Abstract
Multidrug-resistant tuberculosis (MDR-TB) is a major health threat worldwide, causing a significant economic burden to patients and their families. Due to the longer duration of treatment and expensive second-line medicine, the economic burden of MDR-TB is assumed to be higher than drug-susceptible TB. However, the costs associated with MDR-TB are yet to be comprehensively quantified. We conducted this systematic review and meta-analysis to determine the global burden of catastrophic costs associated with MDR-TB on patients and their households. We systematically searched five databases (CINHAL, MEDLINE, Embase, Scopus, and Web of Science) from inception to 2 September 2022 for studies reporting catastrophic costs on patients and affected families of MDR-TB. The primary outcome of our study was the proportion of patients and households with catastrophic costs. Costs were considered catastrophic when a patient spends 20% or more of their annual household income on their MDR-TB diagnosis and care. The pooled proportion of catastrophic cost was determined using a random-effects meta-analysis. Publication bias was assessed using visualization of the funnel plots and the Egger regression test. Heterogeneity was assessed using I2, and sub-group analysis was conducted using study covariates as stratification variables. Finally, we used the Preferred Reporting Items for Reporting Systematic Review and Meta-Analysis-20 (PRISMA-20). The research protocol was registered in PROSPERO (CRD42021250909). Our search identified 6635 studies, of which 11 were included after the screening. MDR-TB patients incurred total costs ranging from $USD 650 to $USD 8266 during treatment. The mean direct cost and indirect cost incurred by MDR-TB patients were $USD 1936.25 (SD ± $USD 1897.03) and $USD 1200.35 (SD ± $USD 489.76), respectively. The overall burden of catastrophic cost among MDR-TB patients and households was 81.58% (95% Confidence Interval (CI) 74.13–89.04%). The catastrophic costs incurred by MDR-TB patients were significantly higher than previously reported for DS-TB patients. MDR-TB patients incurred more expenditure for direct costs than indirect costs. Social protection and financial support for patients and affected families are needed to mitigate the catastrophic economic consequences of MDR-TB.
Similar content being viewed by others
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is a major health threat globally, affecting nearly half a million people in 20201. MDR-TB disproportionately affects the most vulnerable populations in low- and middle-income countries2,3. Ending catastrophic costs in TB-affected households is one of the targets of the World Health Organization (WHO) end-TB strategy4. However, the widespread dissemination of MDR- and Extensively drug-resistant (XDR)-TB continues to be a significant obstacle to achieving these ambitious targets5.
The WHO defines catastrophic TB costs as total (direct and indirect) costs of TB diagnosis and care above 20% of the household’s annual income6. Direct cost includes medical (registration, consultation, hospitalization, investigation, or medication) and non-medical (food, travel, and nutritional supplements). Indirect costs include overall loss of wages due to productivity loss, missed work, loss of time, loss of income, and caregiving work7. In times of financial hardship, TB patients or TB-affected households implement different coping strategies, such as selling household assets, borrowing money, utilizing savings, and taking children out of school8.
Due to the longer duration of treatment and the need for expensive second-line medications, the catastrophic costs associated with MDR-TB are thought to be higher than the catastrophic costs associated with drug-susceptible TB (DS-TB)9. The economic consequences of MDR-TB are often so severe that patients and affected families can fall into extreme poverty because of high out-of-pocket expenses and income lost during MDR-TB diagnosis and treatment10,11. Studies have shown that MDR-TB patients face considerable financial losses before, during, and after treatment12,13. For instance, a systematic review conducted in several countries showed that the cost of treatment among MDR-TB patients ranges from 2423 United States Dollars (USD) in Peru to USD 14,657 in Tomsk, Russia14.
The burden of catastrophic cost among MDR-TB patients is affected by different factors, including sociodemographic factors, diagnosis delay, length of hospital stay, household wealth status, distance from the health facilities, number of household members, health care setting (private vs public), pre-TB expenditures, and hospitalizations15,16. A systematic review conducted among TB patients (DS- and DR-TB combined) showed that the proportion of catastrophic costs was 43%17. However, the burden of catastrophic costs among MDR-TB patients is expected to be higher because of the longer duration of treatment needed and the high cost of MDR-TB medicines compared with DS-TB patients18. However, the costs associated with MDR-TB are yet to be comprehensively quantified. We conducted this systematic review and meta-analysis to determine the global burden of catastrophic costs associated with MDR-TB on patients and their households.
