Abstract
Systematic review and meta-analysis applying PRISMA guidelines with a PICOS format was constructed to provide an overview of changes in physical performance, body composition and physical training in soldiers during prolonged (≥ 3 months) military operations. Twenty-four studies out of the screened 4431 records filled the inclusion criteria. A small decrease in endurance performance was the most consistent finding (Hedge's g [g] − 0.21, 95% CI − 0.01 to − 0.41) while small overall increases in maximal strength of the lower (g 0.33, 95% CI 0.16–0.50) and upper body (g 0.33, 95% CI 0.19–0.46) were observed. In addition, small increases in strength endurance (push-up, g 0.34, 95% CI 0.15–0.52; sit-up g 0.26, 95% CI 0.07–0.44) were observed. The overall changes in body composition were trivial. Heterogeneity in the outcome variables varied mainly between low to moderate. Large inter-individual variations were observed in physical training volume, including decrements especially in endurance training frequency and volume. A reduction in total training load was often associated with negative changes in body composition and physical performance according to the principle of training specificity. Individuals with higher initial fitness level were more susceptible to decrements in their physical performance during operation.
Similar content being viewed by others
Introduction
Arduous occupational physical demands in various military environments are widely acknowledged. Ground force soldiers commonly perform their occupational duties wearing combat load in a prolonged manner at low intensities which increases energy expenditure mainly through aerobic metabolism. However, duties may also include intensive phases (e.g., combat actions, casualty evacuation, repetitive lifting) which raise the physical activity unexpectedly to very high levels1,2,3,4, requiring higher neuromuscular performance and anaerobic energy production5,6. Under such conditions, soldiers may not have sufficient time for recovery and could thus experience accumulation of physiological stress leading to fatigue. Acute physical fatigue deteriorate cognitive function, physical performance and critical combat skills such as shooting accuracy7,8,9,10,11. In general, dramatic short-term hormonal disturbances and negative changes in body composition as well as physical performance have been reported following demanding military field training3,12,13,14,15.
Occupational physical demands typically increase during military operations. According to Nagai et al.16, 58% of combat aviation support soldiers carried regularly external loads with an average load of 22 kg during a one-year deployment in Afghanistan. The average duration and frequency of load carriage tasks were 3.5 h and 3.7 days per week. Boye et al.17 reported that the prevalence of soldiers performing physically demanding tasks during deployment increased from an average of 12 to 22% when compared to garrison duties. On the other hand, physical training decreased during deployment by 6% (17% vs. 11%), respectively. The duration for an individual soldier being deployed may easily span over several months16,18,19,20,21 and during that time, physical performance may deteriorate significantly with detraining superimposed under military operational stress. For example, decreases of up to 15–20% in maximal aerobic fitness (VO2max)22 and about 10% in maximal muscular strength23 have been observed already four weeks after the cessation of training. Detraining is an important consideration for physically demanding occupations, since a decline in an individual’s physical fitness increases the relative physiological demands of performing a task, reduces overall working capacity during prolonged assignments, and thereby increases the risk of injury24.
High level of physical fitness and optimal body composition in combination with required occupational skills are significant factors for success in an military operational and deployed environment. The importance of endurance performance increases with the duration of the physically demanding task, such as load carriage4,25. Higher endurance performance has also been associated with better stress tolerance and improved ability to maintain cognitive performance8,11,25,26 While endurance performance is associated with military tasks of longer duration, stronger relationships have been observed between high load, short duration tasks and muscular strength and power4,27. In addition, a lower amount of fat mass and higher muscle mass have been reported to be associated with improved occupational performance for various military tasks28,29,30. In addition to having higher occupational performance capacity, physically fit soldiers may be more resilient to operational stressors in demanding military environments31. This is partly explained by improved sensitivity of the neuroendocrine system leading to the ability to recover faster from high operational stress32.
Several studies have shown beneficial effects of physical training on military task performance4. From a career perspective, military performance optimization starts from initial entry recruit or basic training. The following employment training aim to build up a basic level of strength and endurance capacity for recruits, so that minimum standards for deployment are met33. Before the deployment, fitness level can be further enhanced so that peak performance may be reached by the time the soldiers are transported to the operational area33,34. The deployment phase can be compared to the competitive period of an athlete, with the aim of maintenance of physical performance qualities, which have been achieved during preparation period, throughout the operation33,34. However, optimal methods of developing or maintaining physical and occupational performance during a prolonged military deployment are still under debate. While many studies have evaluated training adaptations in non-deployed soldiers, limited information is available from prolonged military deployments3. Therefore, the aim of the present review and meta-analysis is to provide an overview of physical training and changes in body composition, physical performance, as well as their relationships, during prolonged military operations. More specifically, the aim is to review changes in physical performance, body composition and physical training that have been observed in soldiers during longer (≥ 3 months) military deployments and how physical training during deployment impacts changes in physical performance and body composition outcomes. The rationale for this review is to provide knowledge and suggestions for subject matter experts on how physical training should be taken into consideration during prolonged military operations in order to avoid the deleterious effects of detraining and decrements in combat readiness.
Methods
This review and meta-analysis was constructed applying the PRISMA guidelines35 for methodology and reporting. The participant, intervention, comparator, outcome, study design (PICOS) format36 was used to develop eligibility criteria for study inclusion (Table 1). English-written peer-reviewed journal articles with a minimum of 3-month pre-post follow-up design for body composition and physical performance variables in deployed military personnel were eligible to the review. In addition, all available during-deployment training related data were taken into consideration.
