Abstract
Breast cancer treatment leads to physical and psychological changes. The aim of this study was to analyze the incidence of sexual dysfunction and its risk factors in women diagnosed with breast cancer. This retrospective cohort study included women diagnosed and treated for breast cancer (exposed group, n = 90) and healthy women (non-exposed group, n = 93). Data were collected from February 2019 to October 2021 in the state of Rio Grande do Norte (Northeast Brazil), from medical records and using the Female Sexual Function Index (FSFI) questionnaire. Data were collected from medical records and using the Female Sexual Function Index (FSFI) questionnaire. Primary outcomes were analyzed using binary logistic regression. The Mann–Whitney test was used to analyze FSFI domains between groups. The exposed group had a 74% incidence of sexual dysfunction and 3.9 times increased chances of having sexual dysfunction compared with the non-exposed group (OR 3.9, CI 1.8 to 8.2, p < 0.001). Presence of comorbidities increased the chances of sexual dysfunction by 2.5 times (OR 2.5, CI 1.2 to 4.9, p = 0.009). Women diagnosed and treated for breast cancer had a higher incidence of sexual dysfunction than healthy women. Furthermore, comorbidities also increased the chances of sexual dysfunction regardless of exposure to breast cancer.
Similar content being viewed by others
Introduction
Breast cancer ranks first as the most common cancer among women and has high incidence and mortality in low- to high-income countries. In Brazil, 110,000 new cases are estimated for 20301. The prognosis for breast cancer is good when diagnosed early. For example, the 5-year relative survival rate in the United States Canada, and Australia is 90%, whereas in Brazil it is 72.9%1,2.
Common breast cancer treatments, including surgery, chemotherapy, radiotherapy, and hormone therapy, may result in induced menopause and decreased vaginal lubrication, affecting sexual excitement and desire3,4. Moreover, changes in body image5 may also impact sexual function, modifying femininity and sexual identity6. Sexual dysfunction is a disorder of sexual desire and encompasses psychophysiological changes in sexual desire, sexual excitement, orgasm, and satisfaction, leading to interpersonal difficulties and suffering7.
Cross-sectional studies conducted with healthy women indicate a 25% to 50% prevalence of sexual dysfunction.7,8,9,10,11,12 However, up to 75% of women diagnosed with breast cancer report persistent challenges in their sexual life due to the long-term effects of cancer treatment13,14,15,16.
Assessing sexual dysfunction contributes to understanding the experience of cancer diagnosis and its treatment, elucidating impacts on self-esteem and social participation17,18. Therefore, this study aimed to analyze the incidence of sexual dysfunction and its risk factors in women diagnosed and treated for breast cancer living in Northeast Brazil.
Methods
Study design
This study followed a retrospective cohort design19, conducted in accordance with the Strengthening the Reporting of Observational studies in Epidemiology. Groups were formed primarily by identifying the cohort highly exposed to the phenomenon of interest (i.e., breast cancer) and secondarily by selecting the comparison group (i.e., healthy women).
The exposed group consisted of women diagnosed with breast cancer (C-50) according to the International Classification of Diseases (ICD-10) and submitted to one or more oncological treatments (surgery, chemotherapy, radiotherapy, or hormone therapy) between 2013 and 2017 in Natal, Rio Grande do Norte, Brazil that were retrospectively identified and researched 12 months or up to 8 years after cancer diagnosis. The non-exposed group consisted of healthy women from the community.
For sample size calculation, a prevalence of 75% of sexual dysfunction was considered for the exposed group13,15 and 50% for the non-exposed group7,9. The relative risk was considered as 1.5, with a significance level of 5% and statistical power of 80%, resulting in 116 women in the study, 58 in each group19.
