Abstract
Motivated by the complex and multifactorial etiologies of osteoarthritis, here we use a comprehensive approach evaluating knee joint health after unilateral lower limb loss. Thirty-eight male Service members with traumatic, unilateral lower limb loss (mean age = 38 yr) participated in a prospective, two-year longitudinal study comprehensively evaluating contralateral knee joint health (i.e., clinical imaging, gait biomechanics, physiological biomarkers, and patient-reported outcomes); seventeen subsequently returned for a two-year follow-up visit. For this subset with baseline and follow-up data, outcomes were compared between timepoints, and associations evaluated between values at baseline with two-year changes in tri-compartmental joint space. Upon follow-up, knee joint health worsened, particularly among seven Service members who presented at baseline with no joint degeneration (KL = 0) but returned with evidence of degeneration (KL ≥ 1). Joint space narrowing was associated with greater patellar tilt (r[12] = 0.71, p = 0.01), external knee adduction moment (r[13] = 0.64, p = 0.02), knee adduction moment impulse (r[13] = 0.61, p = 0.03), and CTX-1 concentration (r[11] = 0.83, p = 0.001), as well as lesser KOOSSport and VR-36General Health (r[16] = − 0.69, p = 0.01 and r[16] = − 0.69, p = 0.01, respectively). This longitudinal, multi-disciplinary investigation highlights the importance of a comprehensive approach to evaluate the fast-progressing onset of knee osteoarthritis, particularly among relatively young Service members with lower limb loss.
Similar content being viewed by others
Introduction
Knee osteoarthritis (OA) is commonly diagnosed through radiographic indicators, clinical examinations, and patient-reported symptoms1. Upon radiographic evaluation, formation of osteophytes and diminished joint space indicate deterioration of the knee joint, leading to OA pathology2. Furthermore, increased pain severity and functional limitations are clinically meaningful manifestations associated with symptomatic knee OA3,4. While degenerative OA has traditionally been associated with advancing age, earlier onset is more prevalent among younger adults after limb trauma5,6; for example, higher prevalence rates in younger Service members (SM) with (unilateral) lower limb loss compared to the general population (28% versus 12%)5,7,8. Earlier onset of knee OA can substantially reduce long-term functionality and quality of life7; thus, better understanding risk factors for longitudinal changes in knee joint health among SM with lower limb loss is critical for military medicine.
In the non-limb loss population, cross-sectional and longitudinal studies of knee OA have identified/monitored several factors associated with knee OA onset and progression9,10, highlighting the multifactorial nature of the pathology and motivating the need for a comprehensive evaluation. Biomechanical factors play an important role in joint health, and altered joint loading has been associated with both the onset and progression of knee OA11. Altered joint loading is of particular concern for persons with (unilateral) lower limb loss, who typically walk and perform activities with a preference for the contralateral (intact) limb12,13, thus theorized to increase risk for knee joint degeneration due to repetitive exposures to larger and/or prolonged mechanical loads. Considering that such repeated exposures to abnormal loading may not result in near-term structural damage or symptoms, physiological biomarkers can provide additional insights into the underlying molecular and cellular indicators of joint health, perhaps even facilitating early(ier) identification of disease onset14. For example, elevated concentrations of markers indicative of inflammation and/or osteochondral remodeling are present in both serum and synovial fluids with OA15,16. Degraded cartilage material properties, modulated by the composition of the extracellular matrix, have also been related to synovial inflammation and increases in cartilage strain for a given load17. Yet, despite the high prevalence (and seemingly early onset) of knee OA in persons with lower limb loss, coupled with the complex interactions among the biomechanical environment with both joint structure and physiology, to date no studies have utilized a comprehensive approach to explore knee joint health after limb loss.
This study aimed to identify longitudinal changes in associative risk factors for OA, from a comprehensive assessment for knee joint health in SM with unilateral lower limb loss. We hypothesized that decreases in medial tibiofemoral and patellofemoral joint spaces would be positively associated with increased knee joint kinetics and elevated concentrations of physiological markers of bone and cartilage degradation and remodeling, and inflammation. Moreover, we predicted that radioanatomic measures such as bisect offset, patellar tilt, and tibiofemoral joint angle would be associated with greater medial patellofemoral joint space narrowing. Diminished knee joint health would also be associated with poorer patient-reported outcomes (i.e., greater pain, reduced function or quality of life). Better understanding longitudinal relationships among morphological, biomechanical, and physiological factors associated with knee OA after lower limb loss is a necessary first step toward mitigating the deleterious effects of diminishing knee joint health and maximizing long-term outcomes for SM with lower limb loss.
