Abstract
Breast cancer (BC) is the most prevalent malignancy among women worldwide with germline pathogenic variants/likely pathogenic variants (PVs/LPVs) in BRCA1/2 accounting for a large portion of hereditary cases. Recently, heterozygous PVs/LPVs in the ATM serine/threonine kinase or Ataxia-telangiectasia mutated gene (ATM) has been identified as a moderate susceptibility factor for BC in diverse ethnicities. However, the prevalence of ATM PVs/LPVs in BC susceptibility in Arab populations remains largely unexplored. This study investigated the prevalence of ATM PVs/LPVs among BC patients from Saudi Arabia, employing capture-sequencing technology for ATM PVs/LPVs screening in a cohort of 715 unselected BC patients without BRCA1/2 PVs/LPVs. In addition, founder mutation analysis was conducted using the PHASE program. In our entire cohort, four unique PVs/LPVs in the ATM gene were identified in six cases (0.8%). Notably, one recurrent LPV, c.6115G > A:p.Glu2039Lys was identified in three cases, for which haplotype analysis confirmed as a novel putative founder mutation traced back to 13 generations on average. This founder mutation accounted for half of all identified mutant cases and 0.4% of total screened cases. This study further reveals a significant correlation between the presence of ATM mutation and family history of BC (p = 0.0127). These findings underscore an approximate 0.8% prevalence of ATM germline PVs/LPVs in Arab BC patients without BRCA1/2 PVs/LPVs and suggest a founder effect of specific recurrent ATM mutation. These insights can help in the design of a genetic testing strategy tailored to the local population in Saudi Arabia, thereby, enabling more accurate clinical management and risk prediction.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common cancer among women, accounting for a significant share of cancer-related morbidity and mortality worldwide1,2,3. BC incidence varies across ethnicities, thereby emphasizing the need for ethnic-specific genetic risk assessment. In the Middle Eastern population, including Saudi Arabia, BC is the most prevalent malignancy among women4,5,6. Notably, BC appears to manifest at an earlier age and presented with advanced stage in these populations compared to their western counterparts, indicating unique genetic predisposition factors7,8,9,10,11.
Among genetic factors, germline pathogenic variants/likely pathogenic variants (PVs/LPVs) in the BRCA1 and BRCA2 genes account for a large proportion of hereditary breast cancer cases worldwide12,13. Previously, the prevalence of BRCA mutation in Middle Eastern BC patients has been estimated to be 3.4% in overall cases. On the other hand, high-risk BC patients (positive family history, early onset, TNBC, and cases with bilateral breast cancer) demonstrated that 6.4% had BRCA mutation14. However, beyond BRCA1/2, other genes are being recognized as contributors to BC predisposition, with the ATM serine/threonine kinase or Ataxia-telangiectasia mutated gene (ATM) emerging as a notable moderate susceptibility gene15,16,17,18,19. ATM gene plays a crucial role in DNA damage response; cell cycle control as well as telomere maintenance and heterozygous PVs/LPVs of ATM have two to 13- fold-increased risk of BC development20,21,22,23. However, the distribution and the contribution of ATM PVs/LPVs to BC susceptibility in Arab populations remains limited.
Arab genetic architecture is very distinctive with known genetic drift among their population24. The genetic drift and the ensuing founder effect represent significant genetic forces that can shape the genetic landscape of population, contributing to a unique spectrum of disease-causing variants. This phenomenon is particularly evident in populations with high levels of consanguinity such as in the Middle Eastern Arab population25,26. Genetic drift, alongside the population-specific selection pressures and environmental exposures, can result in the enrichment of specific disease-associated PVs/LPVs including those in cancer predisposition genes. Hence, the identification and characterization of these PVs/LPVs and founder mutations can provide valuable insights into the unique genetic underpinnings of BC in the Arab population.
