Abstract
Chronic wasting disease (CWD) is a prion disease affecting cervids. Confirmatory testing of CWD is currently performed postmortem in obex and lymphoid tissues. Extensive evidence demonstrates the presence of infectious prions in feces of CWD-infected deer using in vitro prion-amplification techniques and bioassays. In experimental conditions, this has been achieved as soon as 6-month post-inoculation, suggesting this sample type is a candidate for antemortem diagnosis. In the present study, we optimized the detection of CWD-prions in fecal samples from naturally infected, pre-clinical white-tailed deer by comparing protocols aiming to concentrate CWD-prions with direct spiking of the sample into the PMCA reactions. Results of this screening were compared with similar analyses made in blood. Our data shows that CWD-prion detection in feces using PMCA is best in the absence of sample pre-treatments. We performed a screening of 169 fecal samples, detecting CWD-prions with diagnostic sensitivity and specificity of 54.81% and 98.46%, respectively. In addition, the PMCA seeding activity of 76 fecal samples was compared with that on blood of matched deer. Our findings, demonstrate that CWD-prions in feces and blood are increased at late pre-clinical stages, exhibiting similar detection in both sample types (> 90% sensitivity) when PrP96GG animals are tested. Our findings contribute to understand prion distribution across different biological samples and polymorphic variants in white-tailed deer. This information is also relevant for the current efforts to identify platforms to diagnose CWD.
Similar content being viewed by others
Introduction
Chronic Wasting Disease (CWD) is a prionopathy affecting free-ranging1,2,3,4,5,6 and farmed cervids3,7,8,9. The agent responsible for the transmission and pathological progression of CWD is a misfolded form of the prion protein (PrPSc) that spreads by promoting conformational changes in the host cellular prion proteins (PrPC)3,10,11,12. The abnormal aggregation and deposition of the prion misfolded fibrils occur mostly in the central nervous system1,3,7,8,10. Nonetheless, studies have demonstrated that CWD-prions are also present in extra neural tissues13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28. In addition, infectious prions are known to exist in excreta and bodily fluids of infected animals29,30,31,32,33,34,35,36, which may shed shortly after infection37,38,39,40 and contribute to environmental contamination. Considering the potential role of environmental fomites and humanmade materials in the transmission of CWD, the continuous shedding of prions is thought to play a relevant role in the expansion and recurrent outbreaks of this disease.
Confirmatory testing of CWD is currently performed by enzyme-linked immunosorbent assays (ELISAs) and/or immunohistochemistry (IHC) in biopsies of medial retropharyngeal lymph node (MRLPN) and obex tissues collected postmortem. In addition, recto-anal mucosa-associated lymphoid tissue (RAMALT) and MRLPN collected antemortem and tested by IHC are used to test white-tailed deer under specific situations41. A limitation in using antemortem diagnostic tests is that the collection of the samples is invasive, requiring the sedation of animals that in some cases die due to surgery-associated complications. In addition, these techniques require trained veterinarians and have limited sensitivity at early CWD post-exposure periods15,31,42.
The protein misfolded cyclic amplification (PMCA) and the real-time quaking-induced conversion (RT-QuIC) techniques are ultra-sensitive prion replication methods43,44,45,46,47,48 able to identify infectious prions in different sample types15,19,31,32,48,49. PMCA mimics the self-propagation of infectious prions in vitro through multiple sonication/incubation cycles, increasing the number of prion particles present in a given sample. The detection of proteinase K (PK)-resistant PrPSc by PMCA has been performed extensively in experimental and natural samples that may or may not harbor sub-infectious levels of prions22,23,31,32,39,47,49,50,51,52,53,54,55,56,57,58. Our group has explored the use of the PMCA technique as a tool to detect CWD-prions in multiple environmental and biological specimens (Refs.23,56,57,58,59 and unpublished). Importantly, this technique has been studied for its potential use in samples amenable with antemortem testing such as blood56,57, saliva55, feces39,53, and urine32.
As mentioned above, extensive evidence demonstrates the presence of infectious prions in excreta from deer29,60. Importantly, prion detection in these samples can be achieved long before the onset of clinical signs29,38,39,40,61. For example, PMCA analyses of feces from different cervid species (elk, mule deer, and white-tailed deer) demonstrated shedding as soon as 6-months post-infection39. This is relevant considering that PMCA detection identified the CWD status of mule deer using feces 3 months earlier compared to bioassays29. PMCA has also been reported to detect prions in feces 6-months earlier than RT-QuIC in white-tailed deer40. This and other data suggest that feces have the potential to be used in CWD diagnosis. Another relevant sample type that has been extensively explored for antemortem CWD testing is blood. CWD prionemia has been thoroughly demonstrated56,57,62,63. Our group has contributed to the optimization of CWD-prion detection in blood using specimens collected from naturally infected, non-clinical white-tailed deer57. Our published data demonstrate that PMCA is able to diagnose CWD in blood of white-tailed deer with a 100% specificity, and sensitivity that varies according to the progression of the disease and the PrP polymorphic variation at position 9656,57.