Methods
Participants
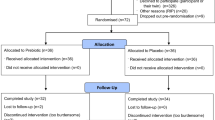
Thirty-eight male SM with unilateral lower limb loss (23 transfemoral and 15 transtibial; mean ± standard deviation age: 37.9 ± 6.5 yr; stature: 1.78 ± 0.05 m; body mass: 89.2 ± 18.4 kg; time since injury: 114.4 ± 70.9 months) were enrolled into a prospective, two-year longitudinal study utilizing a comprehensive evaluation (i.e., clinical imaging, gait biomechanics, physiological biomarkers, and patient-reported outcomes) for identifying the onset, progression, and overall impact of knee OA on functionality and quality of life; eighteen (9 transtibial and 9 transfemoral) SM returned for the two-year follow-up. One participant was removed from subsequent analyses due to receiving a medial knee arthroplasty between baseline and follow-up; thus, seventeen SM with unilateral lower limb loss (9 transtibial and 8 transfemoral) were included (Fig. 1). The Walter Reed National Military Medical Center Institutional Review Board approved all experimental procedures (protocol #500081), performed in accordance with all relevant guidelines and regulations, and written informed consent was obtained from all participants prior to testing.
Clinical imaging
Standard, bilateral weight-bearing anterior–posterior and sunrise-view radiographs of the intact knee were obtained for 15 SM at baseline and follow-up (n = 2 were not medically cleared). Imaging outcomes include clinical grading of knee joint health via Kellgren-Lawrence (KL) classifications and the modified Outerbridge (OC) grading scale (for medial tibiofemoral compartment, lateral tibiofemoral compartment, and patellofemoral joint), measurement of medial and lateral tibiofemoral, medial and lateral patellofemoral joint space narrowing, and radioanatomic positions of the knee and patella. Radiographic OA within the intact knee was considered at KL grade ≥ 2. Patellar tilt angle18, sulcus angle19, bisect offset18, femoral-tibial angle20, knee rotation21, and Insall-Salvati ratio22 were also measured, as described previously.
Gait biomechanics
All SM at baseline (n = 17) and two-year follow-up (n = 17) completed a gait evaluation, walking at a self-selected pace along a 15 m walkway. Full-body kinematics were obtained using an 18-camera motion capture system (Qualisys, Göteborg, SE) to track (120 Hz) the positions of 62 reflective markers. Markers were placed on the head (× 4), C7 and T10 spinal processes, sternal notch, xiphoid, bilaterally across the acromion, humeri, lateral elbows, forearm, radii, ulnae, dorsal hand, posterior and anterior superior iliac spines, calcanei, hallux, and the 2nd and 5th metatarsals. Cluster-based tracking markers were also placed bilaterally on each thigh and shank (4 markers per cluster). Twelve additional calibration-only markers were placed bilaterally on the medial epicondyle of the elbow, lateral and medial epicondyles of the knee and ankle maleoli, and the medial aspect of the 1st metatarsal. Bilateral ground reaction forces were simultaneously sampled (1200 Hz) from six force platforms (AMTI, Watertown, MA, USA) embedded within the walkway. Marker trajectories and ground reaction forces were low-pass filtered (6 and 45 Hz, respectively) with a dual-pass, 2nd order Butterworth filter.
Temporal-spatial parameters, knee joint kinematics, and knee joint kinetics were calculated in Visual3D (C-Motion, Germantown, MD, USA). Briefly, bilateral gait events (i.e., heel strike and toe off) were first determined using a foot kinematic-based detection algorithm23; temporal-spatial outcomes were derived (walking speed, stride length, stride width, and cadence). Peak sagittal knee angles from each gait cycle were extracted to compute joint range of motion (ROM). Joint angles were computed using a flexion–extension, ab/adduction, axial rotation sequence. Inverse dynamics were used to calculate external knee adduction moment (KAM) and knee flexion moment (KFM), resolved relative to the proximal (thigh) segment; peak KAM and KFM were extracted, as were KAM impulse (time integral of KAM) and KAM loading rate (slope between 20 and 80% of time from heel strike to first peak). Knee joint contact forces (total, medial, and lateral) were calculated based on the model of Schipplein and Andriacchi, and developed as described in previous literature24,25. All joint kinematic and kinetic parameters are only reported for the contralateral limb (i.e., non-limb loss).
