Abstract
Asthma control and health related quality of life are an important goal of asthma management, but their association with sputum eosinophilic inflammation has been less firmly established. To investigate the relationship of asthma control and quality of life with sputum eosinophils in clinical practice. Cross-sectional study with a convenience sample, including patients with asthma, aged between 18 and 65 years, attending to outpatient clinic. Patients underwent sputum induction, pulmonary function tests, Juniper’s Asthma Quality of Life Questionnaire (AQLQ), Asthma Control Test (ACT), Global Initiative for Asthma (GINA) criteria for evaluation of asthma control and severity of the disease, blood count analysis, serum IgE and cutaneous prick test. Sputum sample was considered as eosinophilic if the percentage of eosinophils was ≥ 3%. A total of 45 individuals were enrolled, 15 with eosinophilic sputum (≥ 3% eosinophil cells) and 30 with non-eosinophilic sputum (< 3% eosinophil cells). There were no association of ACT an AQLQ scores with sputum eosinophilia (p > 0.05). This study suggested that the finding of sputum eosinophilia was not related to asthma control neither with health-related quality of life in patients with severe asthma.
Similar content being viewed by others
Introduction
Asthma is a common, chronic respiratory disease affecting 1–18% of the population in different countries, characterized by variable symptoms of wheeze, shortness of breath, chest tightness, cough, and by variable expiratory airflow limitation. It is a heterogeneous disease which consists in multiple phenotypes that are distinguished by clinical, function and inflammatory characteristics. The eosinophil is one of the most important cells related to airway inflammation in asthma1.
The type 2 asthma phenotype is based on an eosinophilic inflammation that can occur in the absence of an allergic reaction. This involves biomarkers such as Interleukin (IL) 4, IL-5 and IL-13, Immunoglobulin E (IgE), fractional exhaled nitric oxide (FeNO), peripheral blood eosinophils and eosinophils in induced sputum2. These biomarkers can be employed in several ways to aid treatment decisions3.
Treatment of asthma can reduce or remove symptoms. The assessment of asthma control (control of symptoms and risk of adverse outcomes) is determined by the interaction between the patient’s genetic background, environment exposure, psychological factors, treatments, and the underlying disease process1.
The sputum induction method has been validated and standardized, providing a safe and relatively noninvasive way to collect material from the lower airways4,5. On the other hand, the method of analyzing the samples is laborious and requires well-trained technicians using highly specialized laboratory equipment6.
The importance of examination of sputum cellularity in the management of moderate-to-severe persistent asthma has grown as recent studies have demonstrated that the number of severe exacerbations is lower when induced sputum findings are used to design the anti-inflammatory treatment than when the treatment is based on the current guidelines7.
Considering that asthma control is an important goal of asthma management and that its association with sputum eosinophilic inflammation has been less firmly established, in this exploratory study we sought to analyze the implementation in our institution of conventional method for sputum processing in adult patients with severe asthma, investigating the relationship of asthma control and quality of life with sputum eosinophils in clinical practice.
Methods
Study design
This was a cross-sectional study approved by the ethics and research committee of the Hospital de Clínicas de Porto Alegre (HCPA), protocol number 5.860.232, and Plataforma Brazil, protocol number 5.860.232. All patients signed an informed consent form. Part of the results has been published previously as an original article8. The population studied in the present study was a convenience sample, with no sample size calculation.
Secondarily, this was an exploratory study in which we sought to analyze the preliminary implementation in our institution of conventional method for sputum processing in adult patients with severe asthma. Exploratory research design is conducted for a research problem when the researcher has no past data or only a few studies for reference9.
Population
Patients were recruited from the Asthma Outpatient Clinic of HCPA, Porto Alegre, Rio Grande do Sul, Brazil. This Clinic is responsible of the care of patients with severe asthma.