Therefore, we conducted this study to determine the prevalence, spectrum, and founder effect of ATM germline PVs/LPVs in a large cohort of BRCA1/2 negative BC patients from Saudi Arabia. Such knowledge could contribute to enhancing personalized management strategies for BC patients in the region.
Methods
Sample selection
A total of 715 BRCA PVs/LPVs negative BC cases were included in this study. All these patients were diagnosed and treated at King Faisal Specialist Hospital & Research Centre (KFSH&RC). All clinicopathological data were collected from case records and presented in Table 1. The eighth edition of the American Joint Committee on Cancer (AJCC) staging system was utilized to determine the stage of breast cancer27. The Institutional Review Board of the King Faisal Specialist Hospital & Research Center approved this study. Since only archival tissue specimens and retrospective patient data were utilized, the Research Advisory Council (RAC) provided a waiver of consent under project RAC # 2140 008.
DNA extraction
In our study, Gentra DNA Isolation Kit (Gentra, Minneapolis, MN, USA) was utilized to extract DNA samples from normal formalin-fixed and paraffin-embedded (FFPE) breast cancer or ovarian cancer tissue following the manufacturer's protocols as described in our previous study28. Two pathologists examined the histopathology slides to ensure that normal tissues were obtained from different FFPE blocks such as uninvolved lymph nodes or non-cancerous breast tissue away from the tumor in order to minimize somatic contamination.
Capture sequencing analysis
A custom-designed gene panel was used to perform Targeted capture sequencing on 715 samples29. The DNA samples with A260/A280 ratio between 1.8 and 2.0 were selected for library construction. The preparation of the sequencing library was carried out by randomly fragmenting the DNA sequences as described in the previous study30.The BCL (base calls) produced by Illumina HiSeq 4000 platform were transformed into FASTQ files through the bcl2fastq software (v2.16). Subsequently, the sequence reads in FASTQ format from each sample were aligned to the reference human genome (GRCh37/hg19) using Burrows-Wheeler aligner (BWA)31. We generated BAM files, addressed PCR duplicates, and conducted local realignment using a combination of Picard-tools and Genome Analysis Toolkit (GATK) as described in a previous study32.
Variant calling
GATK was employed for variant calling, followed by the annotation of the variants using ANNOVAR33. Annotations were sourced from databases including dbSNP138, 1000 Genomes, ESP6500, Exome Aggregation Consortium (ExAC), ClinVar and other relevant genome databases. The classification of pathogenic and likely pathogenic variants adhered to the recommended guidelines established by the American College of Medical Genetics and Genomics and the Association of Molecular Pathology (ACMG/AMP)34.
Haplotype analysis
Genotyping was performed on custom designed High-throughput Illumina Infinium SNP Genotyping Array with 778,783 SNPs following manufacturer’s instruction (Illumina Inc). Normalized signal intensity and genotype were computed using Illumina Bead Array Files Python library. Quality checking was done by plotting p10 GC and sample call rate and a text file containing the genotype of entire samples and probes was generated. SNP data for 100 controls was also included from our in-house database.
Haplotype construction was performed on two samples and 100 controls utilizing PHASE version 2.1.1 algorithm35,36. Count of variant positions, nucleotide positions of variants and sample genotypes for each sample and controls at those positions were provided as an input in the algorithm. Following parameter were set: number of iterations = 100, thinning interval = 1, burn-in = 100. DMLE + version 2.337, which is a linkage disequilibrium mapping software, was utilized to estimate the age of variants with founder effect. This software uses Markov Chain Monte Carlo algorithm for Bayesian estimation of mutation age as described previously38. The analysis was performed as described in our previous study39.
Statistical analysis
Contingency table analysis and Fisher’s exact tests were used to analyze the association clinico-pathological variables and ATM mutations. Two-sided tests employed for analyses with a significance threshold set at a p-value < 0.05. All data analyses were carried out using the JMP14.0 software package developed by SAS Institute, Inc., Cary, NC.