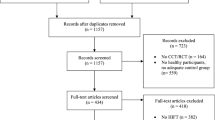
Data for the present review were collected during November 2023 using Medline (PubMed), Google Scholar, and Scopus databases in the mentioned order. The boolean search query ““change*” AND (“body composition” OR “fitness” OR “exercise”) AND (“deployment” OR “military operation”)” was used for screening. In addition, citation search from the records filling the inclusion criteria was performed. Flowchart of the search strategy is presented in Supplement Table S1.
The screening of articles for potential relevance was firstly determined based on the title of the article, and secondly on abstract. The abstract screening and the inclusion selection was performed by two independent researchers who also were subject matter experts in the field of soldier physical performance in different military environments. Possible contradictory opinions on inclusion were decided by a third researcher who carefully read the articles and made the final decision for inclusion or exclusion. Finally, the abstract-screened full text articles were obtained and read, and the relevant ones were included in the review. Additionally, the references from the full text articles were reviewed for potential additional papers. After full-text screening phase, participant demographics, methodological design and main findings regarding physical activity and training, as well as changes in physical performance and body composition were compiled into Table 2.
The participant number, means and standard deviations of the selected outcome measures were entered into a spreadsheet for statistical analysis and Stata version 17.0 (StataCorp, College Station, Texas, USA) was utilized to conduct a meta-analysis along with the systematic review. Due to the low number of deployment studies in general, all studies filling the inclusion criteria were accepted for the review without methodological quality assessment. However, for meta-analysis, studies with overlapping datasets (e.g., same study population and variable in different articles) were eliminated, using only the data with the highest study participant number per case to avoid bias in results. A random-effects restricted maximum likelihood (REML) approach was used to assess inter-study heterogeneity via forest plots, which was formulated by pooling the data from the included studies. Standardized mean differences (i.e., effect size, ES) were calculated using the Hedges g37 and 95% confidence intervals (CI) according to Nakagawa et al.38 to determine the magnitude of pre versus post deployment differences with values of 0.2, 0.5, and 0.8 classified as small, medium, and large levels, respectively. Heterogeneity between the study samples included in the statistical analyses was assessed with values of 25%, 50%, and 75% classified as low, moderate, and high levels, respectively39.
The most commonly reported outcome variables were included in the meta-analysis when reported by four or more studies40. Physical performance variables included endurance performance (spiroergometry, running tests), maximal strength of the lower body (dynamic one repetition maximum i.e., 1RM lifting or squat, static lifting or leg press, knee extension) and the upper body (dynamic 1RM or static bench press, grip strength), lower body power production (vertical or horizontal jump, watt maximum in the Wingate test) and muscle endurance (repeated push-ups and sit-ups). The available anthropometric/body composition variables included body mass, muscle mass (e.g., fat-free mass, lean mass) and body fat (e.g., fat mass, fat percentage). The field running test results were converted into running speed e.g., meters per second for meta-analysis to enable their comparison with the other studies in one forest plot. Similarly, lower and upper body strength measures were converted from relative values (e.g., kg/body mass) to absolute (kg) using the study population mean body mass for the calculation to enable comparison between studies in one forest plot. However, measurement methods that differed significantly from each other (e.g., dynamic vs. isometric force assessment) were analyzed as sub-groups in the meta-analysis. The differences in the abovementioned measurement methods may have influence on the degree of changes and thus, ESs. In addition, the duration of deployments varied, as well as the delay between the deployment and the post-measurement (Table 2), both of which may increase bias to the results.
Results
A total of 4431 records were retrieved from the database searches. The title-screening reduced the number of potential records to 136. Based on inclusion and exclusion criteria, 23 records were selected for the review process. In addition, a citation search from the selected records resulted in inclusion of one more study for the review. Thus, after the final screening, 22 articles and two reviews were included in the present review. However, six of the abovementioned journal articles41,42,43,44,45,46 were excluded as they reported overlapping data (e.g., same study population and variables) with the other studies included in the present review with larger participant number. Therefore, 16 journal articles and two reviews were included in the present review. The main findings of the abovementioned 16 articles are presented in Table 2.
The duration of the deployments varied from 3 to 15 months. Apart from three studies20,30,47 in which body composition and/or physical performance measurements were performed in the middle of the operation, only pre and post measurement results were reported. The post measurements were mainly conducted in homeland after return from the operation with a mean and standard deviation delay of 15.1 ± 15.5 days (median 10 days). The post-measurements were conducted during deployment in five studies18,30,47,48,49.
The number of participants (N = 1426, mean age 26.6 years, mean body mass 81.4 kg, mean height 178 cm) with the pre-post results varied from 20 to 251 between the studies published between the years of 2007 and 2022. The number of original participants reduced significantly in most studies due to voluntary withdrawal, injuries, increased occupational duties and even death in combat. The study participants were mainly male soldiers. Three studies16,51,52 reported that the participants included men and women but due to a low number of female participants, combined results were presented. Another two studies19,20 did not specify the sex of the participants and only one study53 reported combined results but also, men and women separately. The two latter subgroups were used independently in the ES calculations of this review. In addition, one study included a training intervention with a deployed control group47 and thus, these two groups were used separately for the ES calculations. Due to overlap in body composition and physical performance results with three other studies of Pihlainen et al.46,49,50, only the abovementioned47 was used for ES calculations.