Methods
This protocol is reported following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines19, which are detailed in supplementary file S1. The protocol is published in the PROSPERO, with a registration number CRD42021250909.
Searching strategy and eligibility criteria
Systematic searches from five databases (CINAHL (EBSCO), MEDLINE (via Ovid), Embase, Scopus, and Web of Science) were conducted to retrieve relevant studies. We also searched the grey literature using Google and Google Scholar. Reference lists of included studies were searched for additional relevant articles. The search terms included “multidrug-resistant tuberculosis” (multidrug-resistant tuberculosis, MDR-TB), “extensively drug-resistant tuberculosis” (extensively drug-resistant tuberculosis, XDR-TB), and “cost” (cost(s), loss, sales, loan, economics, finance, expense, expenditure, payment, and impoverishment). The search strategy for all databases is provided in supplementary table 2. The search was performed from the inception of each database to 2 September 2022.
Study selection criteria
All potential publications reporting direct or indirect costs incurred by MDR-TB patients and their families and reporting percentages of catastrophic costs were included. Direct costs include direct medical costs (hospitalization, investigation, medication, and registration/consultation) and direct nonmedical costs (food, travel, and special diet). Indirect costs include income loss due to MDR-TB patients and caregivers absent from their jobs20. We excluded conference abstracts, animal studies, case studies, reviews, studies that report only on DS-TB, and studies that did not provide data on catastrophic costs. We also excluded studies that reported only medication cost, hospitalization cost, investigation cost, and cost data from facilities, program, and health system perspectives. However, there were no restrictions to non-English language articles. Google Translate was used to translate non-English language articles.
Outcomes of the study
The primary outcome was the proportion of patients with MDR-TB or their households who incurred catastrophic costs. The secondary outcomes of the review were the proportion of the total costs that were direct costs of MDR-TB treatment among MDR-TB patients and the proportion of individuals resorting to different coping mechanisms. Coping mechanisms include borrowing money, selling assets, and utilization of savings. All costs were reported in USD.
Study selection, data extraction, and quality assessment
After the search, all identified articles were imported into an EndNote version 7 (Thomson Reuters, London) library. Then, duplicated citations were identified and removed. After removing duplication, citations were exported to Rayyan software (Rayyan, Cambridge) for further screening by title and abstract. Two independent reviewers (TYA and HFW) screened the titles and abstracts and reviewed the full text to identify relevant studies. When there were differences between the two authors, a decision was made by consensus. Data were extracted from included studies using a Microsoft Excel (version 2014) spreadsheet on the following information: (1) study characteristics including the name of the first author, year of publication, country of the study, study setting (urban vs rural), and study design, (2) participant's characteristics such as study population (i.e. MDR-TB, XDR-TB, or both), mean or median age, the proportion of male, and sample size; (3) clinical characteristics such as type of MDR-TB medications, duration of treatments, and comorbidity (HIV, diabetes Mellitus); and (4) outcomes of the study, including catastrophic cost and disaggregation of costs. The Newcastle Ottawa Scale (NOS) was used to assess the quality of included studies. The NOS judges the quality of observational studies with three basic perspectives: selection of study groups (4 points), comparability of groups (2 points), and ascertainment of the outcome of interest (3 points). The overall score ranges from zero to nine, with low (0–4), medium (5–7), and high (8–9) quality groupings21,22.
Data synthesis
The choice of a fixed-effect or random-effect meta-analysis model is determined by the presence of heterogeneity. In our case, we used a random-effects meta-analysis to determine the proportions of catastrophic costs among MDR-TB patients due to a significant level of heterogeneity among the included studies with I2 value > 90%. The random-effects meta-analysis assumes the effect size may vary among studies, and it also addresses both the intra-study variation (sampling error) and inter-study variation.