The study included patients aged between 18 and 65 years. The diagnosis of asthma was confirmed by compatible history and evidence of reversible airflow obstruction in spirometry: forced expiratory volume in the first second (FEV1) less than 80% of the predicted value and FEV1/forced vital capacity (FVC) ratio less than 75% plus substantial improvement in airflow after inhalation of short-acting beta2-agonist bronchodilator (BD) (increase in FEV1 greater than 12% in relation to the pre-BD value and greater than 200 mL in absolute value or increase in FEV1 greater than 20% and exceeding 250 mL spontaneously over time or after intervention with medication)1.
Asthmatic subjects exposed to smoking were included. Individuals who reported smoking in the last 30 days and had a smoking index greater than 5 pack-years were considered active smokers. Smokers in cessation or ex-smokers were defined as individuals who had quit smoking in the last 30 days or more and had a smoking index greater than 5 pack-years. Nonsmokers were defined as individuals who reported never having smoked and ex-smokers with a smoking index of less than 5 pack-years. The smoking index (pack-years) was calculated as follows: number of cigarettes smoked per day/20 × the number of years the person had smoked.
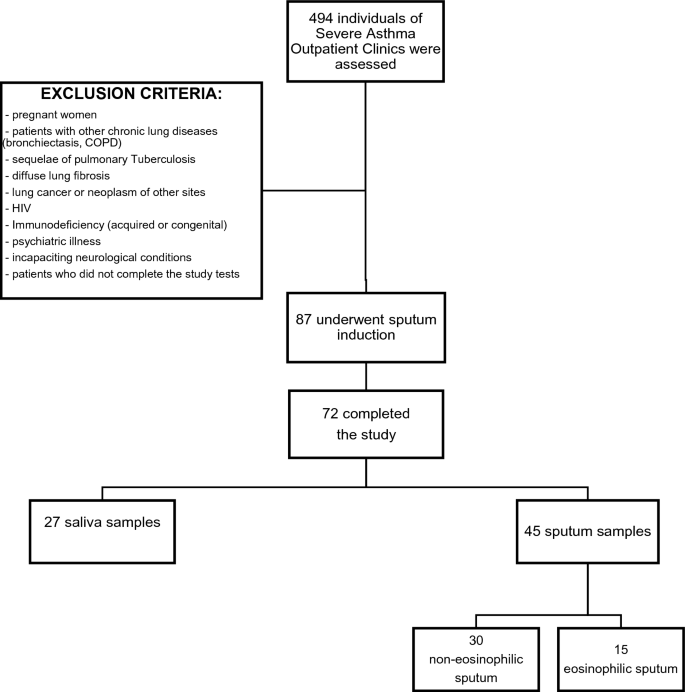
The exclusion criteria from the study were: pregnant women; patients with other chronic lung diseases such as bronchiectasis, sequelae of pulmonary tuberculosis, diffuse lung fibrosis; lung neoplasm or neoplasm of other sites; human immunodeficiency syndrome, acquired immunodeficiency syndrome, or congenital immunodeficiency syndrome; psychiatric illness or incapacitating chronic neurological disease that could prevent the performance of the study procedures; and patients who did not complete the study evaluation tests.
Procedures
Induced sputum samples were collected to evaluate cellularity, following institutional protocols. We considered the sputum sample as eosinophilic if the percentage of eosinophils was ≥ 3%10.
The level of asthma control was assessed using the Asthma Control Test (ACT)11 and the GINA table1.
Spirometry was performed using a Jaeger v 4.31a spirometer (Jaeger, Wuerzburg, Germany). The carbon monoxide diffusing capacity (DLCO) was measured by a single sustained breath of a special gaseous mixture, using Master Screen Diffusion equipment (Jaeger, Wuerzburg, Germany). Pulmonary volumes were measured using the Master Screen Body-Plets (Jaeger, Wuerzburg, Germany)12,13.
All the patients were asked about Health-related Quality of Life using Juniper’s Asthma Quality of Life Questionnaire (AQLQ)14,15. The AQLQ comprises four domains: symptoms, activity limitation, emotional function, and environmental exposure. Each domain is scored from 1 to 7; scores of 1 indicate maximal impairment and scores of 7 indicate no impairment.