A decrease in endurance performance with small but significant standardized mean difference (g − 0.21, 95% CI − 0.01 to − 0.41) has been the most consistently observed negative change in deployed soldiers (Fig. 1). The overall heterogeneity was moderate (57%), while in the sub-group analyses it was small. For example, Lester et al.54 observed a 13% decrement in the 2-mile running test performance (g − 0.89, 95% CI − 0.41 to − 1.38) in male soldiers deployed to Iraq for 13 months. Similarly, 10- to 15-month deployment to Iraq/Afghanistan induced an average decrease of 11% in endurance performance (g − 0.64, 95% CI − 0.24 to − 1.05) in 49 US Army National Guard soldiers51. Five studies reported no changes in endurance performance. In contrast to the abovementioned studies, Sedliak et al.21 reported a mean improvement of 6% (g 0.59, 95% CI 0.04–1.15) in 5 km run combat load run time during a six-month deployment in Afghanistan. In addition, a decrease in military specific endurance test time correlated with the respective decrement in body fat mass (r = 0.49, p < 0.01). Similar results have been reported in other studies50. Only one study reported changes in endurance performance in female soldiers. Warr et al.53 observed a small (3%) but statistically insignificant mean improvement in endurance performance with a trivial ES (g 0.16, 95% CI − 0.61 to 0.94) in 12 female soldiers deployed to Afghanistan/Iraq. The improvement in females was statistically significant when compared to the respective negative change in male soldiers.
Of the available eight studies, an increase in maximal strength of the lower body was reported in five studies while none of the studies reported decreases. The overall increase in lower body strength (g 0.33, 95% CI 0.16–0.50) was small but statistically significant (Fig. 2) and the heterogeneity was low (42%). The sub-group analysis showed moderate heterogeneity across studies using various dynamic lower body muscle strength tests. Similarly, increase in upper body strength (Fig. 3) was reported in five out of available eight studies with small but significant overall ES for change (g 0.33, 95% CI 0.19–0.46). Heterogeneity across the studies assessing upper body strength was low (0%).
A measure of lower body power was reported in seven articles with a small overall g of 0.26 (95% CI − 0.20 to 0.73) and heterogeneity of 89%, revealing high variability especially across studies using various jump tests. With the exception of one study55, the changes were small to trivial (Fig. 4) and without the study of Rintamäki et al.55 ES would have been 0.03 (95% CI − 0.12 to 0.18). A measure of upper body power was reported only in three studies19,21,54 and thus, it was not included in this review.
Improvement in, at least, one muscular endurance test result (e.g., number of repetitions in pull-ups, push-ups and sit-ups) was reported in five out of six available studies18,21,47,51,55, while the rest reported no changes. Push-ups and sit-ups were the most commonly used tests. The overall ESs for push-up and sit-up performances were small, g 0.34 (95% CI 0.15–0.52) and 0.26 (95% CI 0.07–0.44), respectively (Fig. 5). The overall as well as sub-group heterogeneity was 0% in the analyses of muscular endurance.
Summary of meta-analysis results for changes in muscular endurance (push-up and sit-up tests) reported in standardized mean (with 95% CI) and Hedge’s g. Test duration; Dyrstad et al. 2007, RM; Warr et al. 2012, Fallowfield et al. 2014, 2 min RM; Rintamäki et al. 2012, Pihlainen et al. 2022, 1 min RM. Abbreviations; EXP, experimental group; CON, control group.
Body mass changes were reported in 15 studies, fat mass (or fat%) in 13 studies, and muscle mass (or lean mass/fat-free mass) in eight studies. The overall ES for change in body mass was trivial (g -0.05, 95% CI − 0.13 to 0.03). Fat mass (or fat%) increased in three studies19,54,56, decreased in three studies21,51,53, and no changes were observed in seven studies16,20,47,48,49,55,57. Overall, the ES for change in fat mass was trivial with an overall g of − 0.05 (95% CI − 0.21 to 0.10). Likewise, the overall ES for change in muscle mass (g 0.04, 95% CI − 0.12 to 0.20) was trivial. Muscle mass increased in four studies49,53,54,57 and in the experimental training group of one study47, while decreases were observed in one19 study. No changes in muscle mass were observed in two studies20,48 and in the control group of the study of Pihlainen et al.47. Effect sizes for body composition are presented in Figs. 6, 7 and 8.
Physical activity or training was self-reported with various methods by using training diaries or post-deployment surveys in 13 articles. Compared to pre-measurement, physical activity was maintained or decreased during the operation49,52,56. In addition, large inter-individual variations in physical training volume and decrements, especially in endurance training frequency and volume, were observed in the most studies that reported training statistics both preceding and during operation19,50,54. For example, Sharp et al.19 reported that prevalence of soldiers engaging endurance training, at least, three times per week reduced from 80% preceding the operation to 35% during the operation in Afghanistan. Lester et al.54 documented similar reductions (88% vs. 29%) from Iraq. The percentage of soldiers performing strength training, at least, three times per week did not change as markedly as endurance training. The respective pre versus during distributions for strength training were 58% versus 56%19 and 63% versus 44%54. However, one study57 reported increases of 60% (156 ± 106 vs. 250 ± 182 min/week) and 77% (190 ± 101 vs. 336 ± 251 min/week) in endurance and strength training volumes of 35 Special Operations Forces soldiers during 3–6 month deployment in Afghanistan and other undefined locations. Summary of physical activity and training during deployments is presented in Table 2.
