Abstract
Rhabdoid tumors are aggressive tumors that may arise in the kidney, soft tissue, central nervous system, or other organs. They are defined by SMARCB1 (INI1) or SMARCA4 alterations. Often, very young children are affected, and the prognosis is dismal. Four patients with primary atypical teratoid rhabdoid tumor (AT/RT, a rhabdoid tumor in the central nervous system) were treated by resection and high dose chemotherapy. Tazemetostat was introduced after completion of chemotherapy. Three patients have achieved an event free survival of 32, 34, and 30 months respectively. One progressed and died. His overall survival was 20 months. One patient was treated for a relapsed atypical teratoid rhabdoid tumor. The treatment combined metronomic therapy, radiotherapy, tazemetostat and immunotherapy. This patient died of disease progression, with an overall survival of 37 months. One patient was treated for a rhabdoid tumor of the ovary. Tazemetostat was given as maintenance after resection, chemotherapy, and radiotherapy, concomitantly with immunotherapy. Her event free survival is 44 months. Only approximately 40% of patients with rhabdoid tumors achieve long-term survival. Nearly all relapses occur within two years from diagnosis. The event free survival of four of the six patients in our cohort has exceeded this timepoint. Tazemetostat has been mostly tested as a single agent in the relapsed setting. We present promising results when applied as maintenance or add on in the first line treatment.
Similar content being viewed by others
Introduction
Rhabdoid tumors are aggressive malignancies that primarily affect children below the age of 3 years. While these tumors can manifest in various anatomical localizations, the kidney, central nervous system (known as atypical teratoid rhabdoid tumor or AT/RT) or soft tissue are the most commonly affected sites1.
The development of rhabdoid tumors is associated with the inactivation of the SMARCB1 gene (also called INI1), which encodes a critical component of the SWI/SNF ATP-dependent chromatin remodeling complex. Less commonly, inactivation of another SWI/SNF complex component, SMARCA4, is found2. The SWI/SNF complex antagonizes the function of the PCR2 complex, which is responsible for methylating histone H3 at the lysine 27 residue (H3K27me3) leading to the formation of transcriptionally silent chromatin. Inactivation of SMARCB1 or SMARCA4 causes dysfunction of the SWI/SNF complex. This complex would normally suppress the expression of EZH2, it’s dysfunction therefore leads to overexpression of EZH2, which subsequently leads to higher activity of the Polycomb complex 2 of which EZH2 is an important component. This leads to widespread trimethylation of H3K27 and subsequent suppression of gene expression3.
Tazemetostat (EPZ-6438) is a highly selective EZH2 inhibitor with antineoplastic activity4. It has been studied in the treatment of oncologic diseases in adults such as advanced epithelioid sarcoma5 or follicular lymphoma6, and is now emerging for the treatment of other INI1-negative tumors.
Results
From 2019 to the end of 2022 four patients with a primary AT/RT, one patient with a relapsed AT/RT and one patient with a malignant rhabdoid tumor of the ovary were treated by tazemetostat.
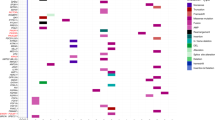
Patients 1 to 4, who were diagnosed with localized SMARCB1 mutated AT/RT, underwent treatment with a combination of maximal safe resection and chemotherapy as per the COG ACNS0333 trial protocol7. Among these patients, complete remission (CR) was achieved in patients 1 and 3, while patients 2 and 4 attained partial remission (PR). Patient and tumor characteristics are shown in Tables 1, and 2 summarizes the treatment prior to the introduction of tazemetostat.
Tazemetostat was introduced after the completion of chemotherapy (dosing details and an overview of the whole treatment in Table 3). Reasons for dose reduction were hematotoxicity in patient 3 and 4 and bromide accumulation in patient 2. Patients 1, and 3 maintained CR, completed two years of tazemetostat treatment and are now in follow-up. Their overall survival (OS) at the last follow-up visit is 32 and 34 months, respectively. Patient 4 maintained PR achieved by resection and chemotherapy, with OS of 30 months at the last follow-up. Patient 2 maintained PR for eight months of tazemetostat treatment (four months full dose, four months reduced dose for toxicity), then progressed. The first event-free survival (EFS1) was 13 months. A second PR was achieved by resection. Tazemetostat was reintroduced in the full dose and given concomitantly with immunotherapy consisting of dendritic cell vaccine and nivolumab. After three months another tumor progression occurred, with EFS2 of 5 months. The patient died of disease progression, with an OS of 20 months.