The proportion of direct and indirect costs was reported. The pooled proportion of catastrophic costs was determined using a forest plot. The presence of heterogeneity between included studies was assessed using Cochran’s Q test and measured quantitatively by the index of heterogeneity squared (I2), with 95% Confidence Interval (CI)23. The level of heterogeneity was classified as low (I2 = < 25%), moderate (I2 = between 25 and 75%) or high (I2 = > 75%). In the presence of heterogeneity, sub-group analysis was conducted to assess the risk of catastrophic costs among groups having adequate and comparable data. The country’s income, study design, year of data collection, type of study population, HIV status, and data collection tool were used for sub-group analysis. Publication bias was reported using visualization of the funnel plot and the Egger regression test24. STATA version 17 software was used to conduct the analysis. All costs were initially presented in US dollars. Sensitivity analysis was conducted by removing low-quality studies.
Role of funder
The study was funded by the Australian National Health and Medical Research Council through an Emerging Leadership Investigator grant and a Curtin University Higher Degree Research scholarship. The funders had no role in study design, data collection, analysis, interpretation, or report writing. The corresponding author had full access to all data in the study. ACAC and KAA were ultimately responsible for the decision to submit for publication.
Ethics approval
Since we used publicly available published data, ethical approval was not required.
Results
Characteristics of included studies
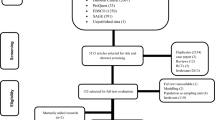
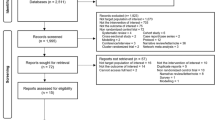
The search strategy identified 6635 potential studies (6566 from electronic databases and 69 using additional records) for evaluation. After duplicates were removed, 3590 citations were screened by title and abstract, and 98 eligible articles had a full-text review. In total, 11 articles16,25,26,27,28,29,30,31,32,33,34 that provided data on 817 MDR-TB patients were included in the meta-analysis. The overall study selection process and reasons for exclusion are presented in Fig. 1.
The included studies were conducted in 11 different countries (seven studies from Low-Middle Income Countries (LMICs), three studies were from Low-Income Countries (LICs) countries, and one study was from an Upper Middle-Income Countries (UMICs)), and the data were collected from 2002 to 2019 (Table 1). The monthly income of households was reported in three studies, and the value ranged from $USD 154.47 in Ghana to $USD 418 in Peru. Self-prepared tools were used to measure catastrophic costs in two included studies, and the other nine used the WHO TB cost survey tool (Table 1).
Cost of MDR-TB treatment
Nine studies16,25,26,27,28,29,30,32,33 reported a detailed cost breakdown for treating MDR-TB. From the nine studies, five studies25,26,27,29,33 reported cost breakdowns for direct and indirect medical costs. The mean direct cost incurred by MDR-TB patients was $USD 1,936.25 (SD ± $USD 1897.03). The mean indirect cost incurred for the treatment of MDR-TB was $USD 1,200.35 (SD ± $USD 489.76). The mean total payment incurred by MDR-TB patients was $USD 3,151.19 (SD ± $USD 2379.15). The direct (medical and non-medical) and indirect costs are summarized in Table 2 and Fig. 2.
Catastrophic costs among MDR-TB patients
All the studies define catastrophic cost at a 20% cut-off point. Based on this definition, the overall pooled percentages of catastrophic costs among MDR-TB patients and households were 81.58% (95% CI 74.13–89.04%) with I2 of 90.0%, indicating considerable heterogeneity of effect estimates between studies (Fig. 3). The proportion of catastrophic costs among MDR-TB patients ranged from 53.76% in Peru to 98.28% in Viet Nam.
Sub-group analysis
The burden of catastrophic costs among MDR-TB patients varied by country income level, with the highest burden of catastrophic costs reported in LMICs (85.18%; 95% CI 76.81–93.55) and the lowest burden reported in UMICs (70.66%; 95% CI 38.13–103.18) with I2 = 96.9%, p = 0.000. The burden of catastrophic cost also varied by HIV status, in which catastrophic cost was significantly higher in studies with a higher prevalence (i.e., above the median value of 50%) of HIV (86.75%; 95% CI 79.16–94.35%) than in studies with a lower HIV prevalence (84.52%; 95% CI; 73.81–95.23) with I2 = 82%, p = 0.000 (Table 3).
Coping mechanisms
Three studies25,29,31 reported different coping mechanisms to alleviate out-of-pocket expenses during MDR-TB treatment. The most used coping mechanisms were borrowing, selling assets, and utilizing savings. The remaining eight studies did not report any forms of coping mechanisms.