Physiological biomarkers
Fifteen SM provided serum samples at baseline and follow-up, while four (of eight at baseline) SM opted to have synovial fluid samples taken at follow-up. Whole blood samples were collected through venipuncture; serum was obtained through centrifugation at 1000RPM for 10 min at room temperature. Synovial fluid was aspirated from the contralateral knee by a trained physician. After collection, both biofluids were aliquoted and stored at − 80 °C for downstream analyses. Subsequently, serum and synovial fluid samples were analyzed for collagen II cleavage (C2C), N-Propeptide of Collagen IIA (PIIANP), hyaluronic acid (HA), and cartilage oligomeric matrix protein (COMP) as markers of osteochondral (bone and cartilage) remodeling; and C-Telopeptide of Type 1 Collagen (CTX-1) and N-telopeptide of Type 1 Collagen (NTX-1) as markers of subchondral bone degradation via Enzyme-Linked Immunosorbent Assays according to manufacturer’s protocol. Serum and synovial fluid samples were also analyzed for various inflammatory and tissue-remodeling markers via multiplex bead assays. For markers related to collagen and mineral metabolism, custom multiplex kits (Invitrogen) were used to evaluate the following markers: matrix metalloprotease (MMP) -2, MMP3, MMP7, MMP8, MMP9, MMP12, MMP13, and tissue-inhibitor or metalloprotease (TIMP)-1. Specifically, for cytokines and chemokines, a ProcartaPlex Human Cytokine/Chemokine kit (Invitrogen, Carlsbad, CA, USA) was used according to manufacturer recommendations.
Patient-reported outcomes
Patient-reported outcomes were obtained from all SM at baseline (n = 17) and follow-up (n = 17), including pain severity by Visual Analog Scale (VAS)26 and PROMIS Pain Interference (SF-8a), Knee Injury and Osteoarthritis Outcome Score (KOOS)27, and Veterans RAND 36-Item Health Survey (VR-36)28.
Statistical analyses
Statistical analyses were performed using Statistical Package for Social Science (SPSS) software (version 25; Chicago, IL, USA). Sample characteristics are presented as numbers, percentages, means ± standard deviations. Normal distribution was evaluated using the Shapiro–Wilk tests. To first compare outcomes between timepoints (i.e., baseline vs. two-year follow-up), paired sample t-tests, or Wilcoxon signed-rank tests (where applicable), were used to compare all outcomes within each domain: (i) demographics, (ii) radioanatomy, (iii) gait biomechanics, (iv) physiological biomarkers, and (v) patient-reported. Effect sizes are reported using Cohen’s d. Chi-square analysis was utilized to compare the proportions of KL classifications. Second, associations between each outcome (at baseline) with changes in tibiofemoral and patellofemoral joint space (from baseline to two-year follow-up) were investigated using Pearson’s correlations. Kendall’s tau-b correlation was utilized for correlations between Insall-Salvati proportions and joint space narrowing. Values of p < 0.05 were considered significant.
Results
Clinical imaging
Upon follow-up (mean time elapsed since baseline evaluation = 26.6 ± 3.2 mo), knee joint health worsened, particularly among seven SMs (2 transtibial, 5 transfemoral) who presented at baseline with no clinical joint degeneration (KL = 0) within the contralateral knee; all returned at two-year follow-up with evidence of degeneration (KL ≥ 1). The proportion of SM with KL ≥ 2, a frequently used threshold for OA diagnoses, was not different at either time point (p = 0.28). Medial tibiofemoral joint space was similar (p = 0.46) between time points, but lateral tibiofemoral joint space decreased by 7.9% (p = 0.01) from baseline to follow-up (Table 1). There were no changes in medial (− 15.1%, p = 0.35) and lateral (− 18.2%, p = 0.20) patellofemoral joint spaces.
At follow-up, there was a greater sulcus angle (Table 2; p = 0.01) and lesser Insall-Salvati measurement (p = 0.03); there were no differences in the proportion of patella alta (Insall-Salvati > 1.2; p = 0.28) or patella baja (Insall-Salvati < 0.8; p = 0.25). Patellar tilt angle at baseline was positively correlated with medial and lateral patellofemoral joint space narrowing; r[12] = 0.70, p = 0.01 and r[12] = 0.71, p = 0.01, respectively. Insall-Salvati at baseline was negatively correlated with narrowing of the medial and lateral tibiofemoral joint spaces; r[13] = − 0.49; p = 0.02, and r[13] = − 0.47, p = 0.04, respectively.
Gait biomechanics
At follow-up, there was lesser sagittal knee ROM (Table 3; p = 0.03) and decreased knee flexion moment (p = 0.05). Peak KAM at baseline was negatively associated with lateral patellofemoral joint space narrowing (r[12] = − 0.58, p = 0.05), and positively associated with lateral tibiofemoral joint space narrowing (r[13] = 0.64, p = 0.02). KAM loading rate at baseline was negatively associated with medial patellofemoral joint space narrowing (r[12] = − 0.80, p = 0.002) and lateral patellofemoral joint space narrowing (r[12] = − 0.75, p = 0.005). KAM impulse at baseline was positively correlated with medial tibiofemoral joint space narrowing (r[13] = 0.61, p = 0.61). Peak medial joint contact forces at baseline were negatively associated with lateral patellofemoral joint space narrowing (r[12] = − 0.66, p = 0.02). Peak lateral joint contact forces at baseline were negatively associated with medial tibiofemoral joint space narrowing (r[13] = − 0.64, p = 0.02).
