Abstract
Arteriovenous fistula (AVF) is the first choice of vascular access in hemodialysis (HD) patients. However, the correlations between patient factors and the arteriovenous fistula patency remain unclear. Therefore, our study investigates the risk factors associated with AVF dysfunction in HD patients. A total of 233 end-stage renal disease (ESDR) patients who met the study inclusion criteria in the Nephrology Department of Hunan Provincial People’s Hospital between December 2020 and June 2022 were included in this study. The baseline demographic, clinical and laboratory parameters were collected at the time of AVF creation and analyzed. Of the 233 ESRD patients, 146 (62.7%) were male and the mean age was 56.11 ± 12.14 (21–82) years. The patients were followed for a median time of 14 months. Kaplan–Meier analysis showed a 6-, 12- and 24-month post-placement survival of 87.1%, 82.8% and 80.7%, respectively. Univariate Cox regression analysis revealed weight (HR, 1.03; P = 0.03) as a predictor for the loss of vascular access functionality. In addition, multivariate Cox regression analysis further demonstrated that sex (HR, 3.41; P = 0.03), weight (HR 1.08; P < 0.01) and phosphorus level (HR: 3.03; P = 0.01) are independent risk factors for AVF dysfunction. AVF dysfunction is highly associated with several risk factors including weight, phosphorus level, and sex. Positive intervention strategies targeting these potential factors, such as weight loss or oral phosphate binders could improve the long-term success of AVF.
Similar content being viewed by others
Introduction
The increasing prevalence of end-stage renal disease (ESRD) has led to a steep rise in the number of patients requiring hemodialysis (HD). Vascular access (VA) is required for the well-being and survival of HD patients and has been referred to as both the “lifeline” and “Achilles’ heel” for HD patients1. There are three types of VA, namely arteriovenous fistula (AVF), arteriovenous graft (AVG), and central venous catheters (CVC). AVF is the optimal VA for HD due to its longevity, lower rates of infection and thrombosis, and greater safety compared with AVG or CVC2,3.
Although the merits of AVF make it a preferred form of renal replacement therapy, it has been revealed that 20–50% of AVFs would fail to mature adequately to vascular access for hemodialysis4. AVF failure consists of three types, including early thrombosis, failure to mature, and late failure5. The characteristic pathology of AVF failure included neointimal hyperplasia, failure to develop outward remodeling or wall thicking5. Accordingly, a unique set of biochemical abnormalities may predispose the vascular wall to inward remodeling and stenosis after AVF creation, including obesity, chronic inflammation, CKD-MBD, hyperphosphatemia, endothelial failure, and lipidemia. In addition, many other variables are also associated with AVF failure, such as patient’s sex, age and comorbidities. However, the exact role of these factors in AVF failure is not completely well defined yet.
In this retrospective study, we aimed to assess the risk factors associated with AVF dysfunction in HD patients, providing new insights for the prevention of AVF failure.
Material and methods
Study design
The AVF patency rate of ESRD patients who had an AVF created between December 2020 and June 2022 at the Nephrology Department of Hunan Provincial People’s Hospital was retrospectively analyzed. A total of 306 ESRD patients were enrolled and assessed for eligibility. Inclusion criteria were listed as follows: (1) Age ≥ 18 years at the time of fistula establishment; (2) ESRD patients who underwent forearm cephalic vein-radial artery end to side anastomosis for the first time; (3) The success of first AVF maturation (blood flow ≥ 200 mL/min) and received initial routine HD (2–3 times per week). Exclusion criteria were listed as follows: (1) Patients who were unable to participate telephone or in-person follow-up for any reason; (2) Patients whose basic data could not be collected; (3) Patients whose AVF had not been utilized.A final total of 233 ESRD patients who met the eligibility criteria and agreed to participate were included in the study (Fig. 1). The demographics and clinical data of the patients were collected at the time of AVF creation. The patients were followed by telephone or in-person, and relevant data were collected from the patients’ records. Patients were divided into patency (n = 191) and dysfunction (n = 42) groups based on whether their AVF was patent or not. AVF dysfunction was defined as lower blood flow during dialysis (≤ 200 mL/min).The study involving human participants adhered to ethical standards set by the institution and the national research committee. It was in accordance with the principles outlined in the 1964 Helsinki Declaration and its later amendments or similar ethical standards. All patients provided written informed consent and the Ethics Committee of Hunan Provincial People’s Hospital approved the study.
Demographic, clinical, and laboratory variables
The demographic, clinical, and laboratory characteristics of the patients were collected from their medical records at the time of AVF creation. These data included personal data (age and sex), smoking status, anthropometric data (height, weight, and body mass index), primary disease of ESRD, preoperative blood pressure, and other comorbidities (hypertension and diabetes). The initial laboratory workup included complete blood count, albumin, uric acid, creatinine, cholesterol, triglycerides, calcium, phosphorus, intact parathyroid hormone, and ferritin. All blood specimens were collected on the day before arteriovenous fistula plasty.
