Abstract
Neonatal mortality and morbidity are often caused by preterm birth and lower birth weight. Gestational diabetes mellitus (GDM) and gestational hypertension (GH) are the most prevalent maternal medical complications during pregnancy. However, evidence on effects of air pollution on adverse birth outcomes and pregnancy complications is mixed. Singleton live births conceived between January 1st, 2000, and December 31st, 2015, and reached at least 27 weeks of pregnancy in Kansas were included in the study. Trimester-specific and total pregnancy exposures to nitrogen dioxide (NO2), particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5), and ozone (O3) were estimated using spatiotemporal ensemble models and assigned to maternal residential census tracts. Logistic regression, discrete-time survival, and linear models were applied to assess the associations. After adjustment for demographics and socio-economic status (SES) factors, we found increases in the second and third trimesters and total pregnancy O3 exposures were significantly linked to preterm birth. Exposure to the second and third trimesters O3 was significantly associated with lower birth weight, and exposure to NO2 during the first trimester was linked to an increased risk of GDM. O3 exposures in the first trimester were connected to an elevated risk of GH. We didn’t observe consistent associations between adverse pregnancy and birth outcomes with PM2.5 exposure. Our findings indicate there is a positive link between increased O3 exposure during pregnancy and a higher risk of preterm birth, GH, and decreased birth weight. Our work supports limiting population exposure to air pollution, which may lower the likelihood of adverse birth and pregnancy outcomes.
Similar content being viewed by others
Introduction
Preterm birth and low birth weight are leading causes of neonatal morbidity and mortality, and also associate with increased morbidity in adulthood1,2. In 2020, the prevalence of preterm birth in the United States (U.S.) was 10.09%, and that of low birth weight was 8.24%3. Preterm birth complications range from early impacts on the digestive, respiratory, and central nervous systems to late consequences on cognitive, motor, auditory, visual, behavioral, and social-emotional function, as well as a variety of implications for growth and health4. The financial burden linked to preterm birth in the U.S. in 2016 was estimated to be on average $76,153 per infant, and low birth weight (< 2500 g) was associated with average expenses of $114,4375. Previous epidemiological studies have suggested that ambient air pollution may increase the risks of preterm birth and low birth weight, although the evidence remains inconsistent across different pollutants and exposure windows1,6,7. Variations in epidemiologic modeling strategies and exposure assessment methods between research may contribute somewhat to these discrepancies8.
Gestational diabetes mellitus (GDM) and gestational hypertension (GH) are the most prevalent maternal medical complications during pregnancy9. Previous research has shown that developing diabetes before the 24th week of pregnancy increases the risk of preterm birth10, and that women with pregnancy-induced hypertension had a 3.89-fold increased (95% CI: 2.66, 5.69) chance of delivering low birth weight newborns11. GDM prevalence varies between 5.8 and 12.9%12, and a previous study reported that 7.1–9.2% of GDM mothers would develop type 2 diabetes within 5 years13. In the U.S., GH complicates up to 10% of pregnancies14 and the prevalence of GDM and GH is increasing globally. Both are related to short- and long-term health risks and a significant burden on maternal and child health, including the risk for cardiovascular and cardiometabolic disorders development in later life15,16. Previous epidemiological research has investigated the connections between air pollution and pregnancy complications (including GDM and GH)17,18,19,20. However, because of differences in population, study design, exposure measurement, and GDM diagnosis, the links between air pollution and pregnancy complications have conflicting results and are less conclusive.
In this study, we investigated the connections between three ambient air pollutants of nitrogen dioxide (NO2), particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5), and ozone (O3), and two birth outcomes (preterm birth and birth weight) and two pregnancy complications (GDM and GH) in the State of Kansas. We also presented the effect modification of those associations by maternal race, maternal ethnicity, maternal age, and maternal residual address zip code level percent below poverty. To the best of our knowledge, this is the first epidemiologic research on air pollution and pregnancy outcomes in Kansas.
Methods
Data and outcome assessments
Birth certificate data were obtained from the Bureau of Epidemiology and Public Health Informatics, Kansas Department of Health for the period 1999–2016. Inform consent was not obtained or required because this study relies on secondary data analysis of registry data, and no contact with participants were initiated. The study was approved by the Institutional Review Board of the Kansas Department of Health. We confirm that informed consent was obtained from all subjects and/or their legal guardian(s) by the Kansas Department of Health. To minimize fixed-cohort bias and establish a cohort defined by conception rather than birth date with constant gestational age distributions over calendar time21, only singleton pregnancies with a gestational age of 27–42 weeks with an estimated date of conception between January 1st, 2000 and December 31st, 2015, were included in our analysis. The date of the last menstrual cycle was utilized to determine gestational age, and 14 days was added to that date to estimate the date of conception. By only including births ≥ 27 weeks, we ensured that all births were followed for the entirety of the first (gestational weeks 1–13) and second trimesters (gestational weeks 14–26). The birth outcomes of interest were preterm birth (gestational age < 37 weeks), term (gestational age ≥ 37 weeks) birth weight, GDM (self-reported), and GH (self-reported). We excluded births with (a) missing census tract information; (b) maternal age < 10 years or > 55 years; (c) birth weight < 250 g or > 7500 g. For the outcomes of GDM and GH, we also excluded mothers with pre-existing diabetes or pre-existing hypertension. Figure 1 describes the number of births retained after applying each exclusion criterion. After exclusions, 596,926 and 554,787 births were eligible for the preterm birth and birth weight analysis, respectively, and 449,871 and 590,574 pregnancies were eligible for the GDM and GH analysis, respectively.
