Abstract
Proton pump inhibitors (PPIs) are commonly used in cancer patients, but their impact on treatment outcomes in multiple myeloma (MM) patients remains unclear. This study investigated the association of PPI use with survival and adverse effects in MM patients across three randomized-control trials initiating daratumumab, lenalidomide, or bortezomib combination treatments. Cox proportional hazard analysis and logistic regression were employed to assess the associations with treatment outcomes, while adjusting for age, sex, weight, MM international staging system stage, ECOG-performance status, comorbidity count, and presence of gastrointestinal disorders. Pooled data involving 1804 patients revealed that 557 (32%) used PPIs at baseline. PPI use was independently associated with worse overall survival (adjusted HR [95% CI] 1.32 [1.08–1.62], P = 0.007) and grade ≥ 3 adverse events (adjusted OR [95% CI] 1.39 [1.03–1.88], P = 0.030). However, the association with progression-free survival did not reach statistical significance (adjusted HR [95% CI] 1.14 [0.97–1.33], P = 0.112). Findings were consistent across trials and treatment arms. PPI use was identified as a negative prognostic factor in MM patients, potentially enhancing clinical decisions regarding its use. Further research is needed to fully comprehend the impacts and safety of PPI use in MM patients.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is the second most common haematological cancer and is characterized by an abnormal proliferation of clonally transformed plasma cells within the bone marrow1. Over the past decade, the treatment of MM has witnessed remarkable advancements, resulting in improved patient outcomes and prolonged survival.
Among the various advancements, the combination of immunomodulatory agents such as lenalidomide or proteasome inhibitors like bortezomib, with dexamethasone, have emerged as standard frontline therapy options for patients with MM1,2. Additionally, the introduction of monoclonal antibodies targeting CD38, such as daratumumab, have further enhanced available options1. However, despite these advancements, the treatment of MM is still associated with considerable heterogeneity in survival outcomes, adverse effects, and likelihoods of treatment resistance and failure3. This highlights the need for continued exploration of factors that can predict likely outcomes and aid in the selection of treatment.
Concomitant medications are commonly used in patients with cancer to manage comorbidities and treatment-related side effects. Proton pump inhibitors (PPIs) are amongst the most widely prescribed drugs, due to the frequency at which patients with cancer experience gastrointestinal (GI) diseases—such as gastroesophageal reflux disease (GERD) and peptic ulcers4. However, in combating these symptoms, PPIs may disrupt the gut microbiota, increase susceptibility to infections, and potentially interfere with the dissolution of orally administered medicines. Such impacts have significant potential to affect the gut-immune axis and pharmacokinetic exposures to anticancer medicines, which in turn has the potential to impact the likely survival outcomes of patient with cancer5,6,7,8,9,10,11.
Understanding the potential impact of PPIs in patients with cancer is of great clinical importance as research has demonstrated that while they are often necessary, up to 60% of myeloma patients received PPI prophylaxis during and beyond anticancer therapy without an accepted indication12 (i.e., PPIs are frequently overprescribed due to a presumption that they will not cause any negative impacts). Notably, much recent research indicates that PPIs are likely associated with significant changes in the efficacy of immune checkpoint inhibitors used in the treatment of solid tumours13,14,15. Yet, despite the immunomodulatory foundations of many agents used in the treatment of MM, the relationship between PPI use and survival outcomes remains largely unexplored in patients with this disease. This study aimed to investigate the association of PPI use with survival outcomes and the incidence of grade ≥ 3 adverse events in patients with MM.
Methods
Patient population
Individual patient data was pooled from 3 randomized, open-label trails: MAIA (NCT02252172, data cut-off: February 19, 2021)16, POLLUX (NCT02076009, data cut-off: March 7, 2016)17, and CASTOR (NCT02136134, data cut-off: January 11, 2016)18.
All studies enrolled adult patients aged 18 years or older. POLLUX and CASTOR assessed the efficacy of daratumumab on patients with relapsed or refractory MM who had received at least one prior line of therapy. The MAIA trial included newly diagnosed MM patients who were not eligible for high dose chemotherapy or autologous stem cell transplantation due to age (≥ 65 years) or the presence of coexisting conditions that may result in unacceptable side effects18.
In the MAIA and POLLUX trials, daratumumab was administered as a 16 mg/kg intravenous (IV) infusion in combination with lenalidomide (25 mg capsule orally) and dexamethasone (40 mg orally or intravenously) (DRd) compared to lenalidomide plus dexamethasone (Rd). In the CASTOR trial, daratumumab (16 mg/kg IV infusion) was administered in combination with bortezomib (1.3 mg/m2 subcutaneously) and dexamethasone (20 mg orally) (DVd) compared to bortezomib plus dexamethasone alone (Vd).