Quality and publication bias assessment
The quality of included studies ranged from low to high, with a median score of 4 points and a maximum score of 8 points. Of the included 11 studies, there is no study, regarded as a good-quality study. Five studies had a score of five to seven points, regarded as moderate-quality studies, and the remaining six studies had a score of less than five points, regarded as low-quality studies (Supplementary file 3). There was evidence of publication bias in the statistical and visual appraisal of funnel plots (Fig. 4).
Sensitivity analysis
The burden of catastrophic cost after removing low-quality studies was 77.45% (95% CI 66.28–88.62) with I2 = 87.1%, p = 0.000 (Fig. 5). However, the finding showed no difference from the original that includes all studies (81.58%; 95% CI 74.13–89.05).
Discussion
To our knowledge, this systematic review and meta-analysis is the most comprehensive data synthesis reported to date, presenting the economic burden of MDR-TB faced by patients and affected households at a global level. Our systematic review identified only 11 eligible studies reporting the economic consequences of MDR-TB, including 817 MDR-TB patients with available data. This finding indicates that studies quantifying the economic impacts of MDR-TB remain inadequate despite the growing burden of MDR-TB, and more data are urgently needed to inform options for mitigating the economic consequences of MDR-TB on patients and their families. The proportion of catastrophic cost, as measured by the proportion of patients with total costs exceeding 20% of their annual household income, was almost double in MDR-TB (81.58%) than in DS-TB (43%) patients17. This finding is related to the fact that a longer duration of treatment is required for the management of MDR-TB (9 months to 2 years) than DS-TB (6 months)6,35. Moreover, the treatment options for MDR-TB are very limited and expensive, and sometimes recommended medicines are not easily available, particularly in low- and middle-income countries36,37. MDR-TB patients also experience many adverse effects from the second-line medications required for the treatment of MDR-TB, and these adverse effects sometimes prolong hospital stays and incur additional medical expenses38. The stigma associated with MDR-TB is also higher than the stigma associated with DS-TB39, which sometimes leads patients to job loss38. Despite basic MDR-TB services being provided free of charge, this has not been sufficient in mitigating the economic consequences of MDR-TB in Brazil, which showed that 18% of MDR-TB patients are exposed to financial hardship40. Despite MDR-TB medications being available in public sectors free of charge, a study done in ten high-burden MDR-TB countries showed that 4.8 out of 17 second-line anti-TB recommended drugs are not available through the public health care system41. Therefore, patients are obliged to get treatment from the private sector, where TB medications are not available free of charge. A study conducted in India also showed that 70% of MDR-TB patients preferred treatment in private facilities, in which TB medicines are not available free of charge, and the cost of the drugs is the major treatment expense (37%) among MDR-TB patients42. However, detailed information about which drugs and investigations are freely provided in the public sector and whether the study was conducted in public or private health facilities were not contained among the included studies.
Our systematic review and meta-analysis showed that the direct costs incurred for treating MDR-TB patients were higher than the indirect costs. This finding is inconsistent with a systematic review conducted among DS-TB patients, in which only 15% of the total cost was incurred from direct costs17. Another systematic review and meta-analysis among DS-TB patients conducted in LICs and LMICs countries revealed that direct cost covers only 40% of the total costs43. This could be because the component of the direct costs among MDR-TB patients is significantly affected by the longer duration of the treatment44. The second possible reason could be due to longer hospitalization, multiple visits to physicians for diagnosis and investigation, purchasing medications, and looking for a special diet in MDR-TB patients45. To cover these expenses, MDR-TB patients used different coping mechanisms, including selling assets, borrowing money, and taking children out of school.
By quantifying the burden of catastrophic costs, the current systematic review and meta-analysis provided important information crucial for patients, policymakers, and researchers for designing appropriate strategies to achieve the WHO End-TB Strategy targets of no TB patients or their households facing catastrophic costs. Several interventions can be applied to eliminate catastrophic costs associated with MDR-TB, including early diagnosis and treatment initiation, creating awareness of the availability of free diagnosis and treatment for MDR-TB at public health facilities, and providing social protection and financial support such as nutritional supplementation, comprehensive health cover, and reimbursement for diagnosis, medication, hospitalization, and transportation costs43,46,47,48. Ensuring the implementation of universal health coverage at all levels is also critical to minimize direct and indirect medical costs and to avoid economic consequences on MDR-TB patients and their families6,49,50.