Decrements in training volume were often related to negative changes in physical performance and body composition following the principle of training specificity (Table 2). Dyrstad et al.18 observed large individual variations in training volume and changes in aerobic capacity (r = 0.46, p < 0.001) during a 12-month deployment in Kosovo. One third (n = 20) of the Norwegian soldiers, whose VO2max decreased (− 3 ± 4%), performed physical training on average 77 ± 48 min/week (endurance 31 ± 22, strength 46 ± 35 min/week) during the operation. In contrast, the other third (n = 19) whose VO2max was improved (+ 4 ± 4%) during the operation, trained 169 ± 76 min per week (endurance 48 ± 46, strength 121 ± 71 min/week), e.g., more than twice as much as the group with a decreased aerobic capacity18. Warr et al.53 compared training adaptations in National Guard soldiers who engaged in training ≥ 3 times/week versus those performing less than that during deployments in Iraq and Afghanistan. In general, no changes were observed in VO2max during deployment, but interaction between group and time was observed between soldiers performing endurance training ≥ 3 times per week and those training less than that (∆ VO2max + 2% vs. − 8%, p < 0.05). Pihlainen et al.50 observed that deployed soldiers (n = 24) whose endurance performance decreased during a 6 month operation in Lebanon reported that their endurance training frequency was 40% lower (2.6 ± 1.6 vs. 1.4 ± 1.1 times/week) than preceding the operation. On the contrary, soldiers who improved their endurance performance during the operation, increased endurance training frequency (2.3 ± 1.4 vs. 2.4 ± 1.0 times/week) from the pre-deployment level. Also, correlation between the relative pre-during operation change in endurance training frequency and percent change in 3000 m run time was observed (r = − 0.57, p < 0.001).
Regarding strength training during deployment, Lester et al.54 reported a relationship between strength training frequency and change in 1RM bench press (r = 0.61, p < 0.05). Warr et al.53 found an interaction between group and time (p < 0.001) in change of 1RM bench press result between soldiers performing strength training ≥ 3 times per week (+ 15%) and those performing less than three times per week (+ 3%). Pihlainen et al.50 found a moderate correlation between relative pre-during operation change in strength training frequency and respective change in muscle mass (r = 0.31, p < 0.05). Moderate correlations between strength training frequency and changes in muscle mass have been reported also in earlier deployment studies19,54.
As for physical performance, also body composition changes seem to be, at least partly, related to their baseline values. For example, Sharp et al.19 reported significant mean decreases in body mass and fat-free mass only in soldiers in the highest initial quartiles, whereas no changes were found in the lowest quartiles. According to Farina et al.57, mean body mass did not change in the British Royal Marines during their 3–6 month deployment, but when participants were split into quartiles according to their baseline body mass, significant increases were observed in the lowest two groups, whereas the individuals with the highest baseline body mass tended to decrease body mass during operation.
Among the few articles reporting changes between the pre and post measurements, Fallowfield et al.20 observed decreases of 5% (3.9 kg) in body mass and 8% in body fat percentage in the British Royal Marines during the first half of a 6 month operational deployment in Afghanistan. The soldiers regained their body mass and fat percentage by the end of the deployment and thus, no pre-post changes were observed in these variables in the end of the study. Similarly, fat-free mass decreased by 1.9 ± 1.9 kg in the first half and increased by 2.1 ± 1.8 kg during the latter half of the operation. Pihlainen et al.47 also reported a modest increase in body mass during the latter half of a 6 month crisis-management operation in Lebanon in soldiers who were provided a combined strength and endurance training program (+ 0.4 kg, p < 0.05) as well as soldiers in the control group without training program (+ 0.7 kg, p < 0.05). While no pre-post changes in body mass or fat mass were observed, respective increases were reported in muscle mass (+ 0.4 kg, p < 0.05) of soldiers provided with a training program47.
Discussion
The present systematic review and meta-analysis examined changes in physical performance and body composition during military operations with a minimum duration of three months. Additionally, the aim was to report physical activity and training along with their interactions with fitness and body composition. This meta-analysis showed that overall, endurance performance decreased while maximal strength of the lower and upper body as well as muscular endurance increased during deployments. However, the standardized mean differences were mainly trivial or small, with large variation between the studies explaining high statistical heterogeneity values. This is logical as the outcomes varied from negative to positive changes. Also, significant variation existed between the duration of deployments, measurement methods, training facilities, security situations, some to mention.
The first four available deployment studies from 2007 to 201218,19,51,54 reported significant declines in endurance performance, which in general was the most consistent finding from the military operation studies of that time period regarding physical performance. Thereafter, most studies reported no change in endurance performance. However, endurance performance was the only variable in the present review showing negative overall change in terms of mean ES. Therefore, maintenance of this fitness attribute should be in the focus of military commanders during operation.