Patient 5, who harbored germline SMARCB1 alteration, was treated for relapsed AT/RT. The primary tumor had been in the posterior fossa. It had been treated in another country by gross total resection, adjuvant chemotherapy as per the EU-RHAB protocol8 and proton radiotherapy (RT) to the tumor bed (54,0 Gy). Nine months after completing treatment a metastatic relapse in the spinal cord was diagnosed with an EFS1 of 21 months. The subsequent treatment, which was as per the MEMMAT protocol9 administered concomitantly with photon RT to the whole spine (23,0 Gy) with a boost to the metastasis (15,0 Gy), led to a second PR. Tazemetostat was introduced two months after RT and given concomitantly with MEMMAT treatment. After 6 weeks, metastatic progression in the posterior fossa was diagnosed with an EFS2 of 5 months. Following another six months, MRI showed regression of spinal metastasis and a slight progression of the posterior fossa lesions. Proton RT was then administered to the whole brain (30,6 Gy) with a boost to the posterior fossa (23,4 Gy). MRI after RT confirmed a third PR. Tazemetostat was continued concomitantly with immunotherapy consisting of nivolumab and ipilimumab. Immunotherapy had to be discontinued after three months due to grade 3 autoimmune colitis. Subsequently, new tumor sites were found in the pelvic region, liver and lungs and the patient died of tumor progression with an EFS3 of 8 months and an OS of 37 months.
Patient 6 was diagnosed with a stage IIB tumor of the right ovary at the age of 13 years. An R1 resection was performed and an ovarian malignant rhabdoid tumor arising in the context of a germline SMARCA4 alteration was confirmed. The patient underwent adjuvant chemotherapy consisting of three cycles of standard BEP, two cycles as per EpSSG NRSTS 200510 (discontinued for grade IV febrile neutropenia) and RT of the peritoneal cavity (15 Gy) with a boost to the tumor bed (21,6 Gy). Tazemetostat maintenance concomitantly with nivolumab was initiated. This treatment lasted for two years with no significant toxicity. At the last follow-up, 10 months after completing the treatment, the patient was in complete remission, with an EFS/OS of 44 months.
Discussion
Although the introduction of high-dose chemotherapy with RT for AT/RT has significantly increased EFS and OS rates, more than 50% of children diagnosed with AT/RT still die of tumor progression7. The young age of most AT/RT patients renders them particularly susceptible to the toxic side effects of RT, thereby adversely impacting the quality of life of survivors.
The four patients with primary AT/RT in our cohort were all younger than three years of age, with one patient younger than one year. Treatment of these patient involved chemotherapy and high-dose chemotherapy according to the COG ACNS0333 protocol. The focal RT for localized disease was omitted in our patients and up to 2 years of tazemetostat maintenance was employed instead. According to the results of ACNS0333, 91% of the progressions occurred within two years from diagnosis and at four years the EFS was 37%7. Three of four of our patients demonstrated an EFS ranging from 2,5 to 3 years at last follow-up. The patient treated for a metastatic relapse of AT/RT achieved an OS of 37 months (EFS1 = 21 months, EFS2 = 6 months, EFS3 = 8 months). Long-term survival in relapsed patients is rare, as only 5% of early relapsed patients in the European Rhabdoid Registry survived five years from diagnosis11. In such patients, an EFS is mostly counted in weeks rather than months12. The two deceased AT/RT patients were less than one year old at presentation, which is a recognized risk factor. Pertaining the molecular stratification of AT/RT, the non-TYR (= SHH or MYC) subtypes have been postulated to exhibit unfavorable prognosis11. One of the deceased patients was confirmed to belong to the SHH subtype. Conversely, two surviving patients also presented with the SHH subtype.
In the patient with stage II ovarian rhabdoid tumor the EFS was 44 months. Long term survival for stage II patients is described as around 40% and most relapses occur in the first 2 years, essentially none after 5 years13.
Concerning tolerance, the majority of our patients experienced grade 1 or 2 nausea and/or vomiting that did not necessitate dose reduction. Thrombopenia and neutropenia posed greater concern leading to tazemetostat dose reductions. In the relapsed AT/RT patient the combination of metronomic MEMMAT-based peroral chemotherapy and the full dose of tazemetostat was not feasible due to the combined hematologic toxicity. The youngest of our patients experienced grade 2 bromide accumulation accompanied by increased sleepiness, which also resulted in dose reduction. All our patients suffered from repeated respiratory and gastrointestinal infections, with two individuals experiencing one or more herpes zoster infections and hypogammaglobulinemia. An overview of tazemetostat toxicity is shown in Table 4.
Despite these encouraging results, international studies involving larger patient cohorts are warranted to comprehensively evaluate the efficacy of tazemetostat in the treatment of pediatric rhabdoid tumors. Rather than employing tazemetostat as a single agent in the relapsed setting, we believe that it should be further explored as maintenance in a sequential manner, or add-on in the first-line treatment, however based on our experience the single agent maintenance seems to be better tolerated.
Materials and methods
The use of tazemetostat in University Hospital Brno via the Expanded Excess Program was approved by the Ethics Committee of University Hospital Brno. Patients’ parents or guardians have signed informed consent with molecular genetic testing and off-label treatment using tazemetostat. Patients’ parents or guardians have agreed with use of patients’ data for research and publication and signed an informed consent form. All methods were carried out in accordance with relevant guidelines and regulations.