Physiological biomarkers
At follow-up, there were greater serum concentrations of CTX-1 (+ 87.0%; p < 0.001), HA (+ 80.4%; p < 0.001), COMP (+ 148.0%; p < 0.001; d = 1.29), SDF-1 (+ 57.9; p = 0.002), MMP-12 (+ 203.0%; p < 0.001), MMP-13 (+ 70.4%; p = 0.04), MMP-7 (+ 40.8%; p = 0.03), and MMP-8 (+ 156.0%, p = 0.03; Table 4); in contrast, there were lesser concentrations of C2C (− 28.9%; p = 0.01), PIIANP (− 504.1%; p = 0.001), NTX-1 (− 67.8%; p = 0.02), CCL-4 (− 47.7%; p < 0.001), CCL-11 (− 52.2%; p = 0.001), CXCL-10 (− 39.7; p = 0.001), IFN-α(− 60.1%; p = 0.004), and IL-1α (− 85.1%; p < 0.001). Concentrations of CCL-2 and IL-18 at baseline were negatively associated with lateral tibiofemoral joint space narrowing (r[12] = − 0.60, p = 0.04 and r[12] = − 0.74, p = 0.01, respectively). Concentrations of CTX-1 at baseline were positively associated with lateral patellofemoral joint space narrowing (r[11] = − 0.83, p = 0.001).
At follow-up, there were greater synovial fluid concentrations of CCL-11 (+ 133.3%, p = 0.05; Table 5). Moreover, concentrations of MMP-13 at baseline were positively associated with medial tibiofemoral joint space narrowing (r[4] = 0.99, p = 0.001). Concentrations of MMP-8 at baseline were positively associated with lateral patellofemoral joint space narrowing (r[3] = 0.99, p = 0.01). Concentrations of MMP-1 at baseline were negatively associated with lateral patellofemoral joint space narrowing (r[3] = − 0.99, p = 0.04).
Patient-reported outcomes
At follow-up, SM did not report greater pain severity within the contralateral leg (p = 0.34), ipsilateral leg (p = 0.13), contralateral knee (p = 0.27), ipsilateral knee (for transtibial; p = 0.10), back (p = 0.13), or whole body (p = 0.54; Table 6). SM reported decreasing values in KOOS subdomains of Symptoms (p = 0.03) and Quality of Life (p = 0.01) from baseline to follow-up, suggesting worsening symptoms and diminishing quality of life. SM also reported decreasing values in the VR-36 subdomain of General Health (p = 0.03) from baseline to follow-up, again suggesting diminishing general health. There were no differences in PROMIS (all p > 0.05). Changes in medial tibiofemoral joint space were negatively correlated with baseline KOOS-Sport (r[16] = − 0.69; p = 0.01) and VR-36 General Health (r[16] = − 0.69, p = 0.01).
Discussion
This comprehensive, longitudinal evaluation for knee joint health in SM with unilateral lower limb loss aimed to identify relationships among associative risk factors at baseline with knee joint space narrowing. The primary hypotheses that decreases in medial tibiofemoral and patellofemoral joint spaces will be associated with increased knee joint kinetics and elevated serum concentrations, and synovial fluid inflammatory biomarkers of bone and cartilage metabolites, were partially supported.