Exposure assessment
Employing spatiotemporal ensemble models, daily ambient air pollution data for NO2 (1-h maximum), PM2.5 (24-h average), and O3 (8-h maximum) concentrations were calculated at a 1 km × 1 km spatial resolution across the United States from 2000 to 201622. In particular, the ensemble-based models utilized over one hundred predictors and incorporated three machine learning algorithms (including random forest, a neural network, and gradient boosting machine) with good prediction performance23. For each day, air pollution data were averaged to the at-delivery census tract of the mother by averaging the daily concentrations of all 1 km2 grid cells whose centroids were within the census tract boundary.
Statistical analysis
We examined associations between air pollution concentrations and birth outcomes in single-pollutant models for each exposure window of interest for all analyses. We applied logistic regression to determine relationships between preterm birth (< 37 weeks of gestation) and mean air pollution concentrations during the first trimester (weeks 1–13 of gestation), and second trimester (weeks 14–26 of gestation), and a discrete-time survival model to assess relationships during the third trimester (pregnancy week 27 to the end of week t) and total pregnancy (pregnancy week 1 to the end of week t) to avoid bias because of varying exposure periods between preterm and full-term births24. Linear regression models were used to examine term birth weight as a continuous outcome and mean air pollution concentrations for the first trimester, second trimester, third trimester, and total pregnancy exposure windows. For GDM, we utilized logistic regression to assess the connections between GDM and averaged pollutant concentrations during the first trimester, and second trimester because screening for GDM is typically completed between 24 and 28 weeks of gestation25. Logistic regression models were utilized to evaluate the relationship between GH and averaged pollutant levels during the first trimester, second trimester, third trimester, and total pregnancy exposure windows.
For all four outcomes, we controlled for the following covariates: maternal characteristics included education level (< High school, High school or equivalent, Some college/Associate degree, Bachelor’s or above), race (White, African American, Asian, Other), maternal ethnicity (Hispanic, Non-Hispanic), maternal age modeled employing natural cubic spline with 3 degrees of freedom, marital status (Married, Unmarried, Missing), birth parity (1, 2, ≥ 3), and smoking status during pregnancy (Yes or No). Temporal controls included the season of conception (Winter: December–February; Spring: March–May; Summer: June–August; Fall: September–November) and a natural cubic spline with 5 degrees of freedom on the conception date used for a long-term temporal trend. Areal-level covariates included ZIP code-level percent below poverty information from the 2010 Census. Census tract-level land area and water surface area (square meters) as well as percent of greenspace, both were calculated from the National Land Cover Database (NLCD) of the United States Geological Survey. Finally, we included indicators for each of the 106 maternal residential counties in all models.
We stratified the data by maternal race (White, African American, Asian, other), maternal ethnicity (Hispanic versus Non-Hispanic), maternal age (27 years versus > 27 years), and maternal residential address zip code level percent below poverty (12% versus > 12%), with different regression models apply to each stratum, in order to analyze effect modification of the relationship between air pollution and each birth outcome.
Several sensitivity analyses were performed to assess the validity of our key findings. First, we fitted the model by adjusting the long-term temporal trend using a natural cubic spline with 8 and 10 degrees of freedom on the conception date, or adjusted the conception year as a categorical variable. Second, since labor inductions are typically medically indicated, we further restricted to births without maternal induction of labor. Third, to control the confounding by exposures in other windows26, we run a single model to jointly estimate the association with each of the three trimester-average exposures (trimester 1, trimester 2, and trimester 3) for outcomes of preterm birth, birthweight, and GH, and two trimester-average exposures (trimester 1 and trimester 2) for the outcome of GDM. Fourth, we fitted multi-pollutant models by including trimester specific NO2, PM2.5, and O3 in the model simultaneously.
We report odds ratios (ORs) and mean differences for each interquartile range (IQR) increment in air pollution concentrations. Average pollutant levels during pregnancy were utilized to compute the IQRs, and we applied the same IQR values derived from the preterm birth cohort to report results from the birth weight, GDM, and GH outcomes. R software, version 4.0.2, was employed to perform all statistical analyses and all methods were performed in accordance with the relevant guidelines and regulations.
Ethics approval and consent to participate
Human subjects research approved by Emory University IRB #00102330.