Inclusion and exclusion criteria
For the exposed group, inclusion criteria were women diagnosed with breast cancer for at least 12 months, submitted to cancer treatment, and undergoing clinical follow-up, and exclusion criteria were women with intellectual disabilities or unable to understand the questionnaires, who presented cancer recurrences, were under treatment or palliative care, who had sexual dysfunction before the breast cancer diagnosis, or who had debilitating or disabling morbidities not associated with breast cancer and its treatment. The non-exposed group consisted of healthy women without any type of cancer, ideally companions of the participants from the exposed group (i.e., friends, family members, and caregivers), and exclusion criteria were women with intellectual disabilities or unable to understand the questionnaires. Participants were matched by marital status and education level.
Data collection
Data were collected from February 2019 to October 2021 at a non-profit care units for patients with breast cancer (Liga Norte Riograndense Against Cancer, Natal, Rio Grande do Norte, Brazil) and via telephone due to the Covid-19 pandemic.
Data of the exposed group were collected using medical records and transferred to a digital form including sociodemographic information (name, age, body mass index, race, marital status, educational level, income according to Brazilian minimum wage in 2017, and access to health services); life habits (tobacco and alcohol consumption and physical activity frequency); gynecological and obstetric history (pregnancies, deliveries, abortions, breastfeeding, nulliparity, gynecological appointments and pap smear exam frequency, menopausal status, hormonal replacement, mammography, breast self-examination, and family and personal history of cancer); sexual activity (investigated with the questions “are you sexually active?” and “did you have sexual activity before treatment for breast cancer?”); comorbidities (hypertension, diabetes, arthritis, arthrosis, dyslipidemia, and depression); use of medications; and clinical variables (diagnosis, staging, type and duration of treatment, and postoperative complications).
Four trained researchers contacted the participants in-person (before Covid-19 pandemic) or via telephone (during Covid-19 pandemic). Similar data were collected from the non-exposed group, excluding information on cancer and its treatments.
Women from both groups were classified according to the presence or absence of sexual dysfunction based on the Female Sexual Function Index (FSFI) questionnaire. The FSFI was translated and validated for Brazilian Portuguese by Thiel et al. (2008)20. The questionnaire evaluates sexual functioning in the domains of desire, excitement or arousal, vaginal lubrication, orgasm, satisfaction, and pain. The score is based on a 6-point Likert scale where the total score is the sum of each domain multiplied by a correction value. A cutoff point of ≤ 26 was proposed by Wiegel et al. (2005) to classify the presence of sexual dysfunction. The FSFI presented high test–retest reliability and internal consistency21.
The study was approved by the committee for ethics in research with humans of the Liga Norte Riograndense Against Cancer (no 2.197.925). All participants signed the informed consent form, and for illiterate participants, we obtained informed consent from parents/or legal guardians.
Data analysis
The primary outcome encompassed sexual dysfunction and was analyzed as a dichotomous variable, showing a binomial distribution. We opted for a binary logistic regression also due to the retrospective characteristic of data. The significance level of independent variables was p ≤ 0.20 in the gross analysis and added to the final equation according to the theoretical model. The best-adjusted model was chosen using its significance and the lowest value of the Akaike Information Criterion (AIC). The Chi-square test assessed the independent variables for the primary outcome and the effect of measure was the odds ratio (OR). The non-parametric Mann–Whitney test and descriptive analysis were performed for analyzing the FSFI domains between groups. A significance level of 5% was considered in all analyses to minimize possible type I errors. Software SPSS version 20.0 was utilized for statistical analysis of data.
Ethical approval and consent to participate
The study was approved by the committee for ethics in research with humans of the Liga Norte Riograndense Against Cancer (no 2.197.925).
Results
Sample
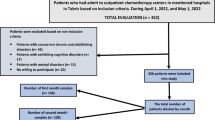
A total of 3645 new cases of breast cancer were registered from 2013 to 2017 (869 in 2013, 857 in 2014, 624 in 2015, 678 in 2016, and 617 in 2017). The first phase of this study encompassed collecting data from medical records of women under clinical follow-up to recruit for inclusion in the exposed group. Women were contacted by telephone and invited to participate in the study. Of 525 women, 386 were not included in the study. The remaining 139 women were interviewed, and 90 were included. For the non-exposed group, 164 women were invited to participate in the study, 98 were interviewed, and 93 were included. Reasons for not participating in the study are shown in Fig. 1.