Blood count analysis was performed, total eosinophils count, and percentage was used. We consider blood count > 300 cell/mm3 as eosinophilia16. Serum IgE dosage was analyzed and considered high if greater than 100 IU/mL, according to the reference value17. The skin prick test for allergy was conducted according to published protocols, if the patient had at least one cross positive, it was considered positive test18.
Patients were asked about symptoms of allergic rhinitis and were classified about its control.
Statistical analysis
This study was made with a convenience sampling. All data were processed and analyzed using the Statistical Package for the Social Sciences, version 22.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed using mean and standard deviation or median and interquartile range. Categorical variables were expressed using absolute and relative frequencies.
The associations between categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test. To compare means, we used Student’s t-test or one-way analysis of variance, supplemented by Tukey’s test. In case of asymmetry, Mann–Whitney or Kruskal–Wallis tests were used and supplemented by Dunn’s test. A p-value ≤ 0.05 was considered statistically significant and all tests were two-tailed.
Ethics statement
This was an exploratory study using data of a cross-sectional study, approved by the ethics and research committee of the Hospital de Clínicas de Porto Alegre (HCPA) and Plataforma Brasil (protocol number 1.139.117). All patients signed an informed consent form. The authors signed a data use agreement protecting the confidentiality of patient information. All study methods were carried out in accordance with international and national guidelines and regulations for clinical studies of humans (Declaration of Helsinki and Brazilian Governmental regulation—Plataforma Brasil).
Results
During a period of 20 months, a total of 494 adults subjects were assessed in the initial evaluation. Out of them, 87 fulfilled all the criteria, but 15 withdraw consent to sputum induction. Thus 72 subjects completed the study (see Fig. 1—Flow diagram of study selection and exclusion criteria).
Despite performing the protocol correctly, 27 individuals could not achieve the necessary volume of sputum to do the analyses. We classified them as non-adequate sputum sample. Table 1 shows the demographic, clinical data, and comparison of patients in groups with inadequate sputum sample and with adequate sputum sample. The individuals with inadequate sputum sample were younger than those with adequate sputum sample (respectively, 42.1 ± 12.9 vs 49.1 ± 12.5 years, p = 0.027). There was no other statistically difference between the 2 groups.
A total of 45 individuals were enrolled in the study (group with adequate sputum samples). Thirty-five patients (77.8%) were female, the mean age was 49.1 ± 12.5 years, the mean BMI was 31.2 ± 6.8 kg/m2 and 73.3% were white. The median ACT was 16 (13–21) points. The mean FVC was 79.5 ± 15.8% of predict, the mean FEV1 was 66.3 ± 18.0% of predict and the mean FEV1/FVC was 67.9 ± 11.0%. Ninety-eight percent of subjects were classified as severe asthma (steps 4 and 5 of GINA classification).
Those patients with adequate sputum samples were divided in two groups: 15 individuals with eosinophilic sputum (≥ 3% eosinophil cells) and 30 with non-eosinophilic sputum (< 3% eosinophil cells). The comparison of demographic and clinical data between patients with non-eosinophilic and eosinophilic sputum is showed in Table 2. There were no statistically significant differences between groups. In both groups female sex was predominant, and the main ethnicity was white. When asthma control was assessed according to GINA, only 13% of all patients had well controlled asthma. The median of ACT score was 15.5 (13–20) in the non-eosinophilic and 16 (12–23) in the eosinophilic sputum group, p = 0.621. The ACT score was ≥ 20 points in only 9 (20%) of patients in non-eosinophilic sputum group and in 6 (13.3%) of patients in eosinophilic sputum group, p = 0.513.