This study has several limitations. First, there needed to be more evidence available for some high MDR-TB burden countries such as Bangladesh, India, Myanmar, Nigeria, Pakistan, Russian Federation, and South Africa, which, together, account for more than two-thirds of the global MDR-TB burden. Second, high heterogeneity among the included studies might affect the interpretation of overall estimates. To overcome this limitation and investigate the source of heterogeneity, we conducted a sub-group analysis. Still, we could not run meta-regression due to the few studies included. Third, factors contributing to catastrophic costs were not assessed in this systematic review as different studies reported different risk factors. Fourth, although most of the included studies used a standard WHO tool, some studies used their tools to measure catastrophic costs, which might over or underestimate the proportion of catastrophic costs. Fifth, the burden of catastrophic cost after MDR-TB treatment completion and patients with XDR-TB were not included in the analysis due to a lack of data, which requires further investigation. As most of the studies didn’t report the MDR-TB treatment administered, it could not limit studies that include patients treated with short-term regimens, which are more relevant in recent times. Lastly, the presence of publication bias may have impacted the representativeness of the findings.
Conclusion
The burden of catastrophic costs was significantly higher among MDR-TB patients than previously reported for DS-TB patients. MDR-TB patients incurred more expenditure for direct costs than indirect costs. Social protection and financial support for patients and affected families are needed to mitigate the catastrophic consequences of MDR-TB. Finally, we recommend that researchers conduct further studies on the cost-effectiveness of home-based care management of MDR-TB patients and its effect on treatment adherence.
Data availability
Data will be available upon request from the corresponding author. All the data supporting the findings of this study are available in the table and figures.
Abbreviations
- CI:
-
Confidence Interval
- DR-TB:
-
Drug-resistant Tuberculosis
- DS-TB:
-
Drug Susceptible Tuberculosis
- HICs:
-
High-Income Countries
- HIV:
-
Human Immune Deficiency Virus
- LICs:
-
Lower Income Countries
- LMICs:
-
Lower Middle Income
- MDR-TB:
-
Multi-Drug Resistant Tuberculosis
- NOS:
-
Newcastle Ottawa Scale
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- RR:
-
Rifampicin Resistant
- TB:
-
Tuberculosis
- UMICs:
-
Upper Middle-Income Countries
- WHO:
-
World Health Organization
- XDR:
-
Extensively Drug-Resistant Tuberculosis
References
World Health O. Global Tuberculosis Report 2020 2020 (World Health Organization, 2020).
Glaziou, P., Sismanidis, C., Floyd, K. & Raviglione, M. Global epidemiology of tuberculosis. Cold Spring Harb. Perspect. Med. 5(2), a017798 (2015).
Tanimura, T., Jaramillo, E., Weil, D., Raviglione, M. & Lönnroth, K. Financial burden for tuberculosis patients in low- and middle-income countries: A systematic review. Eur. Respir. J. 43(6), 1763–1775 (2014).
Kairu, A. et al. Cost of TB services in healthcare facilities in Kenya. Int. J. Tuberc. Lung Dis. 25(12), 1028–1034 (2021).
WHO. Global Tuberculosis Control Surveillance, planning, financing. Geneva: WHO Press; 2008.
World Health Organization. End TB strategy. WHO/HTM/TB/2015.19. Geneva: World Health Organization; 2015.
Lönnroth, K. et al. Beyond UHC: Monitoring health and social protection coverage in the context of tuberculosis care and prevention. PLoS Med. 11(9), e1001693 (2014).
Madan, J. et al. What can dissaving tell us about catastrophic costs? Linear and logistic regression analysis of the relationship between patient costs and financial coping strategies adopted by tuberculosis patients in Bangladesh, Tanzania, and Bangalore, India. BMC Health Serv. Res. 15(1), 1–8 (2015).
Jang, J. G. & Chung, J. H. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam Univ. J. Med. 37(4), 277–285 (2020).
Morris, M. D. et al. Social, economic, and psychological impacts of MDR-TB treatment in Tijuana, Mexico: A patient’s perspective. Int. J. Tuberc. Lung. Dis. 17(7), 954–960 (2013).
van den Hof, S. et al. The socioeconomic impact of multidrug-resistant tuberculosis on patients: Results from Ethiopia, Indonesia, and Kazakhstan. BMC Infect Dis. 16(1), 470 (2016).