Sociodemographic data, life habits, and gynecological, obstetric, and clinical history
Ninety women were included in the exposed group, whereas 93 healthy women composed the non-exposed group (Table 1). The sample was predominantly white or brown, married, and catholic, with complete high school or higher education, and had an income up to one or two to five minimum wages. Most participants performed physical activity and did not smoke or use alcohol. Also, the presence of comorbidities was high in both groups, and BMI indicated overweight in the non-exposed group and obesity in the exposed group. Most participants in the exposed group reported being in menopausal. Reports of good health and satisfaction with body image were frequent. Lastly, most participants denied depression diagnosis. Clinical data of the exposed group indicated that conservative surgery was the most prevalent as a loco-regional treatment, and adjuvant treatment was indicated.
The mean age for the first sexual intercourse was 20.7 (± 3.8) in the exposed group and 20.1 (± 3.9) in the non-exposed group. The number of pregnancies and deliveries was 2.6 (± 1.4) and 2.2 (± 1.1) in the exposed group and 1.9 (± 1.1) and 2.0 (± 3.2) in the non-exposed group, respectively.
Sexual function
Incidence of sexual dysfunction was 74% in the exposed group, and 39% in the non-exposed group. Gross analysis indicated that the exposed group had 4.8 (2.5 to 9.1) times the chances of sexual dysfunction than the non-exposed group. The chances of sexual dysfunction increased by 0.6% for each added year of age and 36% for each pregnancy. Moreover, the chances of sexual dysfunction increased in 2.9 (1.5 to 5.4) times with having comorbidities, 4.8 (1.2 to 18.5) times when participants self-perceived their health as poor or very poor, and 3.0 (1.2 to 7.4) times when smoking. Otherwise, menopause and income higher than one minimum wage reduced the sexual dysfunction by 0.71 and 0.55 times, respectively (Table 2).
Adjusted analysis indicated that cancer and comorbidities significantly increased by 3.9 (1.8 to 8.2) and 2.5 (1.2 to 4.9) times the chances of sexual dysfunction among the exposed group compared to the non-exposed group. We used a model that suggests a correlation between exposure to cancer and the development of sexual dysfunction. The model was adjusted considering menopause, self-perceived health, smoking status, pregnancy, and age variables. However, the final model considered only menopause and age (Table 2).
The non-exposed group scored higher in all domains of the FSFI, indicating better sexual functioning (Table 3).
Discussion
This study investigated sexual dysfunction in women diagnosed and treated for breast cancer. Our results showed a high incidence of sexual dysfunction among this population. We observed that exposure to breast cancer and comorbidities increased the chances of sexual dysfunctions, regardless of menopause and age. Moreover, the exposed group had a worse sexual function in all domains assessed by the Female Sexual Function Index. Our findings corroborate previous studies and highlight the importance of periodically assessing the sexual function of women diagnosed with breast cancer and under treatment22,23,24.
A recent systematic review showed an incidence of 30% to 80% of sexual dysfunction in women with cervical, gynecological, and breast cancer 22. Furthermore, regardless of the category of dysfunction, women had a 2.7 to 3.5 times greater risk of developing sexual dysfunction. Cervical, breast, and endometrial cancers were the most frequently associated with sexual dysfunction22. However, the anatomical location of the tumor is not the only cause of sexual dysfunction in women with cancer as it might also be induced by physiological changes from side effects of cancer treatments22. Reports of gynecological problems including decreased vaginal lubrication, sexual desire and arousal, and difficulty or inability to reach orgasm can result in sexual dysfunction22,23.
A meta-analysis of studies using the FSFI questionnaire conducted by Jing et al. (2019) also showed a higher incidence of sexual dysfunction in women with breast cancer (19.28; 95% CI 17.39–21.16) than in healthy women (FSFI > 26 normal sexual function)25. These results corroborate our findings, in which mean FSFI total scores in the exposed group were lower than the cutoff suggested by Wiegel et al. (2005), thus evidencing sexual dysfunction.