Statistical analysis
All data were analyzed using SPSS 19.0. Continuous data are expressed as mean ± standard deviation and compared using the independent t test (normal distribution) or Mann–Whitney U test (non-normal distribution). Categorical variables are reported in frequency and percentage, and compared using the chi-square test. AVF patency was calculated by the Kaplan–Meier method, and risk factors for AVF dysfunction were identified by univariate and multivariate Cox regression analyses. A P < 0.05 was considered statistically significant.
Results
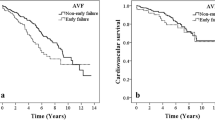
Of the 233 ESRD patients, 146 (62.7%) were male and the mean age was 56.11 ± 12.14 years. The patients were followed for a median time of 14 months. Kaplan–Meier analysis showed the cumulative AVF survival was 87.1% at 6 months, 82.8% at 12 months, and 80.7% at 24 months (Fig. 2). No death was recorded in the follow-up period.
The baseline demographic and clinical characteristics of the patients in the patency group (n = 191) and AVF dysfunction group (n = 42) are summarized in Table 1. Among patients in the AVF dysfunction group, 24 (57.1%) were male, and the mean age was 57.43 ± 12.41 (25–82) years. In the normal AVF group, 122 (63.9%) were male and the mean age was 55.82 ± 12.10 (21–76 years). A total of 20 parameters were collected and compared between the two groups, including weight, diastolic pressure, albumin, and uric acid (Table 2). Compared with the patency group, patients in the AVF dysfunction group had greater weight (68.97 ± 12.23 vs. 63.79 ± 11.61; P = 0.02), lower diastolic pressure (86.10 ± 11.00 vs. 79.71 ± 14.66; P < 0.01) (Table 1) and lower albumin (33.69 g/L vs. 30.29 g/L; P = 0.01) and uric acid (493.0 mmol/L vs. 437.5 mmol/L; P = 0.03) levels. Prothrombin activity (PTA) was significantly higher in AVF patency group than that in AVF dysfunction group (P = 0.04). No significant differences in remaining parameters was detected between the two groups.
Univariate Cox regression analysis revealed that weight (HR, 1.03; P = 0.03) is a predictor for the loss of AVF function(Supplementary Table 1). In addition, multivariate Cox regression analysis further demonstrated that sex (HR, 3.41; P = 0.03) and weight (HR 1.08; P < 0.01) are independent risk factors for AVF failure (Table 3, Fig. 3). Although the difference in blood phosphorus level between the two groups was not statistically significant, multivariate Cox proportional hazard regression showed that blood phosphorus level is an independent risk factor for AVF failure (HR: 3.03; P = 0.01).
Discussion
The increasing demand for HD has been accompanied by a growing consensus on the “fistula first” approach among clinicians. The National Kidney Foundation Guidelines for Vascular Access, Fistula First Breakthrough Initiative (FFBI) established by Centers for Medicare and Medicaid (CMS), and other major renal societies across the globe recommend AVF as the preferred choice for patients requiring maintenance HD (MHD)6,7,8,9. Despite these recommendations, the utilization rate of AVF for dialysis initiation was reported to vary between 15 and 83%10. Furthermore, up to 20–60% of primary AVF failure can be observed in subsequent follow-ups11. A number of risk factors have been reported to affect the success of AVF. However, the detailed role of risk factors in AVF dysfunction remain elusive. This study revealed that sex, weight, and phosphorus level are independent risk factors associated with AVF dysfunction in Chinese ESRD patients, which is in line with previous report.
Hyperphosphatemia is the most common complication in MHD patients, and phosphorus is considered the initiator for vascular calcification. High level of extracellular inorganic phosphate (Pi) stimulates synthetic transformation of vascular smooth muscle cells (VSMCs) and induces the secretion of matrix vesicles to trigger apoptosis pathways, which contributes to VSMC failure and consequently progressive vascular calcification12. Vascular calcification accelerates vessel injury and thrombosis, ultimately leading to AVF failure. Several studies have reported a strong association between hyperphosphatemia and AVF failure. Zhou et al. showed that patients with hyperphosphatemia were more likely to develop AVF failure than their normal counterparts13. Similarly, Yu et al. found that blood Pi level was an independent risk factor for AVF failure12, which is consistent with our finding. Although our univariate analysis did not identify a significant correlation between blood phosphorus level and AVF failure, multivariate analysis revealed that hyperphosphatemia may be associated with a higher risk of AVF failure. Therefore, reducing positive phosphate balance and serum phosphate level by phosphate binders or regular HD may improve AVF patency in ESDR patients.