Ethics approval and consent to participate
The Institutional Review Board of the King Faisal Specialist Hospital and Research Center approved this study and since only archival tissue specimens and retrospective patient data were used, the Research Advisory Council (RAC) of King Faisal Specialist Hospital and Research Center provided waiver of consent under project RAC # 2140 008.
Results
Identification of PVs/LPVs in ATM gene and founder mutation analysis
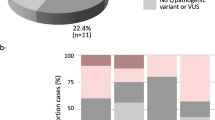
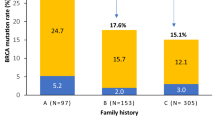
In entire cohort, four unique PVs/LPVs in ATM gene were identified in six cases accounting for 0.8% of all cases. Among these four variants, one recurrent missense LPV was detected in three cases, accounting for 50% of all mutant cases and 0.4% of all sequenced cases. Other three were pathogenic variants including two splicing PVs and one frameshift PV, each one observed in one case, accounting for 0.1% of all cases (Table 2). All these variants were reported previously. Interestingly, four out of six mutant cases were reported to have positive family history of BC, accounting for 3% (4/133) of all family history positive cases (Table 3).
Haplotype construction was performed for two cases with recurrent variant and sufficient DNA sample utilizing PHASE version 2.1.1 algorithm. Our results revealed that the two carriers of c.6115G > A: p.Glu2039Lys in ATM gene shared the same haplotype with length of ~ 1.4 MB (Supplementary Table 1), suggesting that this recurrent mutation is putative novel founder mutation derived from common ancestor. Furthermore, the result of age estimation showed the average age of this founder mutation as 13 generations (10–17 generations; 95% CI).
Clinico-pathological characteristics of BC patients with ATM PV/LPVs
Median age of the ATM mutant cases was 39 years (range = 32–63 years). All the six ATM mutant cases were unilateral tumors. Majority of the ATM mutant cases were larger tumors (T3/T4–83.3%; 5/6), had lymph node metastasis (66.7%; 4/6), were advanced stage (Stage III/IV–50%; 3/6) and hormone receptor positive (83.3%; 5/6). Of the six ATM mutant cases, one patient died due to disease progression. Interestingly, we found a significant association between ATM mutations and family history of breast cancer (p = 0.0127), with three patients having a first-degree relative and one patient having a second-degree relative being diagnosed with BC (Table 3). However, no significant association was noted between ATM mutation and age of onset (early-onset vs. late-onset) of BC, lymph node status, tumor stage, grade, estrogen receptor, progesterone receptor and Her-2 status (Table 4).
Discussion
Our findings underscore the pivotal role of ATM PVs/LPVs in the predisposition of BC in Arab populations, a group traditionally under presented in BC genomics. This study uncovered a recurrent ATM LPV, c.6115G > A:p.Glu2039Lys, identified as novel putative founder mutation within Arab populations. The observation of this recurrent variant draws attention to the specific genetic landscape in this population and emphasizes the unique contribution of ATM PV/LPV to the BC risk.
In our study, 0.8% of 715 unselected Saudi Arabian patients with BRCA mutation-negative BC carried ATM germline PVs/LPVs. BRCA1/2 are well known hereditary breast cancer predisposition genes and have been extensively studied. However, it is now estimated that more than one-half of individuals with a pathogenic variant (PV) who meet the National Comprehensive Cancer Network (NCCN) testing criteria for hereditary breast and ovarian cancer (HBOC) carry PVs in genes other than BRCA1 or BRCA240. According to previous reports, 3.4% of Middle Eastern breast cancer cases carry BRCA1/2 mutation14. However, genetic basis for a large proportion of BC patients is still unknown, therefore, it is crucial to investigate the prevalence of other genes in breast cancer cases to facilitate the development of a genetically-tailored testing strategy for the indigenous population of Saudi Arabia, ultimately enhancing the precision of clinical management and risk prediction. The study further reveals a significant correlation between the presence of ATM PV/LPV and family history of breast cancer. Majority of ATM PVs/LPVs carries have ER and/or PR-positive breast cancer or large tumors.