All studies were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. Participants provided written informed consent. The secondary analysis of de-identified data reported in this study was considered negligible risk research and has been approved by the University of Sharjah Ethics Committee (Approval reference number: REC-23-11-07-01-F). Data were accessed according to the Johnson & Johnson policy and made available through Vivli, Inc. (www.vivli.org).
Outcome and predictor data
Within each of MAIA, POLLUX, and CASTOR progression free survival (PFS) was defined as the time from patient randomization to either disease progression according to the international myeloma working group (IMWG) response criteria or death, whichever occurred first. Overall survival (OS) was defined as the time from the date of randomization to the date of the participant’s death.
Documented use of PPI at baseline (i.e. at the screening visit/prior to treatment initiation) was the primary covariate in this study. Analyses were adjusted for age, sex, weight, MM international staging system (ISS) stage, Eastern Cooperative Oncology Group performance status (ECOG-PS) score, comorbidity count, and presence of gastrointestinal disorders (e.g., GERD, peptic ulcer disease). The rationale behind the selection of the adjustment variables is provided in Supplementary Table 1. Missing data was imputed via the Transcan function in the Hmisc (version 5.1-0) R package. Transcan is a nonlinear additive transformation and imputation function19.
Statistical analysis
Cox proportional hazards regression was employed to examine the associations between PPI use and OS/PFS. The assessment of PPI independence from other prognostic factors was evaluated using univariate and adjusted analyses. Results were reported as hazard ratios (HR) with 95% confidence intervals (95% CI). Statistical significance was set at P-value < 0.05. All models were stratified by clinical trial and treatment arms to account for potential variations between the studies and treatment approaches. Heterogeneity of PPI associations were assessed according to study and treatment interaction analyses. Forest plots were utilized to visually present the HRs (and 95% CI) of subgroups for conducted interaction analyses. Kaplan–Meier plots were employed to graphically depict and estimate survival probabilities based on PPI use. The association between PPI use and any grade ≥ 3 adverse events, occurring within the first 12 months of treatment initiation, was assessed using logistic regression analysis. The results were reported as odds ratios (ORs) along with their corresponding 95% CI. All analyses were performed using R version 4.2.
Ethics approval
Secondary analysis of anonymised clinical-trial data was confirmed as negligible-risk research and has been approved by University of Sharjah Research and Ethics Committee (Approval reference number: REC-23-11-07-01-F).
Results
Patient population
The pooled cohort consisted of 1804 patients, of whom 557 (32%) received PPI at baseline. A summary of patients’ baseline characteristics by study is provided in Table 1 and a summary of baseline chatacteristics by PPI use is provided in Supplementary Table 2. The median follow-up time was 56.2 months for the MAIA, 7.43 months for the CASTOR, and 13.5 months for the POLLUX study.
Regarding missing data, patient weight was missing for 39 (8%) patients in CASTOR and 287 (50%) in POLLUX. For the ECOG score, only 1 patient (< 1%) had missing data in CASTOR, and race had missing data in 11 (2%), 16 (2%), and 57 (10%) patients in CASTOR, MAIA, and POLLUX, respectively. All other variables had complete data. Supplementary Table 3 provides a summary of un-imputed patients' baseline characteristics by study.
Among the patients using PPIs, 335 (58%, P < 0.001) had a GI disorder documented in their medical history. While the lower levels details on the GI disorders were not provided in the MAIA trial data, within CASTOR and POLLUX it was observed that the most frequent GI disorders associated with PPI were gastroesophageal reflux disease (GERD) (n = 45, 13.4%), ulcers encompassing duodenal and/or gastric ulcer (n = 11, 3.2%), gastritis (n = 18, 5.3%), hernia (n = 33, 9.8%), and others (n = 55, 16.4%) including esophagitis, acid peptic disease, dyspepsia, dysphagia, and gastric polyps.
In CASTOR and POLLUX, PPI use was documented by class name—‘proton pump inhibitors’. Within MAIA, it was noted that PPI use (n = 264) related to the specific use were pantoprazole (n = 86, 33%), omeprazole (n = 75, 28%), esomeprazole (n = 65, 25%), lansoprazole (n = 26, 10%), dexlansoprazole (n = 6, 2%), and rabeprazole (n = 6, 2%).
