculionidae) complex revealed by morphometric and phylogenetic analyses. Insect Syst. Divers. 2 (6), 1–11 (2018).
Na, F. et al. Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of kuroshio shot hole borer (Euwallacea sp. nr. fornicatus) cause Fusarium dieback on woody host species in California. Plant Dis. 102, 1154–1164 (2018).
Báez-Vallejo, N. et al. Forest tree associated bacteria for potential biological control of Fusarium solani and of Fusarium kuroshium, causal agent of Fusarium dieback. Microbiol. Res. 235, 126440 (2020).
Gutiérrez-Sánchez, A. et al. Characterization of the exo-metabolome of the emergent phytopathogen Fusarium kuroshium sp. nov., a causal agent of Fusarium dieback. Toxins 13, 268 (2021).
Wang, M. et al. Water balance altered in cucumber plants infected with Fusarium oxysporum f. sp. cucumerinum. Sci. Rep. 5, 7722 (2015).
Nobuchi, A. & Ono, S. Bark beetles from the Bonin islands (Coleoptera, Scolytidae). Entomol. Soc. Jpn. 41, 181–182 (1973).
Yamaguchi, T. et al. Insect pests of the mango plant, Mangifera indica, on the Amami islands, Japan. Kyushu Plant Prot. Res. 52, 60–65 (2006).
Yamaguchi, T. Insect pests of the mango plant in Japan, and their managements. Plant Prot. 66, 612–618 (2012).
Wang, Y. et al. Uncovering the hidden diversity within the Euwallacea fornicatus species complex in China. Entomol. Gen. 42, 631–639 (2022).
Nalim, F. A., Samuels, G. J., Wijesundera, R. L. & Geiser, D. M. New species from the Fusarium solani species complex derived from perithecia and soil in the old world tropics. Mycologia 103, 1302–1330 (2011).
Crous, P. W. et al. Fusarium and allied fusarioid taxa (FUSA). Fungal Syst. Evol. 9, 161–200 (2022).
Batra, L. R. Ambrosia fungi: Extent of specificity to ambrosia beetles. Science 153, 193–195 (1966).
O’Donnell, K. et al. Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallacea ambrosia beetle mutualism. Fungal Genet. Biol. 82, 277–290 (2015).
Carrillo, J. D. et al. Members of the Euwallacea fornicatus species complex exhibit promiscuous mutualism with ambrosia fungi in Taiwan. Fungal Genet. Biol. 133, 103269 (2019).
Carrillo, J. D., Dodge, C., Stouthamer, R. & Eskalen, A. Fungal symbionts of the polyphagous and kuroshio shot hole borers (Coleoptera: Scolytinae, Euwallacea spp.) in California can support both ambrosia beetle systems on artificial media. Symbiosis 80, 155–168 (2020).
Mendel, Z. et al. What determines host range and reproductive performance of an invasive ambrosia beetle Euwallacea fornicatus: Lessons from Israel and California. Front. For. Glob. Change 4, 654702 (2021).
Lynch, S. C. et al. Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.—two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia 108, 313–329 (2016).
Kuroda, K. Xylem dysfunction in Yezo spruce (Picea jezoensis) after inoculation with the blue-stain fungus Ceratocystis polonica. Forest Pathol. 35, 346–358 (2005).
Mendel, Z. et al. The role of Euwallacea nr. fornicatus (Coleoptera: Scolytinae) in the wilt syndrome of avocado trees in Israel. Phytoparasitica 45, 341–359 (2017).
Serrato-Diaz, L. M., Perez-Cuevas, M., Rivera-Vargas, L. I., Goenaga, R. & French-Monar, R. D. First report of Fusarium decemcellulare causing inflorescence wilt and vascular and flower necrosis of rambutan (Nephelium lappaceum), longan (Dimocarpus longan), and mango (Mangifera indica). Plant Dis. 99, 1187 (2015).
Qi, Y. X. et al. First report of dieback of mango caused by Fusarium decemcellulare in China. J. Phytopathol. 161, 735–738 (2013).
Khanzada, M. A., Lodhi, A. M. & Shahzad, S. Pathogenicity of Lasiodiplodia theobromae and Fusarium solani on mango. Pak. J. Bot. 36, 181–190 (2004).
Cognato, A. I. et al. The essential role of taxonomic expertise in the creation of DNA databases for the identification and delimitation of Southeast Asian ambrosia beetle species (Curculionidae: Scolytinae: Xyleborini). Front. Ecol. Evol. 8, 27 (2020).
Swofford, D. L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other methods). Version 4 (Sunderland, 2002).
Jiang, Z. R., Masuya, H. & Kajimura, H. Novel symbiotic association between Euwallacea ambrosia beetle and Fusarium fungus on fig trees in Japan. Front. Microbiol. 12, 725210 (2021).
Jiang, Z. R., Masuya, H. & Kajimura, H. Fungal flora in adult females of the rearing population of ambrosia beetle Euwallacea interjectus (Blandford) (Coleoptera: Curculionidae: Scolytinae): Does it differ from the wild population?. Diversity 14, 535 (2022).
White, T. J., Bruns, T., Lee, S. & Taylor, J. W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (eds Innis, M. A. et al.) 315–322 (Elsevier, 1990).
O’Donnell, K., Kistler, H. C., Cigelnik, E. & Ploetz, R. C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 95, 2044–2049 (1998).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Jiang, Z. R. et al. The role of mycangial fungi associated with ambrosia beetles (Euwallacea interjectus) in fig wilt disease: Dual inoculation of Fusarium kuroshium and Ceratocystis ficicola can bring fig saplings to early symptom development. Microorganisms 10, 1912 (2022).
Lynn, K. M. et al. Euwallacea perbrevis (Coleoptera: Curculionidae: Scolytinae), a confirmed pest on Acacia crassicarpa in Riau, Indonesia, and a new fungal symbiont; Fusarium rekanum sp. nov. Antonie van Leeuwenhoek 113, 803–823 (2020).
Smith, S. M., Gomez, D. F., Beaver, R. A., Hulcr, J. & Cognato, A. I. Reassessment of the species in the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) complex after the rediscovery of the “lost” type specimen. Insects 10, 261 (2019).
García-Avila, C. D. J. et al. First report of Euwallacea nr. fornicatus (Coleoptera: Curculionidae) in Mexico. Fla. Entomol. 99, 555–556 (2016).
Acknowledgements
We wish to thank Nene Tanoue for help with the inoculation test. We also thank Naoki Hijii, Wataru Toki, and other members of the Forest Protection Lab. of Nagoya University for their suggestions to improve our work. This work was supported by the JSPS Grant (KAKENHI: 18KK0180, 19H02994, and 20H03026).
Author information
Authors and Affiliations
Contributions
H.K. and Z.-R.J. conceived this study. N.K. collected the tree sample. H.K. collected the beetle samples and identified them by observing the morphology. S.M.S. and A.I.C. confirmed the identification based on morphological and molecular analysis, respectively. Z.-R.J. isolated the fungi. H.M. sequenced and identified the fungi. K.K. established inoculation method. M.T., Z.-R.J., and H.K. did inoculation test and analyzed data. Z.-R.J. wrote an early MS version. H.K. supervised this study and revised the MS. All authors discussed and contributed to this study, and approved final MS version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, ZR., Tanoue, M., Masuya, H. et al. Fusarium kuroshium is the primary fungal symbiont of an ambrosia beetle, Euwallacea fornicatus, and can kill mango tree in Japan. Sci Rep 13, 21634 (2023). https://doi.org/10.1038/s41598-023-48809-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48809-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.