Patients were treated by surgery, chemotherapy, and in some cases radiotherapy (RT) prior to tazemetostat administration, with tazemetostat being added to standard chemotherapy, RT, or metronomic regimens. Tazemetostat was given in dosing recommended by the distributor: 1200 mg/m2 two times a day for AT/RT and 520 mg/m2 two a day for other rhabdoid tumors. Response assessment was conducted using magnetic resonance imaging. Continuous surveillance of adverse effects was undertaken. Adverse events were graded according to CTCAE 5, toxicity grade 3 led to dose modification. Tumor tissue was subjected to histological examination. To investigate the genetic basis of INI1 inactivation and identify potential concurrent alterations, comprehensive molecular profiling using whole-exome sequencing, array CGH, or MLPA was carried out.
Data availability
Details of the individual patients’ diagnostics, treatment, and follow up are available from the corresponding author upon reasonable request.
References
Geller, J. I., Roth, J. J. & Biegel, J. A. Biology and treatment of rhabdoid tumor. Crit. Rev. Oncog. 20, 199–216 (2015).
Nesvick, C. L. et al. Atypical teratoid rhabdoid tumor: Molecular insights and translation to novel therapeutics. J. Neurooncol. 150, 47–56 (2020).
Kim, K. H. & Roberts, C. W. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 207, 365–372 (2014).
Kuntz, K. W. et al. The importance of being me: Magic methyls, methyltransferase inhibitors, and the discovery of tazemetostat. J. Med. Chem. 59, 1556–1564 (2016).
Gounder, M. et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: An international, open-label, phase 2 basket study. Lancet Oncol. 21, 1423–1432 (2020).
Morschhauser, F. et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: An open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 21, 1433–1442 (2020).
Reddy, A. T. et al. Efficacy of high-dose chemotherapy and three-dimensional conformal radiation for atypical teratoid/rhabdoid tumor: A report from the children’s oncology group trial ACNS0333. J. Clin. Oncol. 38, 1175–1185 (2020).
Nemes, K. et al. Infants and newborns with atypical teratoid rhabdoid tumors (ATRT) and extracranial malignant rhabdoid tumors (eMRT) in the EU-RHAB Registry: A unique and challenging population. Cancers 14, 2185. https://doi.org/10.3390/cancers14092185 (2022).
Slavc, I. et al. Improved long-term survival of patients with recurrent medulloblastoma treated with a “MEMMAT-like” metronomic antiangiogenic approach. Cancers 14, 5128. https://doi.org/10.3390/cancers14205128 (2022).
Ferrari, A. et al. Paediatric non-rhabdomyosarcoma soft tissue sarcomas: The prospective NRSTS 2005 study by the European pediatric soft tissue sarcoma study group (EpSSG). Lancet Child Adolesc. Health. 5, 546–558 (2021).
Frühwald, M. C. et al. Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors. Neuro. Oncol. 22, 1006–1017 (2020).
Steinbügl, M. et al. Clinical evidence for a biological effect of epigenetically active decitabine in relapsed or progressive rhabdoid tumors. Pediatr Blood Cancer 68, e29267. https://doi.org/10.1002/pbc.29267 (2021).
Witkowski, L. et al. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol. Oncol. 141, 454–460 (2016).
Acknowledgements
We acknowledge the Core Facility Bioinformatics of CEITEC Masaryk University and the Core Facility Genomics supported by the NCMG research infrastructure (LM2018132 funded by MEYS CR) for their support in obtaining the scientific data presented in this paper. Tazemetostat was provided by Ipsen (formerly Epizyme). Ipsen had no input into analyses or interpretation of outcomes. Ipsen reviewed this manuscript for scientific accuracy but had no input into the content.
Funding
Supported by the Ministry of Health of the Czech Republic, grant no. NU20-03-00240. Supported by the Ministry of Health of the Czech Republic—DRO (FNBr, 65269705). Supported by the Specific University Research provided by MEYS (MUNI/A/1395/2022). Supported by the project National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102) funded by the European Union—Next Generation EU. This work is generated within the European Reference Network on Paediatric Cancer (ERN PaedCan).
Author information
Authors and Affiliations
Contributions
Writing: K.V., P.P., K.N., A.F., K.D., D.V.; Literature search: A.F., P.P., K.N., K.D.; Investigation, data collection and interpretation: Z.P., V.B., J.S., M.B., P.T., K.V., M.J., H.P., L.K.; Program coordination: MB, ZP; Supervision: D.V., O.S., J.S.; Reviewed/Edited All;
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vejmelkova, K., Pokorna, P., Noskova, K. et al. Tazemetostat in the therapy of pediatric INI1-negative malignant rhabdoid tumors. Sci Rep 13, 21623 (2023). https://doi.org/10.1038/s41598-023-48774-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48774-2
This article is cited by
-
Multiple drugs
Reactions Weekly (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.