Results
Study population characteristics
The preterm birth cohort comprised 596,926 singleton births across 106 Kansas counties, of which 41,936 (7.0%) were preterm. Compared to full-term birth mothers, preterm birth mothers had a high chance to deliver low birth weight babies, have gestational diabetes and chronic diabetes, have gestational hypertension and chronic hypertension, have lower education level, be of younger age, be unmarried, have higher parity, smoke during pregnancy, and live in more impoverished zip code area (Table 1). During the study period, the statewide incidence of preterm birth increased slightly (from 6.7% in 2000 to 7.0% in 2015). Characteristics of the birth weight, GDM, and GH cohorts were presented in Table S1.
Air pollution levels
The census tract-level spatial distributions of three air pollutants (NO2, PM2.5, O3) in Kansas during the sixteen-year study period are presented in Fig. 2. Average NO2 and PM2.5 decreased from 2000–2004 to 2010–2015 (NO2: 12.12 to 10.23 ppb; PM2.5: 9.01 to 7.72 µg/m3), but the daily mean of 8-h maximum O3 increased from 40.82 to 42.43 µg/m3 during the same periods. The interquartile range (IQR) of the of specific pollutants and pregnancy exposure window pairs are: first trimester NO2: 10.90 (ppb); second trimester NO2: 10.88 (ppb); third trimester NO2: 11.01 (ppb); total pregnancy NO2: 10.16 (ppb); first trimester PM2.5: 2.86 (µg/m3); second trimester PM2.5: 2.89 (µg/m3); third trimester PM2.5: 3.04 (µg/m3); total pregnancy PM2.5: 2.86 (µg/m3); first trimester O3: 13.82 (µg/m3); second trimester O3: 13.51 (µg/m3); third trimester O3: 14.26 (µg/m3); total pregnancy O3: 6.23 (µg/m3) (Table S2). Figure S1 shows the correlation matrix of three air pollutants (NO2, PM2.5, O3), and Fig. S2 shows the correlation matrix between different exposure windows for NO2, PM2.5, and O3. Correlations between pollutants in the same exposure window were low to moderate (< 0.45), while correlations between trimesters of the same pollutant can be high due to its seasonality.
Average concentrations of NO2, PM2.5, and O3 over the Kansas state from 2000 to 2015 (from upper row to the lower row represent the average concentrations during 2000–2004, 2005–2009, 2010–2015 for three pollutants) as estimated by using spatiotemporal ensemble models, which utilized over one hundred predictors, integrated three machine learning algorithms, and achieved excellent model performance.
Health effect estimates
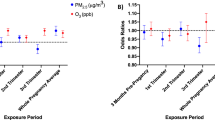
Figure 3 presents the statewide estimated relationships of exposure to three pollutants (NO2, PM2.5, O3) on preterm birth, birth weight, GDM, and GH during different pregnancy exposure windows. We identified elevated risks of preterm birth with IQR increment in second trimester O3 (odds ratio [OR]: 1.05; 95% confidence interval [CI]: 1.01, 1.09), third trimester O3 (OR: 1.04; 95% CI: 1.00, 1.08), and total pregnancy O3 (OR: 1.05; 95% CI: 1.02, 1.08). Per IQR increase in the second and third trimester O3, mean difference in term birth weight were − 9.86 (95% CI: − 14.00, − 5.69) and − 7.93 (− 12.34, − 3.53) grams. Second-trimester exposure to NO2 was positively related to the risk of GDM (OR: 1.06; 95% CI: 1.00, 1.12). O3 in the first trimester (T1) of pregnancy were connected to increased risk of GH (T1: 1.11; 95% CI: 1.05, 1.17). We didn’t observe any associations related to PM2.5 exposure. Effect estimates and 95% CI for all outcomes, pollutants and exposures are given in Table S3.
Statewide trimester-specific and total pregnancy-adjusted odds ratios (ORs) and mean difference and 95% confidence intervals per interquartile range (IQR) increase in the concentration of 3 pollutants. Models were adjusted for maternal education level, race, ethnicity, age, marital status, smoking, infant parity, zip code level percent below poverty, census tract level greenness, season of conception, and long-term trend using a natural cubic spline on conception date with 5 degrees of freedom. IQRs: First 4 Weeks NO2: 11.26 (ppb); First 6 Weeks NO2: 11.16 (ppb); First Trimester NO2: 10.90 (ppb); Second Trimester NO2: 10.88 (ppb); Third Trimester NO2: 11.01 (ppb); Total Pregnancy NO2: 10.16 (ppb); First 4 Weeks PM2.5: 3.56 (µg/m3); First 6 Weeks PM2.5: 3.30 (µg/m3); First Trimester PM2.5: 2.86 (µg/m3); Second Trimester PM2.5: 2.89 (µg/m3); Third Trimester PM2.5: 3.04 (µg/m3); Total Pregnancy PM2.5: 2.86 (µg/m3); First 4 Weeks O3: 15.81 (µg/m3); First 6 Weeks O3: 15.56 (µg/m3); First Trimester O3: 13.82 (µg/m3); Second Trimester O3: 13.51 (µg/m3); Third Trimester O3: 14.26 (µg/m3); Total Pregnancy O3: 6.23 (µg/m3).
