Abstract
Mucormycosis is a severe complication in critically ill COVID-19 patients. Throughout the pandemic, a notable prevalence of mucormycosis has been observed in the Indian population, whereas lower occurrences have been reported in Europe. However, limited data exist regarding its prevalence in Europe, which is potentially underestimated due to the low sensitivity of bronchoalveolar lavage (BAL) cultures. We aimed to evaluate the prevalence of mucormycosis in a high-risk critically ill COVID-19 population in the Netherlands, and to evaluate the potential benefit of adding Mucor PCR to BAL as part of routine follow-up. In this study, we included 1035 critically ill COVID-19 patients admitted to either one of the two ICUs at AmsterdamUMC between March 2020 and May 2022; of these, 374 had undergone at least one bronchoscopy. Following the AmsterdamUMC protocols, bronchoscopies were conducted weekly until clinical improvement was achieved. We cultured BAL fluid for fungi and used PCR and galactomannan testing to detect Aspergillus spp. Additionally, we retrospectively performed qPCR targeting Mucorales DNA in the BAL of 89 deceased patients. All cultures were negative for Mucorales, whereas 42 (11%) cultures were positive for Aspergillus. Furthermore, qPCR targeting Mucorales was negative in all 89 deceased patients. This study showed that pulmonary mucormycosis was not present in critically ill COVID-19 patients in two tertiary care ICUs. These results indicate routine Mucorales qPCR screening is not clinically necessary in a high-standard-of-care tertiary ICU in a low-endemic area.
Similar content being viewed by others
Introduction
Patients with critically ill Coronavirus disease 2019 (COVID-19) may be prone to fungal pulmonary co-infections, which are associated with increased morbidity and mortality1. Risk factors for fungal infections include an impaired immune response, treatment with glucocorticosteroids, old age, and diabetes mellitus. In addition to COVID-19 associated pulmonary aspergillosis (CAPA), a high prevalence of COVID-19-associated mucormycosis (CAM) was reported in the Indian population2,3. In Europe, case series have indicated a much lower prevalence, which, unlike Indian cases, predominantly includes pulmonary infections in critically ill patients4,5,6. However, CAM may be underdiagnosed due to restricted sampling of the lower respiratory tract and the lack of a broadly available biomarker7. However, recently quantitative polymerase chain reaction (qPCR) on bronchoalveolar lavage (BAL) fluid or serum targeting Mucorales was shown to improve diagnostic sensitivity8. As there is currently limited data on the prevalence of CAM in European ICUs, we investigated the frequency of CAM in a high-risk population of patients with severe COVID-19 using routine BAL cultures and qPCR.
Methods
This observational study was performed in 2 ICUs of the AmsterdamUMC in the Netherlands. Our patient cohort included all patients who had been admitted to the ICU with PCR-proven COVID-19 and had undergone at least one bronchoscopy with BAL during ICU admission. In the participating ICUs, bronchoscopy with BAL was routinely performed weekly, from endotracheal intubation until clinical improvement. Additional bronchoscopies were performed for clinical indications. All BAL fluid samples were cultured for the presence of fungi using Sabouraud dextrose agar (BD Franklin Lakes, NJ, USA; Stock-Keeping Unit 274720) supplemented with 50 mg/L chloramphenicol (Sigma, St-Louis, MO, USA; C-0378) and incubated at 30 and 37 °C. All molds were identified to the species level, using macroscopy, microscopy, and MaldiToF (Bruker Daltonik, Bremen, Germany). In addition, a subpopulation of deceased COVID-19 patients was retrospectively investigated for the presence of CAM using qPCR targeting Mucorales DNA. Of these patients, fungal DNA was extracted from the last available BAL fluid collected within two weeks before death. The BAL samples were stored at − 80 °C, with a maximum storage duration of two and a half years. DNA was extracted using the MagNAPure 96 (Roche Diagnostics, Basel, Switzerland). 300 µL lysis buffer (Roche, MagNA Pure LC Total Nucleic Acid Isolation Kit 03 246 779 001) and 20 µL of internal amplification control (in-house IAC plasmid) were added to the sample. 5 µL of eluate was added to the Mucorales qPCR. This PCR was performed using a commercial CE-IVD assay (MucorGenius®; Pathonostics, Maastricht, The Netherlands) according to the manufacturer’s instructions, with an internal amplification control, a negative control, and a positive control. The negative extraction control consisted of 300 µL of PBS, 300 µL of lysis buffer, and 20 µL of internal amplification control (in-house IAC plasmid), of which 50 µL was added to the MucorGenius®.