Grade ≥ 3 adverse events occurred in 1469 (81%) patients, of whom 502 (87%) were PPI users (Supplementary Table 2). The top ten most common grade ≥ 3 adverse events by study are represented in the Supplementary Table 4. Notably, neutropenia was the most common adverse event across all cohorts, affecting 634 (35%), followed by thrombocytopenia in 336 (19%) and anaemia in 331 (18%) patients.
PPI use and survival outcomes
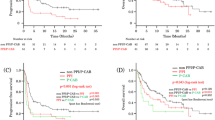
The prognostic associations between PPI use and survival outcomes are presented in Table 2. In pooled univariable analyses, PPI use was statistically associated with worse OS (HR [95% CI] 1.49 [1.22–1.81], P < 0.001) and PFS (HR [95% CI] 1.19 [1.02–1.39], P = 0.03) outcomes. Similarly, in adjusted analyses, PPI use remained statistically associated with worsened OS (HR 1.32, 95% CI 1.08–1.62, P = 0.007). However, the association between PPI use and PFS did not reach statistical significance (adjusted HR 1.14, 95% CI 0.97–1.33, P = 0.1). Kaplan–Meier estimates for the survival outcomes by PPI use are depicted in Fig. 1.
There was no statistically significant differences in the observed associations between PPI use with either OS (P-interaction = 0.2) or PFS (P-interaction = 0.8) between clinical trials, nor between treatment arms (PFS P-interaction = 0.7; OS P-interaction = 0.4). The estimated adjusted HRs (and 95% CI) for the interaction analyses by clinical trial and treatment arms are presented in Fig. 2. PPI use was associated with worse OS outcomes for the pooled estimate (HR [95% CI] 1.32 [1.08–1.62]). Notably, treatment arms incorporating daratumumab exhibited a significant association with worse survival outcomes in both DVd (HR [95% CI] 3.03 [1.38–6.67]) and DRd (HR [95% CI] 1.42 [1.01–2.00]). There was no significant association for treatment arms without daratumumab, however they were trending towards worse outcomes. PPI use did not show a significant association with the pooled estimate for PFS (HR [95% CI] 1.14 [0.97–1.33]). Subgroup specific Kaplan–Meier plots are presented in Supplementary Fig. 2.
PPI use and grade ≥ 3 adverse events
The association between PPI use and grade ≥ 3 adverse events is presented in Table 2. PPI use was statistically significant with patients experiencing adverse events of grade ≥ 3 for both the univariate and multivariate analyses (OR [95% CI] 1.69 [1.27–2.26], P < 0.001) and (adjusted OR [95% CI] 1.39 [1.03–1.88], P = 0.030).
Discussion
This study provides insights about the association of baseline PPI use with survival and adverse events grade ≥ 3 outcomes in MM patients across three distinct cohorts. The findings indicate a significant association between PPI use and worse OS and grade ≥ 3 adverse events across all cohorts. PFS did not show a significant association; however, it was trending towards worse outcomes. To the best of our knowledge, this study is the first to comprehensively examine the relationship between PPI use and outcomes in MM patients receiving multi-drug immunomodulatory combinations. Notably, PPI use was identified as a negative prognostic factor regardless of the study cohort or treatment arm, suggesting a consistent association between PPI use and worse outcomes.
Accumulating evidence links PPI use and increased mortality rates in cancer. A recent study on hematologic malignancies, including MM, revealed significantly higher hazard for cancer-specific mortality (adjusted HR 1.31, 95% CI 1.18–1.44) and 1-year cancer-specific mortality (adjusted HR 1.50, 95% CI 1.29–1.74) in PP users20. Despite differences in outcome measures, these findings align with our results, indicating a negative association between PPI use and survival outcomes. Additionally, studies on solid tumours such as non-small cell lung cancer (NSCLC) and colorectal cancer have similarly demonstrated unfavourable prognostic effects associated with PPI use on survival9,15,21,22,23. Nevertheless, some studies suggest positive correlation between PPI use and cancer outcomes. In a study on untreated head and neck squamous carcinoma patients, the use of PPIs or histamine-2 receptor antagonists (H2Ras), either alone or in combination, was associated with significantly improved overall survival24. However, a limitation of this study was the absence of randomization. Additionally, an experimental study on human MM cells reported that lansoprazole exerted a direct antitumor effect through direct cytotoxicity and apoptotic-like cell death25. However, it is crucial to consider that the study was conducted in vitro and may not fully represent PPI effects in vivo.