Lim, V. W. et al. Cross-sectional study of prevalence and risk factors, and a cost-effectiveness evaluation of screening and preventive treatment strategies for latent tuberculosis among migrants in Singapore. BMJ Open. 11(7), e050629 (2021).
Daftary, A. et al. Dynamic needs and challenges of people with drug-resistant tuberculosis and HIV in South Africa: A qualitative study. Lancet Global Health. 9(4), e479–e488 (2021).
Fitzpatrick, C. & Floyd, K. A systematic review of the cost and cost effectiveness of treatment for multidrug-resistant tuberculosis. Pharmacoeconomics 30, 63–80 (2012).
Wang, Y. et al. Household financial burden among multidrug-resistant tuberculosis patients in Guizhou province, China: A cross-sectional study. Medicine (Baltimore). 99(28), e21023 (2020).
Wang Y, McNeil EB, Huang ZF, Chen L, Lu XL, Wang CQ, et al. Household financial burden among multidrug-resistant tuberculosis patients in Guizhou province, China A cross-sectional study. Medicine. 2020;99(28).
Ghazy, R. M. et al. A systematic review and meta-analysis of the catastrophic costs incurred by tuberculosis patients. Sci. Rep. 12(1), 558 (2022).
Jang, J. G. & Chung, J. H. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam Univ J Med. 37(4), 277–285 (2020).
Moher, D. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4(1), 1 (2015).
Wang Y, McNeil EB, Huang Z, Chen L, Lu X, Wang C, et al. Household financial burden among multidrug-resistant tuberculosis patients in Guizhou province, China: A cross-sectional study. Medicine. 2020;99(28).
Cook, D. A. & Reed, D. A. Appraising the quality of medical education research methods: The medical education research study quality instrument and the Newcastle-Ottawa scale-education. Acad. Med. 90(8), 1067–1076 (2015).
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med. 21(11), 1539–1558 (2002).
Ioannidis, J. P., Stanley, T. D. & Doucouliagos, H. The power of bias in economics research (Oxford University Press, 2017).
Chittamany, P. et al. First national tuberculosis patient cost survey in Lao People’s Democratic Republic: Assessment of the financial burden faced by TB-affected households and the comparisons by drug-resistance and HIV status. PLoS ONE. 15(11), e0241862 (2020).
Fuady, A., Houweling, T. A. J., Mansyur, M. & Richardus, J. H. Catastrophic total costs in tuberculosis-affected households and their determinants since Indonesia’s implementation of universal health coverage. Infect. Dis. Poverty 7(1), 3 (2018).
Kaswa, M. et al. The economic burden of TB-affected households in DR congo. Int. J. Tuberc. Lung Dis. 25(11), 923–932 (2021).
Kilale, A. M. et al. Economic burden of tuberculosis in Tanzania: A national survey of costs faced by tuberculosis-affected households. BMC Public Health. 22(1), 600 (2022).
Muttamba, W. et al. Households experiencing catastrophic costs due to tuberculosis in Uganda: Magnitude and cost drivers. BMC Public Health. 20(1), 1409 (2020).
Nhung, N. V. et al. Measuring catastrophic costs due to tuberculosis in Viet Nam. Int. J. Tuberc. Lung Dis. 22(9), 983–990 (2018).
Pedrazzoli, D. et al. How affordable is TB care? Findings from a nationwide TB patient cost survey in Ghana. Trop. Med. Int. Health 23(8), 870–878 (2018).
Timire, C. et al. Catastrophic costs among tuberculosis-affected households in Zimbabwe: A national health facility-based survey. Trop. Med. Int. Health 26(10), 1248–1255 (2021).
Tomeny, E. M. et al. Patient-cost survey for tuberculosis in the context of patient-pathway modelling. Int. J. Tuberc. Lung Dis. 24(4), 420–427 (2020).
Wingfield T, Boccia D, Tovar M, Gavino A, Zevallos K, Montoya R, et al. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLoS Med. 2014;11(7).
Loveday, M. et al. MDR-TB patients in KwaZulu-Natal, South Africa: Cost-effectiveness of 5 models of care. PloS one. 13(4), e0196003 (2018).
Pooran, A., Pieterson, E., Davids, M., Theron, G. & Dheda, K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa?. PloS one. 8(1), e54587 (2013).