The observed prevalence of ATM PVs/LPVs in our cohort (0.8%) aligns with frequencies reported in other population, reaffirming ATM as a moderate-risk BC susceptibility gene globally. Based on previous reports, ATM mutation frequency ranges from 0.5 to 4%, depending on the population studied41,42,43,44,45,46
In comprehensive sequencing study of BC cases and controls, ATM was identified as one of the several genes with mutations significantly associated with BC risk41. This study reported a similar frequency of ATM PVs/LPVs (0.85%) among BC cases. Another large case–control study found that rare ATM PV/LPV prevalence of 0.4% in Chinese BC patients47. Two previous large-scale gene panel studies in Caucasian BC patients found that the prevalence of ATM PVs/LPVs was approximately 1%40,48.
Remarkably, certain populations have exhibited a higher prevalence of ATM PVs/LPVs. A study from Netherlands has reported a considerably higher frequency of ATM mutation (~ 4%)44. Similarly, a study investigating Irish individuals reported a prevalence of ATM mutations of 2.9%45. Another study based on Spanish population also identified 1.9% frequency of ATM mutation among their BC patients46.
These differences underscore the variable contributions of ATM mutations across different ethnicities, potentially reflecting distinct founder effect. The significant association of ATM mutations with a family history of BC in our study aligns with previous reports49,50. BC patients with family history have been found to carry ATM PVs/LPVs at higher rates, further supporting its role as a hereditary BC susceptibility gene16,22,50. Notably, the frequency and spectrum of ATM mutations can be influenced by genetic drift, population-specific factors, and environmental exposure, warranting further investigation. Interestingly, if we restrict the prevalence of ATM PVs/LPVs among BC patient with positive family history, the frequency will rise to 3% (4/133). Therefore, the family history of breast cancer should be taken into account during genetic counselling.
Similar to patients with any high to moderate risk of BC, surveillance is necessary for patients with ATM PVs/LPVs and their relatives. It was suggested for women with family history of breast to undergo early screening by mammogram and MRI51. According to recent guidelines, women who are carrier for ATM germline PVs/LPVs should go under surveillance by at least age of 40 years since their lifetime risk of BC is likely higher than 25%. Large-scale, age-matched case–control studies are vital to investigate the lifetime risk of BC in carriers of the ATM PVs/LPVs in the Arab population.
Our study findings hold significant clinical implications, besides enhancing our understanding of BC genomic landscape in Arab population, enabling a more inclusive and precise approach to risk assessment, genetic testing and patient management in this population. With the advent of precision medicine, incorporation of ATM genetic testing into standard BC risk assessment, particularly in population where ATM PVs/LPVs are prevalent, can offer more personalized therapeutics and preventive strategies.
In conclusion, our study showed that the prevalence of ATM germline PVs/LPVs in BRCA mutation-negative patients in Arab population was approximately 0.8%. Our findings suggest founder effect for specific recurrent ATM PV/LPV, providing vital insights into the genomics of BC in Arab population. This data could be used to shape a genetic testing strategy customized for Arab population, thereby enabling more accurate clinical management, including risk prediction, surveillance, prevention and treatment of BC.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136(5), E359-386 (2015).
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72(6), 524–541 (2022).
Rao, H. L. et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One 7(1), e30806 (2012).
Mahdi, H. et al. Cancer burden among Arab-world females in 2020: Working toward improving outcomes. JCO Glob. Oncol. 8, e2100415 (2022).
Saadeh, S. & Abdel-Razeq, H. Breast cancer in the Arab world. In Cancer in the Arab World (eds Al-Shamsi, H. O. et al.) 353–362 (Springer Singapore, 2022).
Alrawaji A, Alshahrani Z, Alzahrani W, Alomran F, Almadouj A, Alshehri S, Alzahrani A, Bazarbashi S, Alhashmi H, Almutlaq H Cancer Incidence Report Saudi Arabia 2015. Saudi Cancer Registry (2018).