Radiographically, KL grading suggests overall minimal to moderate joint degeneration (i.e., KL ≤ 3), with worsening of knee joint health from baseline to two-year follow-up (17–42% with KL ≥ 2). Of the seven SM (2 TT, 5 TF) who presented at baseline with no clinical joint degeneration (KL = 0), all returned at two-year follow-up with evidence of degeneration (KL ≥ 1) within the contralateral knee. Although ultimately excluded at follow-up, one participant (42 yrs of age) received a medial knee arthroplasty during the study period (KL = 3 at baseline, with evidence of degeneration in the medial [Outerbridge Grade = 4], lateral [Outerbridge Grade = 2], patellofemoral [Outerbridge Grade = 3] compartments). A secondary hypothesis predicted that radioanatomic measures (e.g., bisect offset, patellar tilt, and tibiofemoral joint angle) would be associated with more significant medial patellofemoral joint space narrowing; this hypothesis was partially supported, as patellar tilt was positively associated with medial and lateral patellofemoral joint space narrowing, consistent with prior literature in persons with patellofemoral pain and OA progression18,29. Furthermore, decreases in the Insall-Salvati ratio, a patellar height measurement, were negatively associated with medial and lateral tibiofemoral joint space narrowing. While the ratio of participants with patella alta/baja was similar between time points, changes in the Insall-Salvati ratio can impact patellofemoral joint mechanisms and often result in diminished function and deterioration of knee joint health30. Changes in patellar tendon length has been extensively studied following anterior cruciate ligament repairs and total knee arthroplasty, but not in the population of SM with limb loss, who present a unique morphological change, absent surgical intervention31. Our results concur with the literature, noting that longer patellar tendons (larger Insall-Salvati ratio) present a situation wherein the patella may be unstable and maltrack, impacting the articular surfaces of the knee and patella32. Within our study sample, decreases in the patellar height could suggest a more proximal patellar positioning, prior to study enrollment. Some potential explanations for a decreased Insall-Salvati ratio may be due to increases in weight or forces upon the contralateral knee, or decreases in the retropatellar fat pad33,34,35. Although literature notes a higher prevalence of patella baja with greater body mass33, it can be surmised that the increased dependence on the contralateral limb after unilateral limb loss may mimic similar overloading conditions of overweight/obesity. Further research is needed to longitudinally track morphological changes in knee radioanatomy and patellar anatomy of the contralateral knee immediately following limb loss, as our study population at enrollment were several years past their injury.
Biomechanically, while cross-sectional evaluations indicate larger knee joint loads in persons with vs. without lower limb loss, here there was a general lack of changes between timepoints in the current study. Common biomechanical adaptations during walking with vs. without knee OA, though variable across studies36, include lesser knee flexion–extension excursion (largely due to joint stiffness or pain) or greater loading metrics (e.g., external KAM peak or loading rate). While prior studies have identified differences in joint loads with vs. without knee OA, such changes are more apparent with more severe stages of OA (i.e., KL ≥ 3)37, and peak KAM is positively correlated with the severity of knee OA (medial tibiofemoral joint space narrowing)38,39. Here, despite the similar (peak) joint loads between timepoints, and minimal-moderate joint degeneration (i.e., KL1-3), there were several moderate associations between KAM loading measures and tibiofemoral or patellofemoral joint space narrowing. We and others have suggested that idiopathic knee OA after limb loss results not necessarily from chronically high joint loads but rather from “unusual” joint loads, defined as loads the joint is not conditioned to sustain frequently24,40,41. Exposure to such unusual loads could be particularly hazardous to long-term joint health if introduced following an extended period of low activity and ostensible structural weakening, such as if an individual with unilateral limb loss hops on one leg (e.g.,42). However, more ecologically valid methods are needed to comprehensively track biomechanical and mobility outcomes for prolonged durations and across numerous activities, at home and in the community (vs. exclusively in a lab during controlled conditions).
Physiologically, while numerous serum biomarkers changed from baseline to follow-up, CTX-1 specifically was positively correlated with lateral patellofemoral joint space narrowing and highlights the presence of increased osteochondral breakdown in systemic biomarkers (i.e., serum). However, we must acknowledge that SM with limb loss often present with multi-site musculoskeletal pain43,44 which may confound joint-specific musculoskeletal research, particularly in the interpretation of some systemic biomarkers and other outcomes (i.e., gait biomechanics, patient-reported function or quality of life). In the current sample, while participants reported multi-site pain (e.g., residual limb, low back), these did not change from baseline to two-year follow-up. Nevertheless, more specific to the knee joint, we identified increases in MMP-13 and MMP-8 among synovial fluid at follow-up, which were respectively associated with greater narrowing of the medial tibiofemoral and lateral patellofemoral joint spaces. These MMP biomarkers indicate joint catabolic activity, ultimately leading to the loss of cartilage via inhibition of cartilage differentiation and promotion of chondrocyte apoptosis45,46. This is a significant finding and novel contribution to both the limb loss and non-limb loss OA populations.
Patient-reported outcomes provide important insights on symptom severity and corresponding influences on well-being and quality of life; however, the ability to accurately assess pain severity is likely hampered in the SM population. For example, while pain experience can be subjective and thus difficult to compare between participants or populations, SM tend to under-report symptoms in both clinical and research settings47. Outcome measures such as the KOOS or VR-36 may be more appropriate to understand the impact and monitor changes in knee joint health among SM with limb loss. Here, KOOS scores generally indicated better outcomes (i.e., scores closer to 100) than reported in the literature across a range of OA pathologies48, for most subdomains. Also, only Symptoms and Quality of Life decreased from baseline to two-year follow-up, again noting a relatively lesser severity of degeneration within the current sample. VR-36 and SF-8A outcomes of the current sample provide a slightly different perspective from acute pain alone, with decreasing General Health from baseline to two-year follow-up, but similarly suggest overall better general health and lesser pain interference in comparison to existing literature49,50.