In this study, most women from the exposed group underwent conservative surgery, followed by chemotherapy and radiotherapy, and, less frequently, hormone therapy. Although treatment side effects might lead to sexual dysfunction, our study showed that exposure to cancer alone is sufficient to increase the risk for sexual dysfunction. This finding might be explained by the fact that psychological factors are involved in the etiology of sexual dysfunction26. Thus, cancer diagnosis and its related fears and apprehensions may affect sexual domains, including sexual desire, arousal, orgasm, and satisfaction.
Surgical cancer treatment may also change body image22,24 and prompt sexual dysfunction23. A prospective study conducted by Aerts et al. (2014) showed that women diagnosed with breast cancer had sexual dysfunction regardless of their surgical procedure (mastectomy or conservative). Moreover, symptoms in women undergoing mastectomy lasted longer (six months to one year after treatment completion) than in healthy women24. Mayer et al. (2018) also observed less sexual satisfaction and lower frequency of sexual activity, in addition to increased sexual discomfort, as reported by women with breast cancer23. These study results support our findings of increased chances of sexual dysfunction among the exposed group.
Sexual function is influenced by psychological and emotional factors, in addition to the endocrine (e.g., androgen, estrogen, progesterone, and prolactin), neuromusculoskeletal (e.g., pelvic floor muscles, fascias, and nerves), and vascular systems, each playing a part in different phases of sexual response25,27. The process of illness from cancer and its treatment can also lead to behavioral changes that influence sexual response, such as a reduction in the frequency of sexual activity23. Women in the exposed group had lower scores in sexual desire and lubrication and higher scores in sexual satisfaction and pain domains of the FSFI. Comparison between groups indicated better scores among the non-exposed group. Therefore, our findings support the hypothesis that breast cancer and its treatments may comprehensively interfere with the physiological function of body systems24.
The analysis of FSFI domains was also performed by Masjoudi et al. (2019)28 in a systematic review of studies including women with breast cancer. The authors showed that the lower scores referred to the sexual desire (2.8) and lubrication (2.8) domains, and the highest score referred to sexual satisfaction (5.09) domain, corroborating our findings. In a prospective longitudinal study, Jensen et al. (2003) observed reduced sexual desire, lubrication, altered vaginal morphology, and dyspareunia in women with cervical cancer compared with healthy women29. Women after mastectomy reported less sexual desire, arousal, and orgasm than before the surgery and showed more sexual dysfunction when compared with healthy women24.
The presence of comorbidities was analyzed in the final model of our study and appeared to increase the chances of sexual dysfunction. Almost half of our participants had chronic diseases, including hypertension, diabetes, arthritis, arthrosis, and dyslipidemia. Regardless of the cancer diagnosis, we observed that diabetes and hypertension were the main contributors to a higher risk of developing sexual dysfunction. Diabetes may lead to neurological impairments and vascular and psychological changes that may affect sexual desire, arousal, lubrication, orgasm and contribute to dyspareunia 30,31,32. Similarly, cardiovascular disease may favor atherosclerosis in the pelvic region arteries, reducing vaginal engorgement and contributing to clitoral erectile insufficiency syndrome, impairing lubrication, and diminishing sexual satisfaction33.
Menopause was not a significant variable in this study, but the literature cites as a relevant factor. Chemotherapy and hormone therapy, especially when administered in the pre-and perimenopause, contribute to hormonal changes that may trigger dryness and early vaginal atrophy and induce a sudden onset of menopause 34,35. More than half of the participants reported being in menopause. However, the stratified analysis indicated that most women in the exposed group were in menopause, which was not the case in the non-exposed group. This might explain the lack of significance of menopause in the final model.
In a prospective study, Abashe et al. (2009) observed that healthy women had better sexual function than women diagnosed with breast cancer who received hormone therapy. the latter group had better sexual function than women who received chemotherapy and radiotherapy36. Despite cancer and its treatment, the authors observed that menopause significantly reduced estrogen, negatively influencing mucosa vascularization, vaginal lubrication, and libido. This may trigger dyspareunia, lubrication dysfunction, and hypoactive desire throughout cancer treatment37.