Siraj, A. K. et al. High expression of Cyclin D1 is an independent marker for favorable prognosis in Middle Eastern breast cancer. Onco Targets Ther. 14, 3309–331814 (2021).
Chouchane, L., Boussen, H. & Sastry, K. S. Breast cancer in Arab populations: Molecular characteristics and disease management implications. Lancet Oncol. 14(10), e417-424 (2013).
Al-Kuraya, K. et al. Predominance of high-grade pathway in breast cancer development of Middle East women. Mod. Pathol. 18(7), 891–897 (2005).
Safiri, S. et al. Burden of female breast cancer in the Middle East and North Africa region, 1990–2019. Arch. Public Health 80(1), 168 (2022).
Alabdulkarim, B., Hassanain, M., Bokhari, A., AlSaif, A. & Alkarji, H. Age distribution and outcomes in patients undergoing breast cancer resection in Saudi Arabia. A single-institute study. Saudi Med. J. 39(5), 464–469 (2018).
Wooster, R. et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 378(6559), 789–792 (1995).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266(5182), 66–71 (1994).
Bu, R. et al. Identification of novel BRCA founder mutations in Middle Eastern breast cancer patients using capture and Sanger sequencing analysis. Int. J. Cancer 139(5), 1091–1097 (2016).
Hu, C. et al. A population-based study of genes previously implicated in breast cancer. N. Engl. J. Med. 384(5), 440–451 (2021).
Renwick, A. et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat. Genet. 38(8), 873–875 (2006).
Easton, D. F. et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 372(23), 2243–2257 (2015).
Young, E. L. et al. Multigene testing of moderate-risk genes: Be mindful of the missense. J. Med. Genet. 53(6), 366–376 (2016).
Dorling, L. et al. Breast cancer risk genes - association analysis in more than 113,000 women. N. Engl. J. Med. 384(5), 428–439 (2021).
Rotman, G. & Shiloh, Y. ATM: From gene to function. Hum. Mol. Genet. 7(10), 1555–1563 (1998).
Matsuoka, S. et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316(5828), 1160–1166 (2007).
Thompson, D. et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J. Natl. Cancer Inst. 97(11), 813–822 (2005).
Liu, B., Tahk, S., Yee, K. M., Fan, G. & Shuai, K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science 330(6003), 521–525 (2010).
Scott, E. et al. Characterization of Greater Middle Eastern genetic variation for enhanced disease gene discovery. Nat. Genet. 48(9), 1071–1076 (2016).
Jaber, L., Halpern, G. J. & Shohat, T. Trends in the frequencies of consanguineous marriages in the Israeli Arab community. Clin. Genet. 58(2), 106–110 (2000).
Bittles, A. H. & Speicher, M. Antonarakis S Consanguinity, genetic drift, and genetic diseases in populations with reduced numbers of founders. In Vogel and Motulsky’s Human Genetics (eds Speicher, Michael R. et al.) 507–528 (Springer Berlin Heidelberg, 2010).
Zhu, H. & Dogan, B. E. American Joint Committee on cancer’s staging system for breast cancer, eighth edition: Summary for clinicians. Eur. J. Breast. Health 17(3), 234–238 (2021).
Abubaker, J. et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J. Clin. Endocrinol. Metab. 93(2), 611–618 (2008).
Siraj, A. K. et al. Expanding the spectrum of germline variants in cancer. Hum. Genet. 136(11–12), 1431–1444 (2017).
Siraj, A. K. et al. PALB2 germline mutations in a large cohort of Middle Eastern breast-ovarian cancer patients. Sci. Rep. 13(1), 7666 (2023).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26(5), 589–595 (2010).
McKenna, A. et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20(9), 1297–1303 (2010).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38(16), e164–e164 (2010).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17(5), 405–423 (2015).