Recently, there has been growing evidence of the role of the gut microbiome on various diseases, including MM. PPIs can alter the gut microbiome, causing gut dysbiosis by reducing gastric acid secretion8. Several studies reported reduced gut microbiota diversity and an increase in Streptococcoceae, Micrococcoceae, and Enterococcoceae in PPI users versus non-users7,26. Additionally, PPIs have been linked to higher risks of Clostridium difficile infections and colonization by drug-resistant organisms, potentially contributing to adverse health outcomes8. Although the precise ways the gut microbiome affects the host systems are not fully elucidated, it impacts processes crucial to hematological malignancies, such as micronutrient processing and immune system activation8,27,28. In a study comparing MM patients to healthy controls, alterations in the gut microbiome were found to actively contribute to MM progression. MM patients exhibited higher levels of nitrogen-recycling bacteria like Klebsiella and Streptococcus that hydrolyse urea for the synthesis of L-glutamine, a key factor in myeloma progression27,28. Furthermore, in MM mouse models, the presence of Prevotella heparinolytica in the gut influenced the immune system through T-helper 17 cells, which causes T cells to migrate to the myeloma environment and fuel tumor progression through IL-17 production29. Growing concern surrounds the impact of PPIs on the efficacy of anti-cancer drugs including immunotherapy and monoclonal antibodies (mAb), whether administered orally or intravenously. A retrospective study highlighted increased adverse events when PPIs were used concomitantly with the mAb’s cetuximab and panitumumab4. As for immunotherapy, multiple studies reported of PPIs affecting drug efficacy and survival outcomes of patients receiving ICIs15 and anti-PD-1/PD-L1 therapies30. Although existing literature doesn't confirm drug interactions between PPIs and daratumumab or lenalidomide, our findings suggest consistent unfavorable outcomes across different treatments, implying a persistent negative association with PPI use irrespective of the therapy employed.
Both lenalidomide and daratumumab operate by mechanisms that rely on the immune system. Lenalidomide can alter cytokine production, regulate T cell co-stimulation, and enhance natural killer (NK) cell-mediated cytotoxicity31,32, while daratumumab induces antibody dependent cell-mediated cytotoxicity (ADCC) and the antibody-dependent cellular phagocytosis (ADCP)33. Hence, interactions between PPIs, the gut microbiota, or the immune system may potentially influence the efficacy of these drugs. In addition to gut dysbiosis, PPIs may also promote T cell tolerance34 and affect immune cell functions. A study on omeprazole revealed that it significantly reduces NK cell functions and cytotoxicity at normal therapeutic doses (20 mg/d)35.
Emerging evidence has highlighted the association between PPI use and various serious adverse events. These include gastrointestinal and extraintestinal complications such as pneumonia, electrolyte imbalances, and vitamin deficiency25,36. Additionally, long-term PPI use is associated with reduced red and white blood cell counts, hemoglobin levels, iron deficiency, and risk of osteoporosis37,38. Some studies also reported of PPI-induced thrombocytopenia or neutropenia39,40,41. These potential adverse events are of great clinical implications for cancer patients, underscoring the need for further research to comprehensively understand the safety profile of PPIs and their potential impact on patients with cancer.
Limitations of this study include the lack of information regarding the specific type and duration of PPI use, hampering the assessment of their potential association with treatment outcomes. Additionally, generalizing study findings to the real-world population is constrained by eligibility criteria applied in clinical trials. For instance, exclusion of patients with smoldering MM or primary amyloidosis and those with prior anti-CD38 therapies or stem cell transplantation, limits broader applicability. Additionally, the evaluation of daratumumab-based combinations in specific patient populations, like refractory or relapsed MM in POLLUX and CASTOR, and newly diagnosed MM in MAIA, may not fully represent the diversity of patients in real-world settings. Another potential limitation is the completeness of the data. Despite relatively low missing data percentages and the use of imputation methods to minimize uncertainty, some bias possibility remains.
In conclusion, this study identified a significant association between PPI and worse OS outcomes and increased odds of experiencing grade ≥ 3 adverse events within a pooled cohort of MM patients treated with contemporary treatment options. These findings may potentially optimize patient care and improve clinicians’ decision-making in prescribing PPIs. This may involve avoiding unnecessary use or considering the shortening of their usage as clinically indicated, given that presuming that they are completely harmless may potentially be inappropriate. However, we also acknowledge the inherent limitations associated with using clinical trial data and these findings need to be validated using real world data. It is also important to investigate whether these associations extend to treatment options beyond daratumumab, lenalidomide, bortezomib, and dexamethasone, and whether the associations become apparent with respect to PFS in a larger cohort. Further research is warranted to elucidate the underlying mechanisms of these associations.