Increases in maximal strength of the lower and upper body as well as muscular endurance may reflect training habits and preferences of soldiers during deployment. The overall change with 95% CI in lower body power varied from increase to decrease. Only one study reported very large ES for increase in lower body power55. Excluding this result, the present meta-analysis reports no overall change in lower body power, despite the increase in maximal strength. The same study55 resulted in high level of heterogeneity in the meta-analysis, and it was considered as potential outlier but due to limited number of studies overall, it was not removed from the analyses. However, if it had been removed, the pooled effect size value of maximal jumping height or distance would have changed from 0.46 (− 0.59 to 1.52) to 0.00 (− 0.21 to 0.21) and, I2 value from 95.2 to 0.0%. Several studies have reported decrements in lower body power after strenuous military field exercise15,58. This may be due to neuromuscular fatigue combined with loss of muscle mass, the two factors contributing to fast force production ability. Based on findings from the reviewed deployment studies, the occupational physical load may not be as high as compared to field exercises, at least for the whole duration of the operation. Since explosive force production is important fitness component, especially, during combat missions59, a special focus should be paid in maintenance of this ability throughout the military operation.
The overall body composition changes were mainly small or trivial, and the results between the studies varied rather evenly from negative change to no change and positive change. A review by McCarthy et al.60 reported effect sizes similar to the present meta-analysis for body mass and fat%. The changes in body composition and physical performance were, at least partly, explained by individual variation in training status and history. Also, although energy balance and nutrition were not in the focus of this review, it is widely known that long-term suboptimal diet combined with insufficient physical activity may lead to negative changes in body composition, physical performance and cardiometabolic health44.
Expected adaptation to decrements in total training load during deployment would be attenuation of physical performance according to the principle of training specificity. This may be the major explanation for decreased endurance performance reported in many deployment studies. Especially endurance training volumes decreased in deployed soldiers19,50,54. In some deployments, military tasks alone were not physically demanding enough to maintain endurance performance, and the total physical activity/work load remained low49. It is also possible that the conditions at the military bases did not support endurance training (e.g., lack of running pathways, treadmills or ergometers, hot climate etc.). Supporting this hypothesis, some studies reported that negative change in endurance performance was associated with either lower than average endurance training frequency19,53 or decreased endurance training frequency from time preceding operation50. Thus, to avoid decrements in endurance performance, endurance-training load should not be dramatically reduced from the level preceding the operation, especially in soldiers with higher initial fitness level, who may be more susceptible to decrements in their physical performance during deployment19,50. In one study with a positive change in endurance performance during deployment, it was speculated that an increase in total training volume, when compared to normative training of soldiers, explained the positive adaptations21. Negative changes in fitness were also associated with other negative outcomes, such as higher number of medical visits51, increases in fat mass18,21,50 and decreased perception of overall health53.
Many of the reviewed articles, as well as other studies17, have reported that the overall external training load (reported training volume, frequency and/or intensity) decreased during the operation as compared to time preceding it. Many reasons may explain this phenomena, including high operational tempo, increased duties and 24 h readiness demand, lack of motivation as well as lack of training facilities or equipment. On the other hand, many studies reported that physical training occurred during operations. Thus, detraining may have been rather an individual choice than result of lacking possibilities. Indeed, one of the likely reasons is that physical training was not compulsory18,21,47,54,55 and maintenance of physical performance was based on the resposibility/motivation of the individual soldier. Interestingly though, many of the reviewed papers18,21,47,54,55 recommended more obligatory and individually tailored physical training, especially during low-tempo phases of deployment to maintain readiness and capability for the possible intensive phases and in general, until the end of the operation period. It must be acknowledged though, that intrinsic, instead of extrinsic, motivation supported maintenance of training habits during operation18. However, it is challenging to change intrinsic motivation towards training during deployment. One simple solution could be guided or supervised compulsory physical training, in addition to general or military specific fitness and body composition assessments, implemented throughout the deployment period. Professionally guided training sessions along with performance assessments might lead to higher motivation, more optimal adaptations, and lower injury rate. It is important to acknowledge that large proportion (23%) of non-combat injuries have been reported to occur during sport activities61, especially during strength training24. Thus, properly guided training could therefore even enhance the occupational performance of soldiers by reducing sports-related injuries.
A recommendation for physical training periodization during deployment has been presented in the literature by Haff34. The starting point is two weekly strength training sessions interspersed with recovery day plus aerobic/anaerobic training session for one-to-two times per week. This is in line with a review from Spiering et al.62 who reported that a minimum dose for maintenance of endurance performance for 15 weeks in general adult population is two weekly training sessions. Strength and muscle mass can be maintained even for longer period (up to 32 weeks) with just one weekly strength training session and one set per exercise. Thus, maintenance of physical performance at least for 15–32 weeks is possible with reduced overall training volume (duration and/or frequency) during deployment, but training intensity should be kept high. Naturally, individualization is required since large variation in training history and fitness level exist between soldiers63. Smith et al.64 reported in their review and meta-analysis that more structured high-intensity strength and endurance training, or their combination (e.g., combined training) is more effective training method to improve endurance performance, strength, power, muscular endurance in soldiers, compared to traditional military physical training typically consisting of running/walking and calisthenics with lower intensities. Moreover, high-intensity interval training (HIIT) and/or high-intensity functional training (HIFT) can be considered as a practical training method for soldiers whenever time allocated to training and access to fitness facilities are limited65. HIIT/HIFT may even be performed in operational environments or during operations where decrements in aerobic performance have been observed63. In addition, these “non-traditional” training practices may reduce musculoskeletal injury risk64.