We chose to add age in the adjusted model because of its reported importance on sexual dysfunction. Although our sample was between 45 and 50 years old, no interaction was observed between age and sexual dysfunction, which might be explained by the sample size. Among the general population, sexual dysfunction increases with age and is predominant in the reproductive phase38. Compared to healthy women, women exposed to cancer had lower sexual activity with increased age, suggesting a physiological effect of ageing35. Furthermore, young women with cancer showed a high incidence of sexual dysfunction related to body image and treatment type39.
One of the limitations of the study includes memory bias, which may have influenced the answers provided during the interviews. Also, we faced difficulties in accessing the participants by telephone due to the COVID-19 pandemic. Furthermore, patients were evaluated in different survival phases, both in the short survival phase (closer to the end of the oncological treatment) and in the long survival phase (5 years after diagnosis), which could modify the way the individual perceives the symptoms assessed. Therefore, these results should be interpreted with caution. Lastly, although we observed a considerable difference in the mean age between groups, this variable was added as an adjusted variable in the multivariate analysis and was insignificant in the final model.
This study has practical implications and highlights the importance of a multidisciplinary follow-up of women with sexual dysfunction. A clinical evaluation considering our findings may inform effective interventions. Moreover, early diagnosis of sexual dysfunction improves therapeutic outcomes. Our findings contribute to the body of evidence supporting the development of public policies for women with breast cancer in Brazil and countries with similar sociocultural and economic characteristics.
Conclusion
This study showed that women diagnosed and treated for breast cancer had a higher incidence of sexual dysfunction than healthy women. Therefore, breast cancer was a risk factor for sexual dysfunction. Comorbidities also increased the chances of sexual dysfunction regardless of exposure to breast cancer.
Data availability
The datasets produced and examined in this study are not publicly accessible due to patient privacy concerns. However, interested parties can obtain access to these datasets by contacting the corresponding author through reasonable requests.
Abbreviations
- FSFI:
-
Female sexual function index
- AIC:
-
Akaike information criterion
- OR:
-
Odds ratio
References
Ferlay, J., et al. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer. Accessed 02 Feb 2023. https://gco.iarc.fr/tomorrow.
Tao, Z. et al. Breast cancer: Epidemiology and etiology. Cell Biochem. Biophys. 72, 333–338 (2015).
Bartula, I. & Sherman, K. A. The Female Sexual Functioning Index (FSFI): Evaluation of acceptability, reliability, and validity in women with breast cancer. Support Care Cancer. 23(9), 2633–2641 (2015).
Silva, R. F., Conde, D. M., Freitas-Júnior, R. & Martinez, E. Z. Comparison of quality of life, satisfaction with surgery and shoulder-arm mordity in breast cancer survivors submitted to breast-conserving therapy or mastectomy followed by immediate breast reconstruction. Rev. Clinics. 65(8), 781–787 (2010).
Guedes, T. S. R. et al. Body image of women submitted to breast cancer treatment. Asian Pac. J. Cancer Prevent. 19, 1487–1493 (2018).
Bartula, I. & Sherman, K. A. Development and validation of the Female Sexual Function Index adaptation for breast cancer patients (FSFI-BC). Breast Cancer Res. Treat. 152, 477–488 (2015).
Rogers, R. C. et al. An international Urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int. Urogynecol. J. 29(5), 647–666. https://doi.org/10.1007/s00192-018-3603 (2018).
Erdős, C. et al. Female sexual dysfunction in association with sexual history, sexual abuse and satisfaction: A cross-sectional study in Hungary. J. Clin. Med. 12, 1112. https://doi.org/10.3390/jcm12031112 (2023).
Nobre, J. P. & Pinto-Gouveia, J. Dysfunctional sexual beliefs as vulnerability factors for sexual dysfunction Universidade de Coimbra. Portugal. J. Sex Res. 43, 68–75 (2006).