Additionally, data on other fungal infections (culture, galactomannan, and PCR), antifungal therapy, and glucocorticosteroid use were collected from all patients. In accordance with the 2020 ECMM/ISHAM consensus, patients presenting a positive culture, galactomannan, or PCR result in their BAL samples were categorized as having probable CAPA. If Aspergillus spp. was identified during the autopsy, patients were classified as proven CAPA9.
All patients were admitted to dedicated COVID-19 ICUs. Each patient was accommodated either alone in a room or with one other patient, and the rooms were not equipped with self-closing doors. The patient rooms were maintained at a negative pressure. Although HEPA filters were recommended, they were found to be impractical. Therefore, UF 9 air filters were employed instead. In addition, the air in the rooms was changed six times every hour. The study protocol and all study procedures were approved by the Review Committee of the AmsterdamUMC Biobank (reference 2020-065). Written informed consent was obtained from all patients or their legal representatives. This study was conducted in adherence to Good Clinical Practice guidelines and the Declaration of Helsinki.
Results
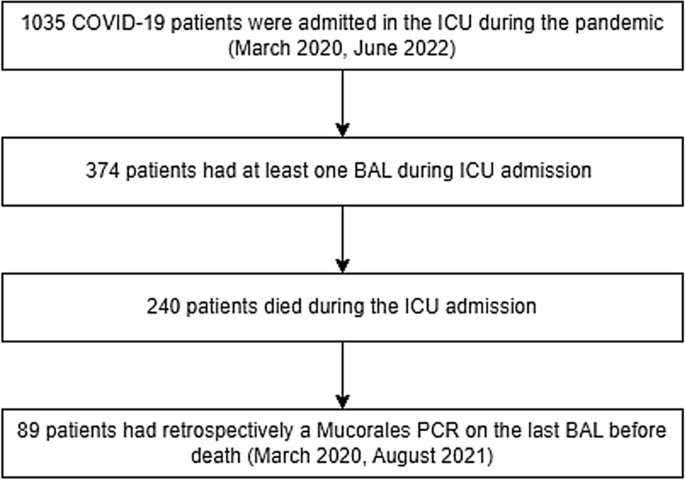
From 06-03-2020 until 27-05-2022, 1035 patients with severe COVID-19 were admitted to one of the participating ICUs. Among these patients, 374 received at least one BAL. The fungal cultures were positive for Aspergillus species in 42 (11%) patients, while all cultures were negative for Mucorales.
Among the 374 patients, 89 (24%) deceased between March 2020 and August 2021 and had undergone a bronchoscopy within 2 weeks prior to death, allowing for retrospective testing with a PCR targeting Mucorales. All 89 patients had died due to respiratory failure. The median time from ICU admission to death was 19 days [15, 30]. At the time of the last BAL, 40 patients (59%) had a PaO2 to FiO2 ratio of < 100 mmHg. The last bronchoscopy was performed at a median of 5 days [4, 9] before death. 85 of 89 patients (96%) were treated with steroids during admission, the median dose per day was 42.6 mg [24.5–57.7] (prednisone equivalents), and antifungal therapy consisting of voriconazole and anidulafungin was initiated in 26 patients (29%) (Table 1). Mucorales PCR performed on BAL was negative for all 89 patients. However, Aspergillus spp. were detected in the BAL of 18 patients (18%). These patients were classified as probable CAPA based on positive BAL culture (3 patients), positive BAL Aspergillus PCR (11 patients), and positive BAL-galactomannan (7 patients)9. No other fungal infections were observed. An Autopsy was performed on 12 patients, all of whom had no mycological evidence of invasive fungal disease prior to death. At autopsy, no histopathological evidence of invasive fungal disease was observed in these patients. No significant differences were observed between patients with probable CAPA and non-CAPA patients in terms of admission characteristics, comorbidities, and clinical outcomes.