In the present study, we (i) evaluated three methods to detect PrPSc in white-tailed deer feces by PMCA; (ii) screened fecal samples from captive, naturally CWD-infected and pre-clinical white-tailed deer; and (iii) compared the CWD-prion detection using two extra neural samples (feces and blood) considering disease progression, genotyping variation, and sex. Our data provides valuable information regarding the use of blood and feces for CWD detection, and contributes to the understanding of prion accumulation in different sample types during the course of the incubation period.
Materials and methods
Fecal and blood samples collection and preparation
Fecal samples from 169 farmed white-tailed deer were opportunistically collected postmortem by USDA personnel. Fecal samples from each individual were collected with clean gloves, placed in 50 mL conical tubes and stored at − 80 °C until aliquoted into 1.5 mL microcentrifuges tubes. The samples were shipped to Dr. Morales’ laboratory at The University of Texas Health Science Center at Houston (UTHealth) and stored at − 20 °C until use. Fecal samples were thawed at room temperature and processed as described in Plummer et al.39 with minimal modifications. Briefly, 10% (w/v) feces homogenates were prepared in ultra-pure water containing a cocktail of protease inhibitors EDTA free (Roche, Basel, Switzerland) in MP Biomedical Lysing Matrix G 2 mL tubes containing a mix of glass beads and silicon carbide particles (MP Biomedicals, Irvine, CA, USA) and homogenized in a Bertin Precellys 24 dual homogenizer (MP Biomedicals, Irvine, CA, USA). Samples were placed in ice between homogenization cycles and stored at − 20 °C until being processed. Additionally, a subset of 76 white-tailed deer were blood-sampled. Blood specimens were collected postmortem from the jugular vein by USDA personnel and stored at -80 °C in tubes containing EDTA as described in Refs.56,57. PMCA results for these samples were previously described in these articles.
Sodium phosphotungstic acid (NaPTA) treatment
The treatment was performed as described previously39 with slight modifications. One mL of feces homogenates was placed in 2 mL Lo-bind tubes (Eppendorf, Enfield, CT, USA) and supplemented with SDS (Sigma-Aldrich, Inc., St. Louis, MO, USA) to a final concentration of 1% (v/v). This mixture was vortexed, incubated in rotation for 1 h at room temperature, and then centrifuged for 1 h at 15,000×g and 10 °C. The supernatants were transferred to clean 1.5 mL Lo-bind tubes (Eppendorf, Enfield, CT, USA) and submitted to a new centrifugation step under the same conditions mentioned above. The supernatants were transferred to new 2 mL Lo-bind tubes (Eppendorf, Enfield, CT, USA) and 4% sarkosyl prepared in PBS was added in a 1:1 ratio. Afterward, a 4% sodium phosphotungstate hydrate solution prepared with ultra-pure water at pH 7.1 was added to the samples to reach a final concentration of 0.57%. The samples were incubated overnight at 37 °C in agitation (600 rpm) using an Eppendorf thermomixer (Eppendorf, Enfield, CT, USA). Products were again centrifuged for 30 min at room temperature and 15,000×g. The pellets were carefully rinsed with PMCA conversion buffer (1% triton X-100, and 150 mM NaCl prepared in PBS) containing a cocktail of protease inhibitors with EDTA (Roche, Basel, Switzerland) and centrifuged for additional 10 min at room temperature and 15,000×g. The final pellets were resuspended in PMCA substrate and submitted to in vitro prion replication.
Sarkosyl treatment
Fecal and blood samples were treated with sarkosyl to concentrate their PrPSc content as previously described by our group57. Briefly, 200 µL of 10% (w/v) feces homogenates or blood were placed in 4.0 mL polycarbonate thick ultracentrifuge tubes (Beckman Coulter, Inc., Brea, CA, USA) containing 20% (w/v) sarkosyl (Thermo Fisher Scientific, Waltham, MA, USA) (prepared in PBS) in a 1:1 ratio. The mixtures were centrifuged for 1 h at 100,000×g and 4 °C and pellets were rinsed with 400 µL of 1× PBS containing a cocktail of EDTA-free protease inhibitors (Roche, Basel, Switzerland). Pellets were centrifuged again at 100,000×g for 30 min at 4 °C. Finally, pellets were resuspended in PMCA substrate and submitted to in vitro prion replication as described below.