Ideally, training emphasis and prescription would be based on occupational task/demands analysis. It also needs to be taken into consideration that soldiers may encounter several external stressors during deployment which may impair their ability to recover from training load and therefore, more recovery may be required for periodization of training during deployment. If these additional stressors are not considered, risks for non-functional overreaching and injury are increased3,34,63. One potential option is flexible non-linear periodization model, which allows soldiers to take into consideration the occupational stress and modulate training accordingly. Flexible periodization does not mean that workouts are selected by personal preferences but instead, sessions that are targeted to develop qualities that require training in a recovered state (e.g., maximal and explosive strength), are performed accordingly. If the occupational duties do not enable longer (one-hour) consecutive training sessions, they may also be performed in shorter bouts (i.e., micro-training) without the risk of attenuated training adaptations66.
Following limitations of reviewed studies were identified. A large variation in research methodology including differences in measurement methods, delay between post measurements and unclearly reported sex-distribution complicate the inference of results. Moderator analyses to determine which factors contribute to the variability in effect sizes may have improved the quality of the present meta-analysis. However, due to the complexity of moderating factors across the studies, this could not be performed thoroughly. Thus, conflicting findings between the studies are likely, at least partly, explained by differences in security situation, resources, possibilities, and motivation for physical training, as well as duration of the follow-up. In addition, decrements in study population was common finding of the reviewed studies. This needs to be considered when planning future military operation studies. Finally, this review did not consider the effects of nutrition in body composition or physical performance changes since studies focusing on effects of nutrition during deployment are still scarce.
Conclusion
Each deployment is a unique challenge for soldiers to maintain their initial fitness level and body composition, which requires individually tailored training programs for optimization of physical performance and readiness throughout the operation. Overall, special attention should be paid in maintenance of aerobic endurance, which was the most likely performance variable to decrease during deployment. Regarding neuromuscular performance, lower and upper body strength and muscular endurance are less likely to decrease. Body composition changes were mainly small and varied from negative to positive changes in muscle and fat mass. Detraining seems to be problem, especially, for soldiers with high initial fitness level. To minimize declines in performance and readiness, soldiers should be encouraged to perform frequent endurance and strength training, depending on their pre-deployment training status, at least 2–4 times per week using flexible non-linear periodization. At least, a part of physical training should be supervised or preferably guided to optimize training adaptations and minimize injury risk. Finally, the number of peer-reviewed articles on changes in body composition and fitness changes and, especially in physical training during military operation is still limited. Thus, more deployment studies are warranted.
Data availability
This systematic review and meta-analysis has no original data to provide as they have been compiled from previously published journal articles. Most of the data have been reported within the main text and are available for copying for further analyses.
References
Henning, P. C., Park, B. S. & Kim, J. S. Physiological decrements during sustained military operational stress. Mil. Med. 176(9), 991–997 (2011).
Sharp, M., Patton, J. & Vogel, J. A database of physically demanding tasks performed by U.S. army soldiers. Military Performance Division. U.S. Army Research Institute of Environmental Medicine, Natick MA. 1998. Technical Report T98. Available at: https://apps.dtic.mil/sti/pdfs/ADA338922.pdf. Accessed April 6, 2023.
Nindl, B. C. et al. Physiological Employment Standards III: Physiological challenges and consequences encountered during international military deployments. Eur. J. Appl. Physiol. 113(11), 2655–2672 (2013).
Vaara, J. P. et al. Physical training considerations for optimizing performance in essential military tasks. Eur. J. Sport Sci. 22(1), 43–57 (2022).
Nindl, B. C. et al. Executive summary from the National strength and conditioning association’s second blue ribbon panel on military physical readiness: Military physical performance testing. J. Strength Cond. Res. 29(Suppl 11), S216–S220 (2015).
Friedl, K. E. et al. Perspectives on aerobic and strength influences on military physical readiness: report of an international military physiology roundtable. J Strength Cond Res. 29(Suppl 11), S10–S23 (2015).
Knapik, J. et al. Soldier performance and mood states following a strenuous road march. Mil. Med. 156(4), 197–200 (1991).
Martin, K., Périard, J., Rattray, B. & Pyne, D. B. Physiological factors which influence cognitive performance in military personnel. Hum. Factors 62(1), 93–123 (2020).
O’Leary, T. J., Wardle, S. L. & Greeves, J. P. Energy deficiency in soldiers: The risk of the athlete triad and relative energy deficiency in sport syndromes in the military. Front. Nutr. 7, 142 (2020).
Conkright, W. R. et al. Sex differences in the physical performance, physiological, and psycho-cognitive responses to military operational stress. Eur. J. Sport Sci. 22(1), 99–111 (2022).
Sekel, N. M. et al. Military tactical adaptive decision making during simulated military operational stress is influenced by personality, resilience, aerobic fitness, and neurocognitive function. Front. Psychol. 14, 1102425 (2023).
Church, D. D., Gwin, J. A., Wolfe, R. R., Pasiakos, S. M. & Ferrando, A. A. Mitigation of muscle loss in stressed physiology: Military relevance. Nutrients 11(8), 1703 (2019).
Conkright, W. R. et al. Neuromuscular performance and hormonal responses to military operational stress in men and women. J. Strength Cond. Res. 35(5), 1296–1305 (2021).
Beckner, M. E. et al. Circulating biomarkers associated with performance and resilience during military operational stress. Eur. J. Sport Sci. 22(1), 72–86 (2022).