Limitations
Several limitations are present and should be considered. Acknowledging a general paucity of longitudinal data, many patients with knee OA enrolled in longitudinal studies do not progress radiographically during ~ two-year trials, highlighting that OA progression is highly variable by individual and stage of disease9,51,52; in the absence of existing characterization of disease trajectory among SM with limb loss, we ultimately chose a two-year follow-up window to balance additional pragmatic considerations (i.e., attrition). Moreover, our broad cross-sectional recruitment strategy (i.e., no a priori grouping by joint health or other factors upon enrollment) generated a convenience sample with somewhat heterogeneous characteristics. Despite the likelihood for knee joint health to be influenced by time since injury and/or “severity” of injury (i.e., transtibial vs. transfemoral limb loss), neither the study design nor final dataset support the evaluation of such factors within the longitudinal framework. The prevalence of new amputations within the military has decreased every year since peaking in 201153; while our sample is more reflective of SM injured within this time period (time since injury = 119.8 ± 89.3 months), without baseline measures obtained more proximal (and/or prior) to time of injury we are unable to determine the causality of observed associations. While attrition is an inherent challenge for longitudinal studies, this challenge was magnified during the COVID-19 global pandemic as travel and research restrictions prevented many participants from returning for follow-up. Importantly, compared to participants who completed follow-up, participants lost to follow-up were not different by baseline demographics, medical history, or knee joint health grading (Supplementary Table 1); thus, mitigating potential confounding effects of attrition beyond sample size alone.
Conclusion
This study establishes a comprehensive approach for evaluating (contralateral) knee joint health after unilateral lower limb loss and emphasizes the need for early identification/intervention to mitigate the fast-progressing degeneration among SM with lower limb loss. In particular, SM with traumatic limb loss are typically younger at time of injury and more active following limb loss, increasing the likelihood of suffering long-term consequences of poor knee joint health on function and quality of life. Future research should continue to employ this multi-faceted approach to evaluate knee joint health and encourage the development of optimal interventions to maximize quality of life and long-term functional outcomes for SM with limb loss.
Data availability
All data associated with this study are present in the paper. Availability of raw clinical imaging, biomechanical data, or serum samples are not available for public use.
Abbreviations
- C2C:
-
Collagen II cleavage
- COMP:
-
Cartilage oligomeric matrix protein
- CTX-1:
-
C-Telopeptide of type 1 collagen
- HA:
-
Hyaluronic acid
- KAM:
-
Knee adduction moment
- KFM:
-
Knee flexion moment
- KL:
-
Kellgren–Lawrence
- KOOS:
-
Knee injury and osteoarthritis outcomes score
- MMP:
-
Matrix metalloprotease
- MRI:
-
Magnetic resonance images
- NTX-1:
-
N-telopeptide of type 1 collagen
- PIIANP:
-
N-propeptide of collagen IIA
- OA:
-
Osteoarthritis
- OC:
-
Outerbridge classification
- SM:
-
Service members
- TIMP:
-
Tissue-inhibitor or matelloprotease
- VAS:
-
Visual analog scale
- VR36:
-
Veterans RAND 36 item health survey
References
Heidari, B. Knee osteoarthritis diagnosis, treatment and associated factors of progression: part II. Caspian J. Intern. Med. 2, 249 (2011).
Felson, D. et al. Osteophytes and progression of knee osteoarthritis. Rheumatology 44, 100–104 (2005).
Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 21, 1145–1153 (2013).
Øiestad, B. E. et al. Longitudinal course of physical function in people with symptomatic knee osteoarthritis: Data from the multicenter osteoarthritis study and the osteoarthritis initiative. Arthr. Care Res. 68, 325–331 (2016).
Cameron, K. L., Hsiao, M. S., Owens, B. D., Burks, R. & Svoboda, S. J. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthr. Rheum. 63, 2974–2982 (2011).
Felson, D. T. et al. Occupational physical demands, knee bending, and knee osteoarthritis: Results from the Framingham study. J. Rheumatol. 18, 1587–1592 (1991).
Gailey, R., Allen, K., Castles, J., Kucharick, J. & Roeder, M. Review of secondary physical conditions associated with lower-limb. J. Rehabil. Res. Dev. 45, 15–30 (2008).
Rivera, J. C., Wenke, J. C., Buckwalter, J. A., Ficke, J. R. & Johnson, A. E. Posttraumatic osteoarthritis caused by battlefield injuries: The primary source of disability in warriors. JAAOS-J. Am. Acad. Orthop. Surg. 20, S64–S69 (2012).