Sotiropoulos, K. et al. Phylogeny and biogeography of the alpine newt Mesotriton alpestris (Salamandridae, Caudata), inferred from mtDNA sequences. Mol. Phylogenet. Evol. 45(1), 211–226 (2007).
Stephens, M. & Scheet, P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am. J. Hum. Genet. 76(3), 449–462 (2005).
Reeve, J. P. & Rannala, B. DMLE+: Bayesian linkage disequilibrium gene mapping. Bioinformatics 18(6), 894–895 (2002).
Lu, R., Zhu, H. & Wu, X. Estimating mutation rates in a Markov branching process using approximate Bayesian computation. J. Theor. Biol. 565, 111467 (2023).
Siraj, A. K. et al. Prevalence, spectrum, and founder effect of BRCA1 and BRCA2 mutations in epithelial ovarian cancer from the Middle East. Hum. Mutat. 40(6), 729–733 (2019).
Buys, S. S. et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 123(10), 1721–1730 (2017).
Minion, L. E. et al. Hereditary predisposition to ovarian cancer, looking beyond BRCA1/BRCA2. Gynecol. Oncol. 137(1), 86–92 (2015).
Soukupova, J., Dundr, P., Kleibl, Z. & Pohlreich, P. Contribution of mutations in ATM to breast cancer development in the Czech population. Oncol. Rep. 19(6), 1505–1510 (2008).
Dork, T. et al. Spectrum of ATM gene mutations in a hospital-based series of unselected breast cancer patients. Cancer Res. 61(20), 7608–7615 (2001).
Broeks, A. et al. ATM-heterozygous germline mutations contribute to breast cancer-susceptibility. Am. J. Hum. Genet. 66(2), 494–500 (2000).
Aloraifi, F. et al. Detection of novel germline mutations for breast cancer in non-BRCA 1/2 families. The FEBS journal 282(17), 3424–3437 (2015).
Tavera-Tapia, A. et al. Almost 2% of Spanish breast cancer families are associated to germline pathogenic mutations in the ATM gene. Breast Cancer Res. Treat. 161, 597–604 (2017).
Yang, Z. et al. Prevalence and characterization of ATM germline mutations in Chinese BRCA1/2-negative breast cancer patients. Breast Cancer Res. Treat. 174(3), 639–647 (2019).
Couch, F. J. et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 3(9), 1190–1196 (2017).
Teraoka, S. N. et al. Increased frequency of ATM mutations in breast carcinoma patients with early onset disease and positive family history. Cancer 92(3), 479–487 (2001).
Thorstenson, Y. R. et al. Contributions of ATM mutations to familial breast and ovarian cancer. Cancer Res. 63(12), 3325–3333 (2003).
Daly, M. B. et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017. J. Natl. Compr. Cancer Netw. 15(1), 9–20 (2017).
Acknowledgements
We would like to thank department of Pathology & Laboratory Medicine and department of Surgery for their assistance in this study. We would also thank Mark Ranier Diaz, Allianah Benito and Maria Angelita Sabido for their technical assistance.
Author information
Authors and Affiliations
Contributions
K.S.A. participated in study design, manuscript drafting. R.B., A.K.S., participated in experiment design, data acquisition, data analysis, and manuscript preparation. S.A., Z.Q., W.H. contributed to perform experiments and data validation. A.T., F.A., O.A. provided patient samples. M.A., K.I. participated in data collection and data analysis. All authors read and approved the final manuscript. Since only archival tissue specimens and retrospective patient data were used, the Research Advisory Council (RAC) of King Faisal Specialist Hospital and Research Center provided waiver of consent under project RAC # 2140 008.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bu, R., Siraj, A.K., Al-Rasheed, M. et al. Identification and characterization of ATM founder mutation in BRCA-negative breast cancer patients of Arab ethnicity. Sci Rep 13, 20924 (2023). https://doi.org/10.1038/s41598-023-48231-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48231-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.