Monedero, I. & Caminero, J. A. Management of multidrug-resistant tuberculosis: An update. Therap. Adv. Respir. Dis. 4(2), 117–127. https://doi.org/10.1177/1753465810365884 (2010).
Bharty, S. et al. Initiation of MDR TB treatment: Is hospitalization worth?. Indian J. Tuberc. 61(1), 57–64 (2014).
Alene, K. A. et al. Mental health disorders, social stressors, and health-related quality of life in patients with multidrug-resistant tuberculosis: a systematic review and meta-analysis. J. Infect. 77(5), 357–367 (2018).
Rudgard WE, das Chagas NS, Gayoso R, Barreto ML, Boccia D, Smeeth L, et al. Uptake of governmental social protection and financial hardship during drug-resistant tuberculosis treatment in Rio de Janeiro, Brazil. Eur Respir J. 2018;51(3).
Wells, W. A. et al. Size and usage patterns of private TB drug markets in the high burden countries. PLoS One. 6(5), e18964 (2011).
Mullerpattan, J. B., Udwadia, Z. Z., Banka, R. A., Ganatra, S. R. & Udwadia, Z. F. Catastrophic costs of treating drug resistant TB patients in a tertiary care hospital in India. Indian J. Tuberc. 66(1), 87–91 (2019).
Tanimura, T., Jaramillo, E., Weil, D., Raviglione, M. & Lönnroth, K. Financial burden for tuberculosis patients in low-and middle-income countries: A systematic review. Eur. Respir. J. 43(6), 1763–1775 (2014).
Kristina, S. A., Andayani, T. M. & Wulandari, G. P. A systematic review of the direct and indirect costs among tuberculosis patients. Res. J. Pharm. Technol. 13(1), 456–460 (2020).
Pantoja, A. et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part I. Socio-economic profile and costs among tuberculosis patients. Int. J. Tuberc. Lung. Dis. 13, 698–704 (2009).
Fuady, A., Houweling, T. A., Mansyur, M., Burhan, E. & Richardus, J. H. Effect of financial support on reducing the incidence of catastrophic costs among tuberculosis-affected households in Indonesia: Eight simulated scenarios. Infect. Dis. Poverty 8(1), 1–14 (2019).
Kundu, D. et al. Innovative social protection mechanism for alleviating catastrophic expenses on multidrug-resistant tuberculosis patients in Chhattisgarh, India. WHO South-East Asia journal of public health. 4(1), 69–77 (2015).
Oh KH, Rahevar K, Nishikiori N, Viney K, Choi H, Biermann O, et al. Action towards universal health coverage and social protection for tuberculosis care and prevention: Workshop on the End TB Strategy Pillar 2 in the Western Pacific Region 2017. MDPI; 2018.
International labour office. A joint crisis initiative of the UN chief executives board for co-ordination on the social protection floor (International Labour Office, 2009).
International Labour Office. World Social Security Report 2010/11: providing coverage in times of crisis and beyond (International Labour Office, 2010).
Acknowledgements
This work was supported by the Australian National Health and Medical Research Council (NHMRC) through an Emerging Leadership Investigator Grant APP1196549. KAA is a senior researcher at Curtin University who received the fund. TYA is also supported by the Curtin University Higher Degree Research (HDR) Scholarship and acknowledges Curtin University for providing support.
Funding
KAA is funded by an Australian National Health and Medical Research Council Investigator Grant (APP1196549). TYA is also supported by the Curtin University Higher Degree Research (HDR) Scholarship. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: T.Y.A., K.A.A., A.C.A.C. Data curation: T.Y.A., H.F.W., A.C.A., K.A.A. Formal analysis: T.Y.A. Investigation: T.Y.A., H.F.W., A.C.A., K.A.A. Methodology: T.Y.A. & K.A.A. Supervision: A.C.A.C., K.A.A. Validation: T.Y.A., A.C.A.C., K.A.A. Writing – original draft: T.Y.A. Writing – review & editing: A.C.A.C., H.F.W., K.A.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akalu, T., Clements, A., Wolde, H. et al. Economic burden of multidrug-resistant tuberculosis on patients and households: a global systematic review and meta-analysis. Sci Rep 13, 22361 (2023). https://doi.org/10.1038/s41598-023-47094-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47094-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.