Heilbronn, B., Doma, K., Sinclair, W., Connor, J., Irvine-Brown, L. & Leicht, A. Acute fatigue responses to occupational training in military personnel: A systematic review and meta-analysis. Mil. Med. usac144 (2022).
Nagai, T. et al. Effects of deployment on musculoskeletal and physiological characteristics and balance. Mil. Med. 181(9), 1050–1057 (2016).
Boye, M. W. et al. Army physical demands study: Prevalence and frequency of performing physically demanding tasks in deployed and non-deployed settings. J. Sci. Med. Sport 20(Suppl 4), S57–S61 (2017).
Dyrstad, S. M., Miller, B. W. & Hallén, J. Physical fitness, training volume, and self-determined motivation in soldiers during a peacekeeping mission. Mil. Med. 172(2), 121–127 (2007).
Sharp, M. A. et al. Physical fitness and body composition after a 9-month deployment to Afghanistan. Med. Sci. Sports Exerc. 40(9), 1687–1692 (2008).
Fallowfield, J. L. et al. Energy expenditure, nutritional status, body composition and physical fitness of Royal Marines during a 6-month operational deployment in Afghanistan. Br. J. Nutr. 112(5), 821–829 (2014).
Sedliak, M., Sedliak, P. & Vaara, J. P. Effects of 6-month military deployment on physical fitness, body composition, and selected health-related biomarkers. J. Strength Cond. Res. 35(4), 1074–1081 (2021).
Swank, A. & Sharp, C. Adaptations to aerobic endurance training programs. In Essentials of Strength Training and Conditioning 4th edn (eds Haff, G. G. & Triplett, T.) 131 (Human Kinetics, 2016).
Häkkinen, K., Alén, M. & Komi, P. V. Changes in isometric force- and relaxation-time, electromyographic and muscle fibre characteristics of human skeletal muscle during strength training and detraining. Acta Physiol. Scand. 125(4), 573–585 (1985).
Roy, T. C., Knapik, J. J., Ritland, B. M., Murphy, N. & Sharp, M. A. Risk factors for musculoskeletal injuries for soldiers deployed to Afghanistan. Aviat. Space Environ. Med. 83(11), 1060–1066 (2012).
Drain, J., Billing, D., Neesham-Smith, D. & Aisbett, B. Predicting physiological capacity of human load carriage—A review. Appl. Ergon. 52, 85–94 (2016).
Beckner, M. E. et al. Impact of simulated military operational stress on executive function relative to trait resilience, aerobic fitness, and neuroendocrine biomarkers. Physiol. Behav. 236, 113413 (2021).
Hauschild, V. D. et al. Fitness tests and occupational tasks of military interest: A systematic review of correlations. Occup. Environ. Med. 74(2), 144–153 (2017).
Crawford, K. et al. Less body fat improves physical and physiological performance in army soldiers. Mil. Med. 176(1), 35–43 (2011).
Lyons, J., Allsopp, A. & Bilzon, J. Influences of body composition upon the relative metabolic and cardiovascular demands of load-carriage. Occup. Med. (Lond.) 55(5), 380–384 (2005).
Pihlainen, K., Santtila, M., Häkkinen, K. & Kyröläinen, H. Associations of physical fitness and body composition characteristics with simulated military task performance. J. Strength Cond. Res. 32(4), 1089–1098 (2018).
Szivak, T. K. & Kraemer, W. J. Physiological readiness and resilience: Pillars of military preparedness. J. Strength Cond. Res. 29(Suppl 11), S34–S39 (2015).
Szivak, T. K. et al. Adrenal stress and physical performance during military survival training. Aerosp. Med. Hum. Perform. 89(2), 99–107 (2018).
Billing, D. C. & Drain, J. R. International Congress on Soldiers’ Physical Performance 2017: Research priorities across the service members operational lifecycle. J. Sci. Med. Sport 20(Suppl 2), S1–S3 (2017).
Haff, G. Periodization for tactical populations. In NCSA´s Essentials of Tactical Strength Training and Conditioning (eds Alvar, B. et al.) 181–205 (Human Kinetics, 2017).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Methley, A. M., Campbell, S., Chew-Graham, C., McNally, R. & Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 21(14), 579 (2014).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863 (2013).
Nakagawa, S. & Cuthill, I. C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 82(4):591–605 (2007). doi: https://doi.org/10.1111/j.1469-185X.2007.00027.x. Erratum in: Biol. Rev. Camb. Philos. Soc. 84(3):515 (2009).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003).
Jackson, D. & Turner, R. Power analysis for random-effects meta-analysis. Res. Synth. Methods 8(3), 290–302 (2017).
Hill, N. E. et al. Changes in gut hormones and leptin in military personnel during operational deployment in Afghanistan. Obesity (Silver Spring) 23(3), 608–614 (2015).
Farina, E. K. et al. Effects of deployment on diet quality and nutritional status markers of elite U.S. Army special operations forces soldiers. Nutr. J. 16(1), 41 (2017).
Fallowfield, J. L. et al. Serum 25-hydroxyvitamin D fluctuations in military personnel during 6-month summer operational deployments in Afghanistan. Br. J. Nutr. 121(4), 384–392 (2019).