Effect modifications
Table S4 shows subgroup-specific results stratified by maternal race, maternal ethnicity, maternal age, and maternal residential address zip code level percent below poverty. We did not observe consistent results across all outcomes and pollutants. However, mothers living in higher ZIP code level percent below poverty tend to have worse birth outcomes and pregnancy complications related to air pollution exposure. For example, the associations between NO2 and preterm birth are 0.98 (95% CI: 0.94, 1.02) and 1.04 (95% CI: 1.00, 1.08) for mothers living in ZIP codes with lower versus higher percent below poverty, respectively.
Sensitivity analysis
Our findings were robust to a number of sensitivity analyses. First, we examined the sensitivity of results to the approach for adjusting for long-term trend by fitting the model using a spline with 8 and 10 degrees of freedom on the conception date, or adjusting the conception year as a categorical variable. The estimated effects remained largely stable compared to the main analysis (Table S5). Second, we observed consistency in our preterm birth results after excluding births with induced labor (Table S6). Third, in comparison to the primary analysis, the estimated effects stayed consistent after including all related trimester-average exposures in the model (Table S7). Fourth, under multi-pollutant models, we observed consistency in our results (Table S8).
Discussion
This retrospective cohort study aimed to examine the relationship between ambient air pollution (NO2, PM2.5, O3) exposure during pregnancy and the risks of adverse birth outcomes (preterm birth and birth weight) and pregnancy complications (GDM and GH) using spatially-resolved exposure assessment methods. Our findings indicate that higher O3 exposure during pregnancy elevates the risk of preterm birth, lower birth weight, and gestational hypertension. We also observed that early-pregnancy NO2 exposure elevated the risk of GDM.
Our observation that exposure to O3 was positively connected to the risk of preterm birth is similar with prior studies. Previous research from various parts of the world have discovered that O3 exposures raise the risk of preterm birth27,28. A recent systematic review of 15 studies across 4 continents (Australia, Asia, Europe, and North America) reported that the pooled effect per 10 µg/m3 increment in second trimester exposure to O3 was 1.05 (95% CI: 1.02, 1.08)28. This finding is consistent with our results—an elevated 5% risk during the second trimester per IQR of 13.51 µg/m3. For the associations of whole-pregnancy O3 exposure and preterm birth, one previous paper also reported a 3% higher risk of birth before 37 weeks and a 13% enhanced risk of preterm birth deliveries per IQR of 7.1 µg/m329. The biological mechanisms underlying the link between O3 exposure and preterm birth are primarily mediated by oxidative stress and systemic inflammation30. Animal models have shown that O3 exposure alters the circulating serum cytokines, which may affect normal placentation, uterine artery vascularity, impaired trophoblast invasion, migration, and decreased trophoblast metabolic capability30. These biological mechanisms establish a rational explanation for how exposure to O3 during pregnancy might trigger preterm birth.
We detected a negative relationship between O3 exposure during the second and third trimesters and term birth weight. Our results are generally consistent with earlier research31,32,33,34. For example, one previous study discovered that the OR of low birth weight for each IQR increase in ozone concentration was 1.04 (95% CI: 1.02, 1.05) in a California population31. However, some previous research reported no relationship between exposure to O3 and birth weight1. These inconsistent results could be attributed to the various study designs, diverse study populations, and various levels of ozone exposure. Our results suggested significant effect of O3 exposure in the second and third trimester on birth weight in the trimester-specific analyses. This finding partially matched the previous results. According to earlier research, the first and third trimesters may be the most vulnerable time period for exposure to adverse birth outcomes35,36. However, Salam et al.34 reported a link between O3 exposure in the second and third trimesters and decreased birth weight which is consistent with our birth weight results. The underlying reasons for the differences remained unknown. Still, to assess the accurate effect of O3 exposure on birth weight, additional studies using various epidemiological approaches, different geographic locations, and more participants are needed. The possible underlying biological mechanisms by which O3 exposure may lead to reduced birth weight, including inflammatory reactions, blood flow, placental development and function, and oxidative stress37,38. Additionally, because pregnancy is characterized by organ formation, high levels of cell proliferation, and changing metabolic capacity, pregnant mothers are more susceptible to the impacts of air pollution39,40.