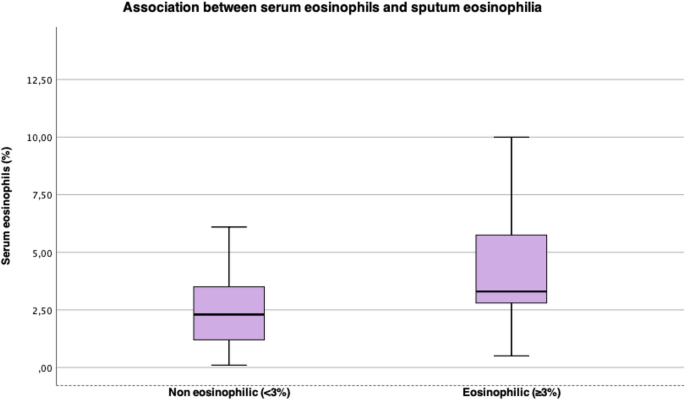
Table 3 presents comparison between lung function, cutaneous prick test, serum eosinophils and serum IgE between patients with non-eosinophilic and eosinophilic sputum. The individuals with eosinophilic sputum had higher serum eosinophils (%) levels than the non-eosinophilic group (respectively, 3.3% vs 2.1%, p = 0.037) and when considering serum eosinophilia as eosinophils count > 300 mm3, there was association with sputum eosinophilia too (p = 0.044), Fig. 2. There were no other significant statistical differences between groups. The proportion on patients with blood eosinophilia was 33.3%.
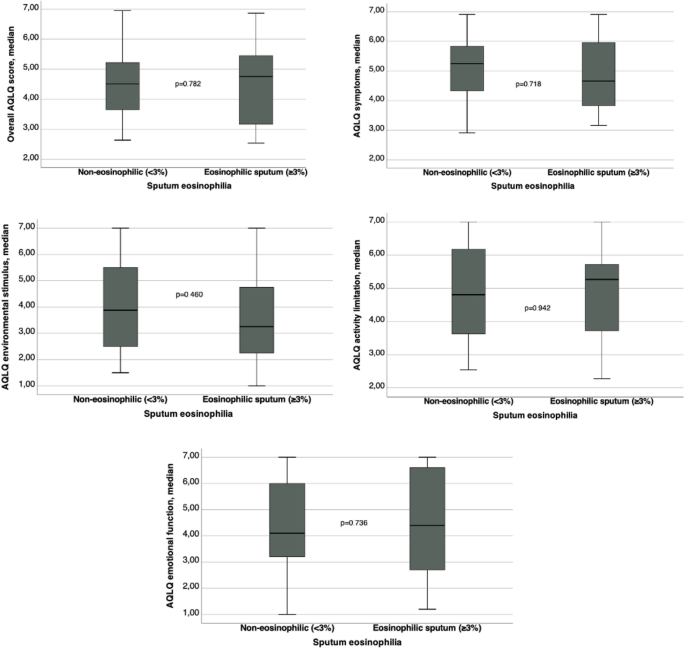
Table 4 shows comparison between ER visits, hospital admissions and AQLQ domain scores between patients with non-eosinophilic and eosinophilic sputum. Graphics comparing AQLQ scores are on Fig. 3. There were no significant statistical differences between groups for ER visits, estimated number of days away from job or school, number of hospital admissions and scores of AQLQ. The ER visits and hospital admission rates were low in both groups in the present study. The AQLQ domain scores showed a moderate impairment in quality of life in both groups.
Discussion
The knowledge about sputum cellularity in inflammatory respiratory diseases allows more comprehensive approach and care for patients with moderate or severe conditions7. This cross-sectional study suggested that the finding of eosinophilic sputum was not related to asthma control neither with health-related quality of life in a population with severe asthma attending to an asthma outpatient clinic in a tertiary and academic hospital in Southern Brazil.
Duncan et al. used the measures of inflammatory cells in induced sputum to search for asthma severity relationship with eosinophils. This was research with chronic stable asthma19 which included former smokers, comparing forced expiratory volume, symptoms, and sputum eosinophils apoptosis. It was demonstrated that reduced eosinophil apoptosis and sputum eosinophil load are correlated with degree of self-reported symptoms and severity of the disease.