Nykänen, T. et al. Diet macronutrient composition, physical activity, and body composition in soldiers during 6 months deployment. Mil. Med. 184(3–4), e231–e237 (2019).
Nykänen, T., Pihlainen, K., Kyröläinen, H. & Fogelholm, M. Associations of nutrition and body composition with cardiovascular disease risk factors in soldiers during a 6-month deployment. Int. J. Occup. Med. Environ. Health 33(4), 457–466 (2020).
Pihlainen, K. et al. Training-induced acute neuromuscular responses to military specific test during a six-month military operation. Int. J. Environ. Res. Public Health 18(1), 215 (2020).
Pihlainen, K. et al. Effects of combined strength and endurance training on body composition, physical fitness, and serum hormones during a 6-month crisis management operation. J. Strength Cond. Res. 36(9), 2361–2370 (2022).
Psutka, J., Pavlík, V., Fajfrová, J., Urban, M. & Halajčuk, T. Monitoring of anthropometric changes in the armed forces of the Czech Republic personnel during the deployment in Afghanistan. Mil. Med. Sci. Let. 84(4), 166–169 (2015).
Pihlainen, K., Santtila, M., Vasankari, T., Häkkinen, K. & Kyröläinen, H. Evaluation of occupational physical load during 6-month international crisis management operation. Int. J. Occup. Med. Environ. Health 31(2), 185–197 (2018).
Pihlainen, K., Häkkinen, K., Santtila, M., Raitanen, J. & Kyröläinen, H. Differences in training adaptations of endurance performance during combined strength and endurance training in a 6-month crisis management operation. Int. J. Environ. Res. Public Health 17(5), 1688 (2020).
Warr, B. J., Heumann, K. J., Dodd, D. J., Swan, P. D. & Alvar, B. A. Injuries, changes in fitness, and medical demands in deployed National Guard soldiers. Mil. Med. 177(10), 1136–1142 (2012).
Carlson, A. R., Smith, M. A. & McCarthy, M. S. Diet, physical activity, and bone density in soldiers before and after deployment. US Army Med. Dep. J. 25–30 (2013).
Warr, B. J., Scofield, D. E., Spiering, B. A. & Alvar, B. A. Influence of training frequency on fitness levels and perceived health status in deployed National Guard soldiers. J. Strength Cond. Res. 27(2), 315–322 (2013).
Lester, M. E. et al. Effect of a 13-month deployment to Iraq on physical fitness and body composition. Mil. Med. 175(6), 417–423 (2010).
Rintamäki, H. et al. From the subarctic to the tropics: Effects of 4-month deployment on soldiers’ heat stress, heat strain, and physical performance. J. Strength Cond. Res. 26(Suppl 2), S45-52 (2012).
Frank, L. L. & McCarthy, M. S. Telehealth coaching: Impact on dietary and physical activity contributions to bone health during a military deployment. Mil. Med. 181(5 Suppl), 191–198 (2016).
Farina, E. K. et al. Effects of combat deployment on anthropometrics and physiological status of U.S. army special operations forces soldiers. Mil. Med. 182(3), e1659–e1668 (2017).
Nindl, B. C. et al. Physical performance responses during 72 h of military operational stress. Med. Sci. Sports Exerc. 34(11), 1814–1822 (2002).
Mala, J., Szivak, T. K., Flanagan, S. D., Comstock, B. A., Laferrier, J. Z., Maresh, C. M., & Kraemer, W. J. The role of strength and power during performance of high intensity military tasks under heavy load carriage. US Army Med. Dep. J. 3–11 (2015).
McCarthy, M. S., Elshaw, E. B., Szekely, B. M. & Pflugeisen, B. Health promotion research in active duty army soldiers: The road to a fit and ready force. Nurs. Outlook 65(5S), S6–S16 (2017).
Sanders, J. W. et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am. J. Trop. Med. Hyg. 73(4), 713–719 (2005).
Spiering, B. A., Mujika, I., Sharp, M. A. & Foulis, S. A. Maintaining physical performance: The minimal dose of exercise needed to preserve endurance and strength over time. J. Strength Cond. Res. 35(5), 1449–1458 (2021).
Kyröläinen, H., Pihlainen, K., Vaara, J. P., Ojanen, T. & Santtila, M. Optimising training adaptations and performance in military environment. J. Sci. Med. Sport 21(11), 1131–1138 (2018).
Smith, C., Doma, K., Heilbronn, B. & Leicht, A. Effect of exercise training programs on physical fitness domains in military personnel: A systematic review and meta-analysis. Mil. Med. 187(9–10), 1065–1073 (2022).
Helén, J. et al. High-intensity functional training induces superior training adaptations compared with traditional military physical training. J. Strength Cond. Res. 37, 2477 (2023).
Kilen, A. et al. Distribution of concurrent training sessions does not impact endurance adaptation. J. Sci. Med. Sport 24(3), 291–296 (2021).
Author information
Authors and Affiliations
Contributions
K.P. and M.S selected articles for systematic review, H.K. was the third referee for inclusion criteria, J.R. performed the statistical analyses, K.P. and J.R. prepared figures and tables, All authors participated in writing and reviewing of the original and revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pihlainen, K., Santtila, M., Nindl, B.C. et al. Changes in physical performance, body composition and physical training during military operations: systematic review and meta-analysis. Sci Rep 13, 21455 (2023). https://doi.org/10.1038/s41598-023-48712-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48712-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.