Data availability
Data were accessed according to YODA policy and process for clinical study data sharing and is available for request at https://yoda.yale.edu/.
References
De Luca, F. et al. Monoclonal antibodies: The greatest resource to treat multiple myeloma. Int. J. Mol. Sci. 24, 3136 (2023).
Mian, A. et al. Incidence of second primary malignancies in patients with multiple myeloma receiving anti-CD38 monoclonal antibodies: A systematic review and meta-analysis. Leuk. Res. https://doi.org/10.1016/j.leukres.2023.107324 (2023).
Bhatt, P., Kloock, C. & Comenzo, R. Relapsed/refractory multiple myeloma: A review of available therapies and clinical scenarios encountered in myeloma relapse. Curr. Oncol. 30, 2322–2347 (2023).
Uchiyama, A. A. et al. Proton pump inhibitors and oncologic treatment efficacy: A practical review of the literature for oncologists. Curr. Oncol. 28, 783–799 (2021).
Fessler, J., Matson, V. & Gajewski, T. F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 7, 1–15 (2019).
Bavishi, C. & Dupont, H. Systematic review: The use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment. Pharmacol. Ther. 34, 1269–1281 (2011).
Imhann, F. et al. Proton pump inhibitors affect the gut microbiome. Gut 65, 740–748 (2016).
Meriggi, F. Controversial link between proton pump inhibitors and anticancer agents: Review of the literature. Tumori J. 108, 204–212 (2022).
Lee, J. E. et al. Concomitant use of proton pump inhibitors and palbociclib among patients with breast cancer. JAMA Netw. Open 6, e2324852. https://doi.org/10.1001/jamanetworkopen.2023.24852 (2023).
Husain, M. et al. Proton pump inhibitor use (PPI) in patients treated with immune checkpoint inhibitors (ICI) for advanced cancer: Survival and prior therapy. https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.15_suppl.2633 (Wolters Kluwer Health, 2021).
Bridoux, M., Simon, N. & Turpin, A. Proton pump inhibitors and cancer: Current state of play. Front. Pharmacol. 13, 798272. https://doi.org/10.3389/fphar.2022.798272 (2022).
Leitinger, E., Hui, L. & Grigg, A. Is there a role for proton pump inhibitor prophylaxis in haematology patients?. Intern. Med. J. 49, 694–701 (2019).
Liu, C. et al. An up-to-date investigation into the correlation between proton pump inhibitor use and the clinical efficacy of immune checkpoint inhibitors in advanced solid cancers: A systematic review and meta-analysis. Front. Oncol. 12, 753234 (2022).
Hopkins, A. M. et al. Efficacy of atezolizumab in patients with advanced NSCLC receiving concomitant antibiotic or proton pump inhibitor treatment: Pooled analysis of five randomized control trials. J. Thorac. Oncol. 17, 758–767 (2022).
Hopkins, A. M. et al. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of IMpower150. Br. J. Cancer 126, 42–47 (2022).
Facon, T. et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 1582–1596 (2021).
Dimopoulos, M. A. et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 375, 1319–1331 (2016).
Palumbo, A. et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 375, 754–766 (2016).
Hmisc: Harrell Miscellaneous (2022).
Vilmar, A. et al. Increased mortality in patients with hematologic malignancies treated with proton pump inhibitors: A nationwide cohort study. Leuk. Lymphoma https://doi.org/10.1080/10428194.2023.2216324 (2023).
Hopkins, A. M., Kichenadasse, G., Karapetis, C. S., Rowland, A. & Sorich, M. J. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with Atezolizumab Proton Pump Inhibitors and Immunotherapies. Clin. Cancer Res. 26, 5487–5493 (2020).
Wang, X. et al. Proton pump inhibitors and survival in patients with colorectal cancer: A Swedish population-based cohort study. Br. J. Cancer 125, 893–900 (2021).
Wei, N., Zheng, B., Que, W., Zhang, J. & Liu, M. The association between proton pump inhibitor use and systemic anti-tumour therapy on survival outcomes in patients with advanced non-small cell lung cancer: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 88, 3052–3063 (2022).
Papagerakis, S. et al. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Cancer Prev. Res. 7, 1258–1269 (2014).
Canitano, A., Iessi, E., Spugnini, E. P., Federici, C. & Fais, S. Proton pump inhibitors induce a caspase-independent antitumor effect against human multiple myeloma. Cancer Lett. 376, 278–283 (2016).