In a retrospective longitudinal study of 187 patients, Demarche et al. demonstrated that asthma control was associated with fluctuations in sputum eosinophilic inflammation20. Furthermore, they have calculated a minimal important increase and decrease in sputum eosinophils associated with a change of at least 0.5 in the Asthma Control Questionnaire (ACQ). The age and smoking status were like the present study, but the asthma severity was lower than that identified in our study.
Pizzichini et al. studied a population of 130 patients with asthma that had been receiving treatment for asthma for at least a year and were lifelong non-smokers or ex-smokers, who were assessed to induced sputum and to answer quality of life questionnaires21. The sputum was labelled eosinophilic if sputum eosinophils were ≥ 3%, neutrophilic if neutrophils were ≥ 60%, and pauci-granulocytic if neither. It was found that near 70% of subjects with controlled asthma had pauci-granulocytic sputum, suggesting that answering “No” to the four questions of GINA table (1) is a good indicator of control of air inflammation. On the other hand, in patients with partially controlled or uncontrolled asthma, there was no difference between the groups with eosinophilic, neutrophilic, or pauci-granulocytic sputum. The population of this research differs from ours in some features: they did not include active smokers and they had number of individuals with controlled asthma higher than ours. In our population, with GINA criteria, only 6.7% had control of the disease.
Recently, Athanazio et al. evaluated prevalence of eosinophilic phenotype in patients with severe asthma, with blood eosinophils count. The prevalence of patients with severe asthma and eosinophils > 300 cell/mm3 in Brazil was 40%16. Our research found similar data, 33% of our severe asthma population studied had sputum eosinophilia (defined as sputum eosinophils ≥ 3%). When comparing serum and sputum eosinophils, blood eosinophils count > 300 cell/mm3 was associated with sputum eosinophilia (p = 0.044), secondarily there was association between percentual of serum eosinophils and sputum eosinophilia.
Our study has some limitations. First, this was a cross-sectional study, so it was not possible to establish a temporal link between sputum eosinophilia and asthma control and health related quality of life. Second, the sample size was too small with a convenience sample and would be associated with low statistical power. Third, our study did not encompass all the spectrum of asthma severity, and the study only included steps 4 and 5 of GNA severity classification. Also, the study population was selected from patients referred to a reference center and was probably biased toward the more severe disease. It is important to emphasize that the patients in our sample were on high doses of inhaled steroids, the majority was using more than 1600mcg of budesonide. Fourth, the study population includes only those patients who were able to produce a sputum sample of sufficient quality.
Another important issue we found was to implement the induced sputum in our service. Although we followed straightly a standardized protocol5 almost 40% of the first study sample could not achieve adequate sputum to do the analyzes. This group’s characteristics had no statistical differences when compared to who expectorated adequate sputum. The proportion of inadequate samples in our research was higher than other studies5,21,22, probably because induced sputum to investigate inflammatory features is laborious as it demands time, trained professionals, and a qualified laboratory, able to process and count inflammatory cells7. It is not performed in our daily practice.
In conclusion, this cross-sectional study suggested that the finding of sputum eosinophilia in a population with severe asthma and in use of high doses of inhaled steroids was not related to asthma control, neither with health-related quality of life.
Data availability
The datasets generated and/or analysed during the current study are available in the file “datasets.xlsx”, which is attached on “Supplementary Material”.
References
GINA-Main-Report-2022-FINAL-22-07-01-WMS.
de Carvalho-Pinto, R. M. et al. Recomendações para o manejo da asma da Sociedade Brasileira de Pneumologia e Tisiologia—2021. J. Bras. Pneumol. 47, 1–24 (2021).
Robinson, D. et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: Current knowledge and therapeutic implications. Clin. Exp. Allergy 47, 161–175. https://doi.org/10.1111/cea.12880 (2017).
Pizzichini, M. M. et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am. J. Respir. Crit. Care Med. 154(4), 866–869. https://doi.org/10.1164/ajrccm.154.4.8887576 (1996).