We discovered significant relationship between first trimester NO2 exposure and GDM. Previous studies reported inconsistent associations between NO2 and GDM. For example, a birth cohort of 256, 372 subjects in New York City found NO2 exposure in the first trimester was significantly linked to higher odds of GDM (OR: 1.05 per 7.96 ppb, 95% CI: 1.01, 1.09)41. But another study from Southern California with 239,574 subjects reported a weak but not statistically significant relationship between NO2 first trimester exposure and GDM (OR: 1.02 per 10.40 ppb, 95% CI: 0.99, 1.05)18. The heterogeneity between studies is likely influenced by differences in exposure assessment approaches, or differences in outcome assessment, medical care, population characteristics, or pollution mixtures. Our results also show significant negative associations between early pregnancy O3 exposure and GH. One previous study reported that O3 exposure during the first two trimester is associated with hypertension during pregnancy (HDP), and early pregnancy is a critical window of exposure42. Multiple biological pathways have been proposed to underlie O3 and HDP. Hypertension is linked to physiologic reactions to environmental variables, according to previous animal and toxicological research43. Pregnant women have been demonstrated to have higher levels of oxidative stress, and inflammation when exposed to air pollution43,44. These inflammatory reactions may also result in endothelial dysfunction, autonomic instability, and altered blood rheology, all of which have been associated to an elevated risk of hypertension45,46.
We didn’t obverse consistent associations between adverse pregnancy and birth outcomes with PM2.5 exposure. Previous meta-analysis studies suggested consistent associations between ambient PM2.5 and reduced birth weight, especially the effect estimates based on the entire pregnancy exposure1. However, the associations between PM2.5 and preterm birth1, GDM47, and GH48 are inconsistent. For example, Tang et al.47 did a meta-analysis study based on 1,547,154 individuals, and they concluded increased exposure to PM2.5 was not associated with the increased risk of GDM (adjusted OR: 1.03, 95% CI: 0.99, 1.06). The possible explanations for our results include Kansas is a relatively underdeveloped state in the U.S., and it has many rural areas compared to other states, so the PM2.5 components of black carbon and sulfate should be relatively lower compared to states with more urban areas. Black carbon and sulfate have been linked to several adverse birth and pregnancy outcomes49,50.
Our study has several strengths. First, our analysis was based on a large population of over 500,000 births, and exposure assessment using spatiotemporal ensemble models with high temporal and spatial resolutions23, which provided complete coverage over the entire Kansas state geographic region. This approach differs from previous methods that were limited to air quality metrics from only stationary monitors, which lack spatial and temporal resolution. Second, we used a time-to-event framework to model the effect of the third trimester and total pregnancy exposure to air pollution on the risk of preterm birth24. This design enables us to evaluate risk for third trimester effectively by minimizing the potential bias brought on by variations in the length of third trimester averaging period between preterm and full-term births51. Last, the Kansas birth records contain comprehensive maternal information, which removes the potential recall bias associated with gestational age based on the last menstrual period52.
Our research also has a few limitations. First, maternal address at birth is the only address available in our birth certificate data. However, some women may have resided at a different address during pregnancy. This may result in nondifferential exposure misclassification, which might bias the results toward the null. Previous research reported exposure misclassification due to pregnant women’s home address change was limited because most pregnant women did not move throughout their pregnancy53,54. Additionally, among mothers who moved, they remained close enough to their previous home address. Therefore, mobility is unlikely to have significantly impacted our findings55. Second, GDM and GH were identified through medical records, is subject to outcome misclassification. According to a previous study56, GDM misclassification using birth certificate data would lead to an underestimation of the GDM prevalence and further underestimate the relative risk. Third, while we controlled for multiple risk factors in our models, including maternal clinical characteristics and ZIP code level percent below poverty, there are still issues with residual confounding. Known risk factors, such as maternal alcohol use, illicit drug use, mother’s mobility information during pregnancy, previous preterm birth, and previous preeclampsia information, were unavailable from the Kansas birth certificate data. Fourth, we had about 25% GDM missingness from the birth records data, and the GDM results were based on subjects without GDM missingness. We did not perform any imputation for this missingness because we did not know the mechanism of this missingness. Even though we did not expect the missingness to be correlated with air pollution levels, the GDM results should be interpreted carefully. Last, our results were based on live birth only, and we didn’t consider the effects of air pollutants on pregnancy loss and fetal death. However, air pollution to fetal death is an emerging field, and current studies do not provide consistent results57,58,59,60.
For future research, it is possible that the true exposure window does not fit neatly within the boundaries of a trimester, and our trimester specific analysis may miss this association. Therefore, future work can explore exposures at finer temporal scale (e.g., months or weeks) to better identify critical exposure window. Also, since the Kansas air pollutant exposure ranges are relatively small, we were unable to assess the non-linearity associations. But we recommend future studies consider this.
Conclusions
In summary, our findings indicate significant relationships between higher O3 exposure during pregnancy and the increased risk of preterm birth and gestational hypertension, as well as decreased birth weight. We also noted that early-pregnancy NO2 exposure elevated the risk of GDM. Our study contributes to the body of knowledge regarding O3 detrimental impact on public health. Additionally, it demonstrates the significance of reducing exposure to air pollution in pregnant women for the purpose of lower the risk of adverse birth outcomes and pregnancy complications. Personal exposure assessment studies will be required to validate these connections.