Felson, D. T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 21, 10–15 (2013).
Sandell, L. J. Etiology of osteoarthritis: Genetics and synovial joint development. Nat. Rev. Rheumatol. 8, 77–89 (2012).
Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 25, 815–823 (2011).
Wasser, J. G., Acasio, J. C., Miller, R. H. & Hendershot, B. D. Overall greater demands on the musculoskeletal system at multiple walking speeds in Service members with lower limb loss. J. Appl. Biomech. 37, 522–530 (2021).
Teater, R. H., Wolf, D. N., McDonald, K. A. & Zelik, K. E. Unilateral transtibial prosthesis users load their intact limb more than their prosthetic limb during sit-to-stand, squatting, and lifting. Clin. Biomech. 108, 106041 (2023).
Madry, H. et al. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 24, 1753–1762 (2016).
Daghestani, H. N. & Kraus, V. B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 23, 1890–1896 (2015).
Petersson, I. F., Boegård, T., Svensson, B., Heinegård, D. & Saxne, T. Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br. J. Rheumatol. 37, 46–50 (1998).
Waldstein, W., Perino, G., Jawetz, S. T., Gilbert, S. L. & Boettner, F. Does intraarticular inflammation predict biomechanical cartilage properties?. Clin. Orthop. Relat. Res.® 472, 2177–2184 (2014).
Hunter, D. et al. Patella malalignment, pain and patellofemoral progression: The health ABC study. Osteoarthr. Cartil. 15, 1120–1127 (2007).
Qin, J. et al. Evaluation of the effect of the sulcus angle and lateral to medial facet ratio of the patellar groove on patella tracking in aging subjects with stable knee joint. BioMed Res. Int. 2018 (2018).
Chang, C. et al. What should be considered in using standard knee radiographs to estimate mechanical alignment of the knee?. Osteoarthr. Cartil. 18, 530–538 (2010).
of the OARSI, R. W. G. et al. Assessment of the radioanatomic positioning of the osteoarthritic knee in serial radiographs: comparison of three acquisition techniques. Osteoarthr. Cartil. 14, 37–43 (2006)
Lu, W., Yang, J., Chen, S., Zhu, Y. & Zhu, C. Abnormal patella height based on Insall-Salvati ratio and its correlation with patellar cartilage lesions: An extremity-dedicated low-field magnetic resonance imaging analysis of 1703 Chinese cases. Scand. J. Surg. 105, 197–203 (2016).
Zeni, J. Jr., Richards, J. & Higginson, J. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 27, 710–714 (2008).
Miller, R. H., Krupenevich, R. L., Pruziner, A. L., Wolf, E. J. & Schnall, B. L. Medial knee joint contact force in the intact limb during walking in recently ambulatory service members with unilateral limb loss: A cross-sectional study. PeerJ 5, e2960 (2017).
Schipplein, O. & Andriacchi, T. Interaction between active and passive knee stabilizers during level walking. J. Orthop. Res. 9, 113–119 (1991).
Delgado, D. A. et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2 (2018).
Roos, E. M. & Lohmander, L. S. The knee injury and osteoarthritis outcome score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 1, 1–8 (2003).
Brazier, J., Roberts, J. & Deverill, M. The estimation of a preference-based measure of health from the SF-36. J. Health Econ. 21, 271–292 (2002).
Kannus, P. Long patellar tendon: Radiographic sign of patellofemoral pain syndrome–a prospective study. Radiology 185, 859–863 (1992).
Meneghini, R. M. et al. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J. Arthroplast. 21, 116–120 (2006).
Neogi, D. S., Bae, J. H., Seok, C. W. & Lim, H. C. Impact of patellar height on unicompartment knee arthroplasty: Does patella baja lead to an inferior outcome?. J. Orthop. Traumatol. 15, 47–54 (2014).
Eijkenboom, J. F. et al. Patellofemoral alignment and geometry and early signs of osteoarthritis are associated in patellofemoral pain population. Scand. J. Med. Sci. Sports 30, 885–893 (2020).
Francois, E. L. et al. Incidence of patella baja before and after primary total knee arthroplasty based on body mass index. Orthopedics 42, 90–94 (2019).
Hamarneh, G., Chu, V., Bordalo-Rodrigues, M. & Schweitzer, M. in Medical Imaging 2005: Physiology, Function, and Structure from Medical Images. 527–534 (SPIE).
Meneghini, R. M. et al. The effect of retropatellar fat pad excision on patellar tendon contracture and functional outcomes after total knee arthroplasty. J. Arthroplast. 22, 47–50 (2007).