The impact of weight on AVA patency is currently unclear. Obesity-associated inflammation and advanced atherosclerosis may lead to endothelial injury, resulting in lower initial intraoperative blood flow and higher failure rate of primary AVF maturation14. The DOPPS reported that successful AVF placement was associated with a lower BMI8. Chan et al. reported that a BMI ≥ 35 kg/m2 significantly increased the risk of AVF failure by 3.66-fold15. Kim et al. revealed that obese patients had significantly longer maturation time and higher early maturation failure rate. A BMI ≥ 25 kg/m2 conferred a relative risk of 2.4 for AVF failure16. In line with these underlying mechanisms and previous clinical studies, we have discovered that increased weight may be correlated with a higher risk of AVF failure.
It has been convinced that gender profoundly contributes to AVF outcome. Our study suggested that female patients have a decreased AVF patency rate than men, which was in line with Bashar et al.’s finding that AVF non-maturation was associated with female gender. Several studies revealed that women have lower AVF maturation rates and longer maturation time than men17,18. Due to smaller mean vessel diameters in women, decreased vasodilation capacity, and insufficient outward remodeling15,17, female patients need more effort to salvage nonfunctioning AVFs to promote AVF maturation outcomes19.
Although diabetes is a well-established risk factor for advanced atherosclerosis and cardiovascular diseases, it was not a significant risk predictor for AVF failure in our study. A meta-analysis by Almasri et al. revealed that patients with diabetes and cardiovascular disease had a shorter duration of AVF patency compared with non-diabetic patients17. However, several studies were consistent with our findings, showing that there was no difference in AVF patency between diabetic and non-diabetic patients20,21.
In addition to the above parameters we included, a number of variables such as surgeon experience, AVF sites and vessel histology were associated with AVF maturation. The experience of surgeon is a vital cause for AVF success and patency. Fassiaadis et al. revealed that the primary AVF success was 13% higher when the senor surgeon who performed 15–18 AVF procedure per week, suggesting the impact of surgical experience on AVF maturation22. In addition, the sites of AVF created also affect the vascular access success. Sultan et al. has demonstrated distal placement of AVF need more intervention to salvage AVF patency and are linked to lower cumulative survival rate compared to those created proximally23. Venous diameter is considered to be an independent predictor for AVF maturation. The AVF maturation can be highly expected if the diameter of vein measured more than 4 mm in preoperative assessment, whereas AVF performed with vein with less than 2.5 mm in diameter had a high rate of non-maturation24. Moreover, Mendes et al. reported that 22 fistulae created with a cephalic vein less than 2 mm in diameter had 19 non-maturation, whereas 19 of 25 (76%) fistulae created using a cephalic vein diameter more than 2 mm successfully matured, verifying the predictive value of vein diameter in AVF failure25.
This study has several limitations. Firstly, this is a single-center retrospective study with a small sample size, which may have limited the statistical power of the analysis. Secondly, other factors that may influence AVF patency such as the status of blood vessels, surgical technique, AVF sites and vessel histology have not been included in our study. Therefore, further studies focusing on these parameters are warranted.
Conclusion
AVF dysfunction is strongly associated with a number of risk factors including weight, phosphorus level, and sex. Thus, intervention strategies targeting these potential factors such as weight loss, or oral phosphate binders may improve the long-term success of AVF. Further multi-center randomized-controlled trials are needed to confirm these findings.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Kjellstrand, C. M. The Achilles’ heel of the hemodialysis patient. Arch. Intern. Med. 138(7), 1063–1064 (1978).
Ravani, P. et al. Associations between hemodialysis access type and clinical outcomes: A systematic review. J. Am. Soc. Nephrol. 24(3), 465–473 (2013).
Vascular Access Work Group. Clinical practice guidelines for vascular access. Am. J. Kidney Dis. 48(Suppl 1), S176-247 (2006).
Masengu, A., Maxwell, A. P. & Hanko, J. B. Investigating clinical predictors of arteriovenous fistula functional patency in a European cohort. Clin. Kidney J. 9(1), 142–147 (2016).
Roy-Chaudhury, P. & Kruska, L. Future directions for vascular access for hemodialysis. Semin. Dial. 28(2), 107–113 (2015).
NKF-DOQI clinical practice guidelines for vascular access. National kidney foundation-dialysis outcomes quality initiative. Am. J. Kidney Dis. 30(4 Suppl 3), S150-191 (1997).
Ethier, J. H. et al. Clinical practice guidelines for vascular access. Canadian Society pf Nephrology. J. Am. Soc. Nephrol. 10(Suppl 13), S297-305 (1999).