Data availability
The exposure datasets generated and/or analyzed during the current study are available in the NASA EARTHDATA repository, https://sedac.ciesin.columbia.edu/data/set/aqdh-pm2-5- concentrations-contiguous-us-1-km-2000–2016; https://sedac.ciesin.columbia.edu/data/set/aqdh-o3-concentrations-contiguous-us-1-km2000-2016; https://sedac.ciesin.columbia.edu/data/set/aqdh-no2-concentrationscontiguous-us-1-km-2000-2016
References
Stieb, D. M., Chen, L., Eshoul, M. & Judek, S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ. Res. 117, 100–111. https://doi.org/10.1016/j.envres.2012.05.007 (2012).
Heikkila, K. et al. Preterm birth and the risk of chronic disease multimorbidity in adolescence and early adulthood: A population-based cohort study. PLoS One 16, e0261952. https://doi.org/10.1371/journal.pone.0261952 (2021).
Osterman, M., Hamilton, B., Martin, J. A., Driscoll, A. K. & Valenzuela, C. P. Births: Final data for 2020. Natl. Vital Stat. Rep. 70, 1–50 (2021).
In (eds. Behrman, R. E. & Butler, A. S.) Preterm Birth: Causes, Consequences, and Prevention The National Academies Collection: Reports funded by National Institutes of Health (2007).
Beam, A. L. et al. Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008–2016. J. Perinatol. 40, 1091–1099. https://doi.org/10.1038/s41372-020-0635-z (2020).
Li, X. et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ. Pollut. 227, 596–605. https://doi.org/10.1016/j.envpol.2017.03.055 (2017).
Hao, H. et al. Air pollution and preterm birth in the U.S. State of Georgia (2002-2006): Associations with concentrations of 11 ambient air pollutants estimated by combining community multiscale air quality model (CMAQ) simulations with stationary monitor measurements. Environ. Health Perspect. 124, 875–880. https://doi.org/10.1289/ehp.1409651 (2016).
Shah, P. S. & Balkhair, T. Knowledge Synthesis Group on Determinants of Preterm, L. B. W. b. Air pollution and birth outcomes: A systematic review. Environ. Int. 37, 498–516. https://doi.org/10.1016/j.envint.2010.10.009 (2011).
Istrate-Ofiteru, A. M. et al. The influence of gestational diabetes mellitus (GDM) and gestational hypertension (GH) on placental morphological changes. Rom. J. Morphol. Embryol. 61, 371–384. https://doi.org/10.47162/RJME.61.2.07 (2020).
Buchanan, T. A., Xiang, A. H. & Page, K. A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 8, 639–649. https://doi.org/10.1038/nrendo.2012.96 (2012).
Getaneh, T., Negesse, A., Dessie, G. & Desta, M. The impact of pregnancy induced hypertension on low birth weight in Ethiopia: Systematic review and meta-analysis. Ital. J. Pediatr. 46, 174. https://doi.org/10.1186/s13052-020-00926-0 (2020).
Bai, W. et al. Association between ambient air pollution and pregnancy complications: A systematic review and meta-analysis of cohort studies. Environ. Res. 185, 109471. https://doi.org/10.1016/j.envres.2020.109471 (2020).
Zhang, H. et al. Ambient air pollution and gestational diabetes mellitus: A review of evidence from biological mechanisms to population epidemiology. Sci. Total Environ. 719, 137349. https://doi.org/10.1016/j.scitotenv.2020.137349 (2020).
Zhu, Y. & Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diab. Rep. 16, 7. https://doi.org/10.1007/s11892-015-0699-x (2016).
George, M. G., Tong, X. & Bowman, B. A. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 74, 695–703. https://doi.org/10.1001/jamaneurol.2017.0020 (2017).
American College of, O., Gynecologists' Presidential Task Force on, P., Heart, D. & Committee on Practice, B.-O. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet. Gynecol. 133, e320–e356 (2019). https://doi.org/10.1097/AOG.0000000000003243.
Abdo, M. et al. Impact of wildfire smoke on adverse pregnancy outcomes in Colorado, 2007–2015. Int. J. Environ. Res. Public Health 16, 141. https://doi.org/10.3390/ijerph16193720 (2019).
Jo, H. et al. Associations of gestational diabetes mellitus with residential air pollution exposure in a large Southern California pregnancy cohort. Environ. Int. 130, 104933. https://doi.org/10.1016/j.envint.2019.104933 (2019).
Nobles, C. J., Williams, A., Ouidir, M., Sherman, S. & Mendola, P. Differential effect of ambient air pollution exposure on risk of gestational hypertension and preeclampsia. Hypertension 74, 384–390. https://doi.org/10.1161/HYPERTENSIONAHA.119.12731 (2019).
Savitz, D. A. et al. Ambient fine particulate matter, nitrogen dioxide, and hypertensive disorders of pregnancy in New York City. Epidemiology 26, 748–757. https://doi.org/10.1097/EDE.0000000000000349 (2015).