Pavord, I. D., Pizzichini, M. M., Pizzichini, E. & Hargreave, F. E. The use of induced sputum to investigate airway inflammation. Thorax 52, 498 (1997).
Fireman, E., Toledano, B., Buchner, N., Stark, M. & Schwarz, Y. Simplified detection of eosinophils in induced sputum. Inflamm. Res. 60, 745–750 (2011).
Moritz, P. et al. Determination of the inflammatory component of airway diseases by induced sputum cell counts: Use in clinical practice* Determinação do componente inflamatório das doenças das vias aéreas através do escarro induzido: Utilização na prática clínica. J. Bras. Pneumol. 34, 913 (2008).
dos Santos, V. C. H. et al. Association of quality of life and disease control with cigarette smoking in patients with severe asthma. Braz. J. Med. Biol. Res. 55, 1 (2022).
Singh, A. An introduction to experimental and exploratory research. SSRN Electron. J. https://doi.org/10.2139/ssrn.3789360 (2021).
Chakir, J. et al. Monitoring sputum eosinophils in mucosal inflammation and remodelling: A pilot study. Eur. Respir. J. 35, 48–53 (2010).
Petroni Faria Roxo, J. et al. Portuguese-language version of the asthma control test: Validation for use in Brazil* Validação do Teste de Controle da Asma em português para uso no Brasil. J. Bras. Pneumol. 36, 159 (2010).
Crapo, R. O., Morris, A. H. & Gardner, R. M. Reference Spirometric Values Using Techniques and Equipment that Meet ATS Recommendations 13.
Prata, T. A. et al. Spirometry reference values for black adults in Brazil. J. Bras. Pneumol. 44, 449–455 (2018).
Juniper, E. F. et al. Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax 47, 76–83 (1992).
Silva et al. Validação do questionário de qualidade de vida em asma (Juniper) para o português brasileiro (2007).
Athanazio, R. et al. Prevalence of the eosinophilic phenotype among severe asthma patients in Brazil: The BRAEOS study. J. Bras. Pneumol. 48, e20210367 (2022).
Wittig, H. J., Belloit, J., De Fillippi, l., Royal, G. & Gainesville, B. Age-Related Serum Immunoglobulin E Levels in Healthy Subjects and In Patients with Allergic Disease.
Wood, R. A., Phipatanakul, W., Hamilton, R. G. & Eggleston, P. A. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J. Allergy Clin. Immunol. 703, 773–779 (1999).
Duncan, C. J. A., Lawrie, A., Blaylock, M. G., Douglas, J. G. & Walsh, G. M. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur. Respir. J. 22, 484–490 (2003).
Demarche, S. F. et al. Asthma control and sputum eosinophils: A longitudinal study in daily practice. J. Allergy Clin. Immunol. 5, 1335–1343 (2017).
Pizzichini, M. M. M. et al. How does the GINA definition of control correlate with quality of life and sputum cellularity? ERJ Open Res. 5, 1 (2019).
Simpson, J. L., McElduff, P. & Gibson, P. G. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration 79, 147–151 (2009).
Funding
This research was supported by Fundo de Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA), Protocol Number 1.139.117.
Author information
Authors and Affiliations
Contributions
V.A.B.—Conception and design, data collection and analysis, data interpretation, writing of the main manuscript text, preparation of tables, critical review of the article for content, and final approval of the version to be published. V.C.H.S.—Conception and design, data collection and analysis, data interpretation, writing of the main manuscript text, preparation of tables, critical review of the article for content, and final approval of the version to be published. M.A.F.M.—Data collection and analysis, data interpretation, critical review of the article for content, and final approval of the version to be published. P.T.R.D.—Conception and design, analysis, data interpretation, writing of the main manuscript text, preparation of figures, critical review of the article for content, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barcellos, V.A., dos Santos, V.H., Moreira, M.F. et al. Asthma control and sputum eosinophils in adult patients: a cross-sectional study in southern Brazil. Sci Rep 13, 21464 (2023). https://doi.org/10.1038/s41598-023-48381-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48381-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.