Cerentini, M. T. et al. Female sexual dysfunctions: Prevalence and related factors in a sample of young university women—A cross-sectional study. Sex. Relationsh. Ther. 1, 12. https://doi.org/10.1080/14681994.2020.1748592 (2020).
Starc, A., Jukić, T., Poljšak, B. & Dahmane, R. Female sexual function and dysfunction: A cross-national prevalence study in Slovenia. Acta Clin. Croat. 57, 52–60 (2018).
Abdo, C. H. N., Oliveira, W. M. Jr., Moreira, E. D. Jr. & Fittipaldi, J. A. S. Prevalência de disfunções sexuais e condições correlatas em uma amostra de mulheres brasileiras—Resultados do estudo brasileiro sobre comportamento sexual (BSSB). Int. J. Impot. Res. 16(2), 160–166. https://doi.org/10.1038/sj.ijir.3901198 (2004).
Fahami, F., Savabi, M. & Mohamadirizi, S. Relationship of sexual dysfunction and its associated factors in women with genital and breast cancers. Iranian J. Nurs. Midwifery Res. 20(4), 516–520 (2015).
Raggioa, G. A., Butryna, M. L., Arigoa, D., Mikorskia, R. & Palmer, S. C. Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol. Health. 29(6), 632–650. https://doi.org/10.1080/08870446.2013.879136 (2014).
Capodice, J. L. et al. Survey of the prevalence and severity of sexual dysfunction in breast cancer patients. ASCO Meet. Abstr. 26(15), 9557 (2008).
Bartula, I. & Sherman, K. A. Screening for sexual dysfunction in women diagnosed with breast cancer: Systematic review and recommendations. Breast Cancer Res. Treat. 141, 173–185 (2013).
Almeida, T. R., Guerra, M. R. & FiIgueiras, M. S. T. Repercussões do câncer de mama na imagem corporal da mulher: Uma revisão sistemática. Revista de Saúde Coletiva. 22(3), 1003–1029 (2012).
Gonçalves, T. O. et al. Instrumentos para avaliar a imagem corporal de mulheres com câncer de mama. Psicologia teoria e prática 14(2), 43–55 (2012).
Coeli, C. M., & Faerstein E. Estudos de Coorte of referencing in Epidemiologia. (ed. Atheneu) p. 241 (Medronho, 2009).
Thiel, R. R. C. et al. Translation into Portuguese, cross-national adaptation and validation of the Female Sexual Function Index. Rev. Bras. Ginecol. Obstet. 30(10), 504–510 (2008).
Wiegel, M., Meston, C. & Rosen, R. the female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. J. Sex Marital Ther. 31, 1–20 (2005).
Sousa, R. G. T. et al. Sexual dysfunction in women with cancer: A systematic review of longitudinal studies. Int. J. Environ. Res. Public Health. 19, 11921. https://doi.org/10.3390/ijerph191911921 (2022).
Mayer, S. et al. Sexual activity and quality of life in patients after treatment for breast and ovarian cancer. Arch. Gynecol. Obstet. 299, 191–201. https://doi.org/10.1007/s00404-018-4922-2 (2019).
Aerts, L., Christiaens, M., Enzlin, P., Neven, P. & Amant, F. Sexual functioning in women after mastectomy versus breast conserving therapy for early-stage breast cancer: A prospective controlled study. Breast 23, 629–636 (2014).
Jing, L., Zhang, C., Li, W., Jin, F. & Wang, A. Incidence and severity of sexual dysfunction among women with breast cancer: A meta-analysis based on female sexual function index. Support Care Cancer. https://doi.org/10.1007/s00520-019-04667-7 (2019).
McCabe, M. P. et al. Risk factors for sexual dysfunction among women and men: A consensus statement from the fourth international consultation on sexual medicine 2015. J. Sex Med. 13, 153–167. https://doi.org/10.1016/j.jsxm.2015.12.015 (2016).
Ferreira, A. L. C. G., Souza, A. I., Ardisson, C. L. & Katz, L. Female sexual dysfunctions. FEMINA. 35, 11 (2007).