Mills, K., Hunt, M. A. & Ferber, R. Biomechanical deviations during level walking associated with knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res. 65, 1643–1665 (2013).
Hall, M. et al. The knee adduction moment and knee osteoarthritis symptoms: Relationships according to radiographic disease severity. Osteoarthr. Cartil. 25, 34–41 (2017).
Sharma, L. et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthr. Rheum. 41, 1233–1240 (1998).
Davis, E. M. et al. Longitudinal evidence links joint level mechanics and muscle activation patterns to 3-year medial joint space narrowing. Clin. Biomech. 61, 233–239 (2019).
Andriacchi, T. P., Koo, S. & Scanlan, S. F. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J. Bone Joint Surg. Am. 91, 95 (2009).
Seedhom, B. Conditioning of cartilage during normal activities is an important factor in the development of osteoarthritis. Rheumatology 45, 146–149 (2006).
Krupenevich, R. L., Pruziner, A. L. & Miller, R. H. Knee joint loading during single-leg forward hopping. Med. Sci. Sports Exerc. 49, 327–332 (2017).
Ehde, D. M. et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch. Phys. Med. Rehabil. 81, 1039–1044 (2000).
Smith, D. G. et al. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin. Orthop. Relat. Res. 1976–2007(361), 29–38 (1999).
Hu, Q. & Ecker, M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int. J. Mol. Sci. 22, 1742 (2021).
Luo, S., Li, W., Wu, W. & Shi, Q. Elevated expression of MMP8 and MMP9 contributes to diabetic osteoarthritis progression in a rat model. J. Orthop. Surg. Res. 16, 1–9 (2021).
Kanzler, K. E. et al. Mitigating the effect of pain severity on activity and disability in patients with chronic pain: The crucial context of acceptance. Pain Med. 20, 1509–1518 (2019).
Hoorntje, A. et al. More severe preoperative Kellgren–Lawrence grades of knee osteoarthritis were partially associated with better postoperative patient-reported outcomes in TKA patients. J. Knee Surg. 32, 211–217 (2019).
Lundgren-Nilsson, Å. et al. Patient-reported outcome measures in osteoarthritis: A systematic search and review of their use and psychometric properties. RMD Open 4, e000715 (2018).
Lee, A. C. et al. Responsiveness and minimally important differences for 4 patient-reported outcomes measurement information system short forms: Physical function, pain interference, depression, and anxiety in knee osteoarthritis. J. Pain 18, 1096–1110 (2017).
Bartlett, S. J., Ling, S. M., Mayo, N. E., Scott, S. C. & Bingham, C. O. III. Identifying common trajectories of joint space narrowing over two years in knee osteoarthritis. Arthr. Care Res. 63, 1722–1728 (2011).
Collins, J. E., Neogi, T. & Losina, E. Trajectories of structural disease progression in knee osteoarthritis. Arthr. Care Res. 73, 1354–1362 (2021).
Etchegaray, J. M. et al. Core Competencies for Amputation Rehabilitation (RAND Corporation, 2019).
Acknowledgements
The authors acknowledge Emilia-Marie Jaskot, BS for her contributions to project management and coordination. The authors do not have any conflicts of interest to note. The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policies of the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Uniformed Services University of the Health Sciences, the U.S. Departments of the Army, Navy, Air Force, Defense, nor the U.S. Government. Identifying specific products or instrumentation is considered an integral part of the scientific endeavor and does not constitute an endorsement or implied endorsement on the part of the authors, Department of Defense, or any component agency.
Funding
This work was supported, in part, by the Center for Rehabilitation Sciences Research, of the Uniformed Services University of the Health Sciences (awards HU0001-11-1-0004 and HU0001-15-2-0003), and the Extremity Trauma and Amputation Center of Excellence (award HU0001-20-2-0038).
Author information
Authors and Affiliations
Contributions
Conception and Design: B.D.H., A.L.P., R.H.M., L.T.S., M.D.M., and C.L.D. Acquisition, Analysis, and Interpretation of Data: J.G.W., B.D.H., J.C.A., L.D.D., R.L.K., A.L.P., S.M.G., M.S.V., L.T.S., M.D.M., D.A.H., M.G.F., and C.L.D. Drafting of Manuscript: J.G.W., B.D.H., S.M.G., M.S.V., C.L.D. Revising Manuscript and Final Approval of the Article: All Authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wasser, J.G., Hendershot, B.D., Acasio, J.C. et al. Exploring relationships among multi-disciplinary assessments for knee joint health in service members with traumatic unilateral lower limb loss: a two-year longitudinal investigation. Sci Rep 13, 21177 (2023). https://doi.org/10.1038/s41598-023-48662-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48662-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.