Jackson, M. A. et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 65, 749–756 (2016).
D’Angelo, C. R., Sudakaran, S. & Callander, N. S. Clinical effects and applications of the gut microbiome in hematologic malignancies. Cancer 127, 679–687 (2021).
Jian, X. et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 8, 1–21 (2020).
Calcinotto, A. et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 9, 4832 (2018).
Giordan, Q., Salleron, J., Vallance, C., Moriana, C. & Clement-Duchene, C. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front. Immunol. 12, 716317 (2021).
Galustian, C. et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol. Immunother. 58, 1033–1045 (2009).
Gandhi, A. K. et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors I karos and A iolos via modulation of the E 3 ubiquitin ligase complex CRL 4 CRBN. Br. J. Haematol. 164, 811–821 (2014).
Saltarella, I. et al. Mechanisms of resistance to anti-CD38 daratumumab in multiple myeloma. Cells 9, 167 (2020).
Diao, X. Antibiotics and proton pump inhibitors suppress the efficacy of immunotherapy against non-small cell lung cancer. Thorac. Cancer 11, 1763 (2020).
Alkim, H., Unal, S., Okur, H. & Imir, T. Omeprazole inhibits natural killer cell functions. Dig. Dis. Sci. 53, 347–351 (2008).
Numico, G., Fusco, V., Franco, P. & Roila, F. Proton pump inhibitors in cancer patients: how useful they are? A review of the most common indications for their use. Crit. Rev. Oncol. Hematol. 111, 144–151 (2017).
Kaczmarczyk, O. et al. Effect of long-term proton pump inhibitor therapy on complete blood count parameters and selected trace elements: a pilot study. Polskie Archiwum Medycyny Wewnętrznej= Polish Arch. Intern. Med. 130 (2020).
Lespessailles, E. & Toumi, H. Proton pump inhibitors and bone health: An update narrative review. Int. J. Mol. Sci. https://doi.org/10.3390/ijms231810733 (2022).
Binnetoğlu, E. et al. Pantoprazole-induced thrombocytopenia in patients with upper gastrointestinal bleeding. Platelets 26, 10–12 (2015).
Gouraud, A., Vochelle, V., Descotes, J. & Vial, T. Proton pump inhibitor-induced neutropenia: Possible cross-reactivity between omeprazole and pantoprazole. Clin. Drug Investig. 30, 559–563 (2010).
Watson, T. D., Stark, J. E. & Vesta, K. S. Pantoprazole-LNDUCED thrombocytopenia. Ann. Pharmacother. 40, 758–761 (2006).
Acknowledgements
This study, carried out under YODA Project # 2022-5048, used data obtained from the Yale University Open Data Access Project, which has an agreement with JANSSEN RESEARCH & DEVELOPMENT, L.L.C.. The interpretation and reporting of research using this data are solely the responsibility of the authors and does not necessarily represent the official views of the Yale University Open Data Access Project or JANSSEN RESEARCH & DEVELOPMENT, L.L.C.
Funding
This study was supported by University of Sharjah Seed Grant No. 2301110380. A.M.H is supported by an Emerging Leader Investigator Fellowship from the National Health and Medical Research Council, Australia (APP2008119).
Author information
Authors and Affiliations
Contributions
Conception and design: S.A.A., and A.Y.A.; Development of methodology: S.A.A., A.M.H., Z.A., and A.Y.A.; Acquisition of data: S.A.A., A.M.H. and A.Y.A.; Analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computational analysis): S.A.A., A.M.H., Z.A, H.O.A, M.H.S., M.A.Y.A., N.C.S., Y.B., R.A.M., M.J.S., and A.Y.A.; Writing, review, and/or revision of the manuscript: S.A.A., A.M.H., Z.A., H.O.A., M.H.S., M.A.Y.A., N.C.S., Y.B., R.A.M., M.J.S., and A.Y.A.; Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S.A.A., A.M.H., and A.Y.A.; Study supervision: A.M.H. and A.Y.A.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
HOA is on the advisory board of ROCHE, MSD, BMS, ASTRAZENECA, and Novartis. All other authors have no conflict of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almansour, S.A., Alqudah, M.A.Y., Abuhelwa, Z. et al. Association of proton pump inhibitor use with survival and adverse effects outcomes in patients with multiple myeloma: pooled analysis of three clinical trials. Sci Rep 14, 591 (2024). https://doi.org/10.1038/s41598-023-48640-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48640-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.