Masjoudi, M., Keshavarz, Z., Akbari, M. E. & Kashani, F. L. Evaluation of sexual function in breast cancer survivors using female sexual function index: A systematic review. Int. J. Women’s Health Reprod. Sci. 7(4), 434–441. https://doi.org/10.15296/ijwhr.2019.73 (2019).
Jensen, P. T. et al. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 56, 937–949. https://doi.org/10.1016/S0360-3016(03)00362-6 (2003).
Wang, G. L., Wang, L., Wang, Y. L. & Li, M. L. Risk factors for sexual dysfunction among Chinese women with type 2 diabetes. Int. J. Diabetes Dev. Ctries. https://doi.org/10.1007/s13410-014-0241-8 (2014).
Lewis, R. W. et al. Definitions/epidemiology/risk factors for sexual dysfunction. J. Sex. Med. 7, 1598–1607. https://doi.org/10.1111/j.1743-6109.2010.01778.x (2010).
Fatemi, S. S. & Taghavi, S. M. Evaluation of sexual function in women with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 6, 38–39. https://doi.org/10.3132/dvdr.2009.07 (2009).
Steinke, E. E. Sexual dysfunction in women with cardiovascular disease what do we know?. J. Cardiovasc. Nurs. 25(2), 151–158 (2010).
Vargiu, V. et al. Hormone replacement therapy and cervical cancer: A systematic review of the literature. Climacteric. https://doi.org/10.1080/13697137.2020.1826426 (2020).
Soldera, S. V., Ennis, M., Lohmann, A. E. & Goodwin, P. J. Sexual health in long-term breast cancer survivors. Breast Cancer Res. Treat. 172, 159–166. https://doi.org/10.1007/s10549-018-4894-8 (2018).
Abasher, S. M. Sexual health issues in Sudanese women before and during hormonal treatment for breast cancer. Psychooncology. 18, 858–865. https://doi.org/10.1002/pon.1489 (2009).
Lett, C. et al. Is the age at menopause a cause of sexual dysfunction? A Brazilian population-based study. Menopause 25(1), 70–76. https://doi.org/10.1097/GME.0000000000000952 (2018).
Zobar, E. & Kahyaoğlu Süt, H. Relationships among Increasing age, sexual dysfunction, and sexual quality of life in married women of reproductive age. Bezmialem Sci. 9(4), 399–406. https://doi.org/10.14235/bas.galenos.2020.4537 (2021).
Qi, A. et al. Incidence and risk factors of sexual dysfunction in young breast cancer survivors. Ann. Palliat Med. 10(4), 4428–4434. https://doi.org/10.21037/apm-21-352 (2021).
Acknowledgements
Acknowledgment to the North Riograndense Against Cancer League (LNRC) for the collaboration and support for the development of the study, and to the research participants for their valuable time and contributions to this study.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. Dyego Leandro Bezerra de Souza thank CNPq (Brazilian National Council for Scientific and Technological Development) productivity grants 308168/2020-8.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.S.R.G., M.B.O.G.G., J.J.-R., and D.L.B.d.S; methodology, T.S.R.G., M.B.O.G.G., J.J.-R., and D.L.B.d.S.; data curation, T.S.R.G., R.C.S., J.B.V., E.R.M., V.L.C., A.A.G.D., statistical analysis; T.S.R.G., J.M.L., J.J.-R., and D.L.B.d.S.; writing—original draft preparation, T.S.R.G., M.B.O.G.G., and J.M.L. writing—review and editing, T.S.R.G., M.B.O.G.G., J.J.-R., and D.L.B.d.S.; supervision, J.J.-R. and D.L.B.d.S. All authors have read and agreed to the published version of the manuscript. All participants signed the informed consent form.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sousa Rodrigues Guedes, T., Barbosa Otoni Gonçalves Guedes, M., Mikael Lopes, J. et al. Sexual dysfunction in women with breast cancer of Northeast Brazil: a retrospective longitudinal study. Sci Rep 13, 20441 (2023). https://doi.org/10.1038/s41598-023-47684-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47684-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.