Miller, C. D., Robbin, M. L. & Allon, M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 63(1), 346–352 (2003).
Roy-Chaudhury, P., Sukhatme, V. P. & Cheung, A. K. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J. Am. Soc. Nephrol. 17(4), 1112–1127 (2006).
Thi Nguyen, N. et al. Inhibition of mitochondrial phosphate carrier prevents high phosphate-induced superoxide generation and vascular calcification. Exp. Mol. Med. 55(3), 532–540 (2023).
Venkat Ramanan, S. et al. Outcomes and predictors of failure of arteriovenous fistulae for hemodialysis. Int. Urol. Nephrol. 54(1), 185–192 (2022).
Li, Y., Cui, W., Wang, J., Zhang, C. & Luo, T. Factors associated with dysfunction of autogenous arteriovenous fistula in patients with maintenance hemodialysis: A retrospective study. Ann. Palliat. Med. 10(4), 4047–4054 (2021).
Zhou, M. & Lu, F. P. Effect of hyperphosphatemia on patency rate of arteriovenous fistula of patients with late fistula dysfunction/failure after reoperation. Zhonghua Yi Xue Za Zhi 98(42), 3406–3410 (2018).
Chan, M. R., Young, H. N., Becker, Y. T. & Yevzlin, A. S. Obesity as a predictor of vascular access outcomes: Analysis of the USRDS DMMS wave II study. Semin. Dial. 21(3), 274–279 (2008).
Chan, S. M., Weininger, G., Langford, J., Jane-Wit, D. & Dardik, A. Sex differences in inflammation during venous remodeling of arteriovenous fistulae. Front. Cardiovasc. Med. 8, 715114 (2021).
Kim, J. K. et al. Obesity-related decrease in intraoperative blood flow is associated with maturation failure of radiocephalic arteriovenous fistula. J. Vasc. Surg. 62(4), 1010-1017 e1011 (2015).
Almasri, J. et al. Outcomes of vascular access for hemodialysis: A systematic review and meta-analysis. J. Vasc. Surg. 64(1), 236–243 (2016).
Konner, K., Hulbert-Shearon, T. E., Roys, E. C. & Port, F. K. Tailoring the initial vascular access for dialysis patients. Kidney Int. 62(1), 329–338 (2002).
Sedlacek, M., Teodorescu, V., Falk, A., Vassalotti, J. A. & Uribarri, J. Hemodialysis access placement with preoperative noninvasive vascular mapping: Comparison between patients with and without diabetes. Am. J. Kidney Dis. 38(3), 560–564 (2001).
Bahrami-Ahmadi, A., Khavanin Zadeh, M., Chehrehgosha, H. & Abbasi, M. Early failure of arteriovenous fistula (AVF): The effect of diabetes and hypertension in a cross-sectional study. Med. J. Islam. Repub. Iran 36, 89 (2022).
Bashar, K. et al. Arteriovenous fistula in dialysis patients: Factors implicated in early and late AVF maturation failure. Surgeon 14(5), 294–300 (2016).
Fassiadis, N. et al. Does the surgeon’s experience impact on radiocephalic fistula patency rates?. Semin. Dial. 20(5), 455–457 (2007).
Sultan, S., Hynes, N., Hamada, N. & Tawfick, W. Patients on hemodialysis are better served by a proximal arteriovenous fistula for long-term venous access. Vasc. Endovasc. Surg. 46(8), 624–634 (2012).
Bashar, K., Clarke-Moloney, M., Burke, P. E., Kavanagh, E. G. & Walsh, S. R. The role of venous diameter in predicting arteriovenous fistula maturation: when not to expect an AVF to mature according to pre-operative vein diameter measurements? A best evidence topic. Int. J. Surg. 15, 95–99 (2015).
Mendes, R. R. et al. Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J. Vasc. Surg. 36(3), 460–463 (2002).
Funding
The Funding was supported by Natural Science Foundation of Hunan Province of China, 2021JJ40297, Hunan Provincial People’s Hospital RENSHU Funding Project, RS201801, Young Doctor Fund and Young Doctor Fun and Fund for Fostering of the National Natural Science Foundation, BSJJ201806.
Author information
Authors and Affiliations
Contributions
F.Z. and J.L.L. wrote the draft of paper; J.Y. and Y.J. collected the data and follow-up, H.L.X and Y.Y.Y. performed the data analysis; Y.M.L.,K.H.L. and X.L. revised the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, F., Li, J., Yu, J. et al. Risk factors for arteriovenous fistula dysfunction in hemodialysis patients: a retrospective study. Sci Rep 13, 21325 (2023). https://doi.org/10.1038/s41598-023-48691-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48691-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.