Strand, L. B., Barnett, A. G. & Tong, S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med. Res. Methodol. 11, 49. https://doi.org/10.1186/1471-2288-11-49 (2011).
Di, Q. et al. Daily and Annual PM2.5 Concentrations for the Contiguous United States, 1-km Grids, v1 (2000 - 2016). Palisades, New York: NASA Socioeconomic Data and Applications Center (SEDAC) (2021). https://doi.org/10.7927/0rvr-4538.
Di, Q. et al. An ensemble-based model of PM(25) concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 130, 104909. https://doi.org/10.1016/j.envint.2019.104909 (2019).
Chang, H. H., Reich, B. J. & Miranda, M. L. A spatial time-to-event approach for estimating associations between air pollution and preterm birth. J. R. Stat. Soc. Ser. C Appl. Stat. 62, 167–179. https://doi.org/10.1111/j.1467-9876.2012.01056.x (2013).
Rani, P. R. & Begum, J. Screening and diagnosis of gestational diabetes mellitus, where do we stand. J. Clin. Diagn. Res. 10, 01–04. https://doi.org/10.7860/JCDR/2016/17588.7689 (2016).
Wilson, A. et al. Potential for bias when estimating critical windows for air pollution in children’s health. Am. J. Epidemiol. 186, 1281–1289. https://doi.org/10.1093/aje/kwx184 (2017).
Bekkar, B., Pacheco, S., Basu, R. & DeNicola, N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: A systematic review. JAMA Netw. Open 3, e208243. https://doi.org/10.1001/jamanetworkopen.2020.8243 (2020).
Rappazzo, K. M., Nichols, J. L., Rice, R. B. & Luben, T. J. Ozone exposure during early pregnancy and preterm birth: A systematic review and meta-analysis. Environ. Res. 198, 111317. https://doi.org/10.1016/j.envres.2021.111317 (2021).
Ha, S. et al. The effects of air pollution on adverse birth outcomes. Environ. Res. 134, 198–204. https://doi.org/10.1016/j.envres.2014.08.002 (2014).
U.S. EPA. Integrated Science Assessment (ISA) for Ozone and Related Photochemical Oxidants (Final Report, Apr 2020). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-20/012 (2020).
Laurent, O. et al. A statewide nested case-control study of preterm birth and air pollution by source and composition: California, 2001–2008. Environ. Health Perspect. 124, 1479–1486. https://doi.org/10.1289/ehp.1510133 (2016).
Gray, S. C., Edwards, S. E., Schultz, B. D. & Miranda, M. L. Assessing the impact of race, social factors and air pollution on birth outcomes: A population-based study. Environ. Health 13, 4. https://doi.org/10.1186/1476-069X-13-4 (2014).
Ha, E. H. et al. Is air pollution a risk factor for low birth weight in seoul?. Epidemiology 12, 643–648. https://doi.org/10.1097/00001648-200111000-00011 (2001).
Salam, M. T. et al. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: Results from the Children’s Health Study. Environ. Health Perspect. 113, 1638–1644. https://doi.org/10.1289/ehp.8111 (2005).
Klepac, P., Locatelli, I., Korosec, S., Kunzli, N. & Kukec, A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 167, 144–159. https://doi.org/10.1016/j.envres.2018.07.008 (2018).
Lamichhane, D. K., Leem, J. H., Lee, J. Y. & Kim, H. C. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ. Health Toxicol. 30, e2015011. https://doi.org/10.5620/eht.e2015011 (2015).
Glinianaia, S. V., Rankin, J., Bell, R., Pless-Mulloli, T. & Howel, D. Particulate air pollution and fetal health: A systematic review of the epidemiologic evidence. Epidemiology 15, 36–45. https://doi.org/10.1097/01.ede.0000101023.41844.ac (2004).
Kannan, S., Misra, D. P., Dvonch, J. T. & Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect. 114, 1636–1642. https://doi.org/10.1289/ehp.9081 (2006).
Laumbach, R. J. Outdoor air pollutants and patient health. Am. Fam. Phys. 81, 175–180 (2010).
Selevan, S. G., Kimmel, C. A. & Mendola, P. Identifying critical windows of exposure for children’s health. Environ. Health Perspect. 108(Suppl 3), 451–455. https://doi.org/10.1289/ehp.00108s3451 (2000).
Choe, S. A., Eliot, M. N., Savitz, D. A. & Wellenius, G. A. Ambient air pollution during pregnancy and risk of gestational diabetes in New York City. Environ. Res. 175, 414–420. https://doi.org/10.1016/j.envres.2019.04.030 (2019).
Hu, H., Ha, S. & Xu, X. Ozone and hypertensive disorders of pregnancy in Florida: Identifying critical windows of exposure. Environ. Res. 153, 120–125. https://doi.org/10.1016/j.envres.2016.12.002 (2017).
Nagiah, S. et al. Oxidative stress and air pollution exposure during pregnancy: A molecular assessment. Hum. Exp. Toxicol. 34, 838–847. https://doi.org/10.1177/0960327114559992 (2015).