Discussion
In our cohort of 374 patients, having received at least one BAL, no cases of CAM were identified, corresponding to a prevalence of 0% (95% CI: 0–0.010%, Fig. 1). Our cohort included patients that had undergone routine weekly bronchoscopy. Furthermore, in addition to BAL fungal culture, we used Mucorales PCR in a subgroup of patients who died due to respiratory failure. This approach limits the possibility of missing CAM cases; however still, no cases were identified. Risk factors for CAM include diabetes mellitus and corticosteroid use, which were also predominant in our subgroup (Table 1). Furthermore, the CAPA incidence of 18% indicates that our cohort with critically ill COVID-19 was prone to developing secondary fungal infections10.
A case series from European countries, including the Netherlands, indicated that CAM may occur in COVID-19 patients, but an accurate estimate of the prevalence could not be given as a denominator is unknown4,5,6. The baseline epidemiology of mucormycosis is not well studied, but a population-based study from France indicated an incidence of 0.09 cases per 100,000 population per year, prior to the corona pandemic11. For the ICU population, no frequency estimates are available for mucormycosis12; however, in critically ill patients with COVID-19, a CAM prevalence of 1% has been reported4. Our study confirms a very low prevalence of CAM in the ICU despite the presence of risk factors for fungal infections in the majority of patients and intensive sampling.
Strengths of this study were the intensive sampling and the additional use of qPCR, which improved the diagnostic value of culture alone. Recovery of Mucorales through the culture of clinical specimens may be difficult, and the use of qPCR improves diagnostic sensitivity8. Nonetheless, CAM cases were not observed. A limitation of this study was that serum samples were not available for qPCR testing13. However, all patients died primarily because of respiratory failure; therefore, the lungs were expected to be the primary site for CAM. Another limitation is the lack of autopsies in the group of 89 patients receiving a qPCR.
In conclusion, this study showed that pulmonary CAM was not present in critically ill COVID-19 patients in two tertiary care ICUs. These results indicate that there is no clinical need for routine screening with qPCR targeting Mucorales in a high-standard-of-care tertiary ICU in a low-endemic area.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Garcia-Vidal, C. et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 27(1), 83–88 (2021).
Hoenigl, M. et al. The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe 3(7), e543–e552 (2022).
Satija, A. et al. Satellite epidemic of Covid-19 associated mucormycosis in India: A multi-site observational study. Mycopathologia 188, 745 (2023).
Gangneux, J. P. et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir. Med. 10(2), 180–190 (2022).
Seidel, D. et al. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses 65(1), 103–109 (2022).
Buil, J. B. et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Euro Surveill. 26(23), 144 (2021).
Walsh, T. J., Gamaletsou, M. N., McGinnis, M. R., Hayden, R. T. & Kontoyiannis, D. P. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin. Infect. Dis. 54(Suppl 1), S55-60 (2012).
Scherer, E. et al. Quantitative PCR (qPCR) detection of mucorales DNA in bronchoalveolar lavage fluid to diagnose pulmonary mucormycosis. J. Clin. Microbiol. 56, 8 (2018).
Koehler, P. et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 21(6), e149–e162 (2021).
Chong, W. H. & Neu, K. P. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): A systematic review. J. Hosp. Infect. 113, 115–129 (2021).
Bitar, D. et al. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg. Infect. Dis. 20(7), 1149–1155 (2014).
Bassetti, M. & Bouza, E. Invasive mould infections in the ICU setting: Complexities and solutions. J. Antimicrob. Chemother. 72(1), i39–i47 (2017).
Millon, L. et al. Evaluation of serum mucorales polymerase chain reaction (PCR) for the diagnosis of mucormycoses: The MODIMUCOR prospective trial. Clin. Infect. Dis. 75(5), 777–785 (2022).
Author information
Authors and Affiliations
Consortia
Contributions
J.S. and K.D. had full access to all data and guarantee data integrity. J.S., K.D., L.H., P.V. were responsible for the trial design. Data collection was done by J.S. Data management was performed by J.S. and K.D. Statistical analyses, interpretation of the data and drafting of the manuscript were done by J.S., K.D., L.H., P.V., J.S., K.D., L.H., P.V. revised the manuscript and were responsible for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schippers, J.R., Verweij, P.E., Heunks, L.M.A. et al. Absence of COVID-19 associated mucormycosis in a tertiary intensive care unit in the Netherlands. Sci Rep 13, 22134 (2023). https://doi.org/10.1038/s41598-023-47231-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47231-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.