Lee, P. C. et al. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology 22, 524–531. https://doi.org/10.1097/EDE.0b013e31821c6c58 (2011).
Bind, M. A. et al. Air pollution and markers of coagulation, inflammation, and endothelial function: Associations and epigene-environment interactions in an elderly cohort. Epidemiology 23, 332–340. https://doi.org/10.1097/EDE.0b013e31824523f0 (2012).
Huang, W. et al. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: Interactions of systemic inflammation, overweight, and gender. Am. J. Epidemiol. 176, 117–126. https://doi.org/10.1093/aje/kwr511 (2012).
Tang, X. et al. Air pollution and gestational diabetes mellitus: Evidence from cohort studies. BMJ Open Diabetes Res. Care 8, 141. https://doi.org/10.1136/bmjdrc-2019-000937 (2020).
Yu, H., Yin, Y., Zhang, J. & Zhou, R. The impact of particulate matter 2.5 on the risk of preeclampsia: An updated systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 27, 37527–37539. https://doi.org/10.1007/s11356-020-10112-8 (2020).
Cai, J. et al. Prenatal exposure to specific PM(2.5) chemical constituents and preterm birth in China: A nationwide cohort study. Environ. Sci. Technol. 54, 14494–14501. https://doi.org/10.1021/acs.est.0c02373 (2020).
Shen, Y. et al. Prenatal exposure to PM(2.5) and its specific components and risk of hypertensive disorders in pregnancy: A nationwide cohort study in China. Environ. Sci. Technol. 56, 11473–11481. https://doi.org/10.1021/acs.est.2c01103 (2022).
Lewis, C., Hoggatt, K. J. & Ritz, B. The impact of different causal models on estimated effects of disinfection by-products on preterm birth. Environ. Res. 111, 371–376. https://doi.org/10.1016/j.envres.2010.12.004 (2011).
van Oppenraaij, R. et al. Determinants of number-specific recall error of last menstrual period: A retrospective cohort study. BJOG 122, 835–841. https://doi.org/10.1111/1471-0528.12991 (2015).
Chen, L., Bell, E. M., Caton, A. R., Druschel, C. M. & Lin, S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ. Res. 110, 162–168. https://doi.org/10.1016/j.envres.2009.11.001 (2010).
Pennington, A. F. et al. Measurement error in mobile source air pollution exposure estimates due to residential mobility during pregnancy. J. Expo Sci. Environ. Epidemiol. 27, 513–520. https://doi.org/10.1038/jes.2016.66 (2017).
Warren, J. L., Son, J. Y., Pereira, G., Leaderer, B. P. & Bell, M. L. Investigating the impact of maternal residential mobility on identifying critical windows of susceptibility to ambient air pollution during pregnancy. Am. J. Epidemiol. 187, 992–1000. https://doi.org/10.1093/aje/kwx335 (2018).
Kim, S. Y. et al. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004–2007. Prev. Chronic Dis. 9, E88. https://doi.org/10.5888/pcd9.110249 (2012).
DeFranco, E. et al. Air pollution and stillbirth risk: Exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS One 10, e0120594. https://doi.org/10.1371/journal.pone.0120594 (2015).
Faiz, A. S. et al. Ambient air pollution and the risk of stillbirth. Am. J. Epidemiol. 176, 308–316. https://doi.org/10.1093/aje/kws029 (2012).
Leung, M., Kioumourtzoglou, M.-A., Raz, R. & Weisskopf-Marc, G. Bias due to selection on live births in studies of environmental exposures during pregnancy: A simulation study. Env. Health Perspect. 129, 047001. https://doi.org/10.1289/EHP7961 (2021).
Liew, Z., Olsen, J., Cui, X., Ritz, B. & Arah, O. A. Bias from conditioning on live birth in pregnancy cohorts: An illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int. J. Epidemiol. 44, 345–354. https://doi.org/10.1093/ije/dyu249 (2015).
Acknowledgements
We would like to express our gratitude to the Bureau of Epidemiology and Public Health Informatics, Kansas Department of Health for providing the birth certificate data which allowed us to conduct this study.
Funding
National Institute of Environmental Health Sciences [grant number R01ES028346] provided funding for this project.
Author information
Authors and Affiliations
Contributions
H.H. and H.C. led the study design and overview of the project, and interpreted of the data. H.H. drafted the manuscript. R.Y., R.D., and H.Z. contributed to data analyses. M.S., L.D., J.W., S.M. and H.C. led data collection and interpretation of the results. H.W. provided the greenness related exposure data and assisted with data interpretation. All authors have reviewed and made contributions to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hao, H., Yoo, S.R., Strickland, M.J. et al. Effects of air pollution on adverse birth outcomes and pregnancy complications in the U.S. state of Kansas (2000–2015). Sci Rep 13, 21476 (2023). https://doi.org/10.1038/s41598-023-48329-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48329-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.