Protein misfolding cyclic amplification (PMCA)
The PMCA substrate was made using perfused brains from male and female homozygous tg1536 mice64 that were homogenized at 10% (w/v) in PMCA conversion buffer (150 mM NaCl and 1% Triton X-100 prepared in PBS) containing a cocktail of protease inhibitors with EDTA (Roche, Basel, Switzerland). Homogenates were centrifuged for 1 min at 805×g and 4 °C and supernatants were vortexed and aliquoted in 1.5 mL microcentrifuge tubes that were snap-frozen in liquid nitrogen and stored at − 80 °C. The PMCA substrate was supplemented with digitonin (Invitrogen, Carlsbad, CA, USA) and EDTA (Promega Corporation, Madison, WI, USA) at a final concentration of 0.025% and 6 mM, respectively, just prior use in the cyclic prion amplification reaction. Aliquots of 90 µL were transferred in 0.2 mL PCR tubes (Eppendorf, Enfield, CT, USA) containing three 3/32″ PTFE beads (Engineering Laboratories, Inc., Oackland, NJ, USA) and mixed with either 10 µL of 10% (w/v) feces homogenates or feces pellets. The mixtures were submitted to the first round of PMCA in a Qsonica sonicator Q700 with a microplate horn (QSONICA L.L.C, Newtown, CT, USA). The first PMCA round consisted of 144 cycles of incubation/sonication. Aliquots of these PMCA products (10 µL) were mixed with fresh PMCA substrate (90 µL) and subjected to two additional PMCA rounds of 96 cycles. Each PMCA cycle consisted of 29 min and 40 s of incubation, and 20 s of sonication at 37 °C. Each PMCA reaction set included serial dilutions of a CWD brain (10 µL) of known PMCA activity. As a negative control, four unseeded reactions were additionally included in each PMCA set. Each sample was blindly tested, in duplicate. Three different investigators participated in the screening process. The results were evaluated at the third round of PMCA. Fecal samples were considered CWD-positive if at least one of the replicates showed proteinase K (PK) resistant PrPSc signals in western blot (explained below).
Proteinase K (PK) treatment
In order to detect PK resistant PrPSc, PMCA products were treated with this protease (Sigma-Aldrich, Saint Louis, MO, USA) at a final concentration of 100 µg/mL and incubated at 37 °C for 90 min and shaking (450 rpm using an Eppendorf® thermomixer). The digestion reactions were stopped by adding NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA, USA) at a final concentration of 1× and heating the samples at 90 °C for 10 min.
Western blot
To visualize PrPSc in PMCA products after PK treatment, samples were examined via western blot. First, samples were fractionated in NuPAGE 12% Bis–Tris gels (Invitrogen, Carlsbad, CA, USA). Electrophoresis was conducted at 80 V for 20 min and then 140 V for 100 min following the manufacturer’s recommendations. Proteins were transferred to a nitrocellulose membrane (GE Healthcare Amersham, Chicago, IL, USA) using cooled transfer buffer (0.025 M of tris base, 0.192 M of glycine, 10% (v/v) methanol) and blocked for 15 min at room temperature with 10% (w/v) non-fat milk solution. Membranes were probed using the monoclonal Bar224 antibody (Bertin Corp, Rockville, MD, USA) at a 1:10,000 dilution for 60 min at room temperature and incubated with secondary polyclonal Anti-Mouse IgG (whole molecule)-Peroxidase antibody produced in sheep (Sigma-Aldrich, Saint Louis, MO, USA) at a 1:3000 dilution for 60 min at room temperature. After incubation with each antibody, membranes were washed three times for 10 min at room temperature with washing buffer (1× PBS and 0.05% (v/v) Tween 20). The same buffer was used to prepare the milk and antibodies solutions. Membranes were developed using ECL (GE Healthcare Amersham, Chicago, IL, USA) following the manufacturer’s recommendations.
Diagnostic sensitivity and specificity
Diagnostic sensitivity and specificity were calculated as in Parikh et al.65 using the following equations:
Ethical approval
Mice were bred, maintained, and sacrificed in approved facilities at UTHealth Houston. Euthanasia was performed by using CO2 inhalation following federal and local regulations, and approved by the Animal Welfare Committee of UTHealth at Houston (protocol AWC-22-0039). Animal manipulations described in this article followed the recommendations stated in the ARRIVE guidelines (https://arriveguidelines.org/). UTHealth investigators have USDA approval to receive and work with white-tailed deer-derived samples.
Results
Optimization of CWD-prion detection in fecal samples
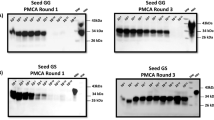
Previous studies showed that NaPTA-based protocols are useful when studying samples harboring low levels of prions14,15,37,39,50,66,67. This has been particularly relevant for fecal samples analyzed with the RT-QuIC technique16,40,61,68. In addition, several reports described that in vitro PrPSc amplification efficiency might be affected by the sample matrix35,52,55,57,67,69 and NaPTA-based protocols may be useful in removing potential inhibitors, particularly the ones present in fecal samples68. In order to perform a CWD-prion screening from feces specimens collected from farmed/naturally-infected white-tailed deer, we evaluated two PrPSc enrichment methods: (i) NaPTA-based, and (ii) the sarkosyl/ultracentrifugation extensively used by our group56,57,58. Both methods were compared with (iii) direct spiking of feces homogenates to the PMCA substrate. For this optimization experiment, a set of nine fecal samples were selected: six specimens collected from late non-clinical white-tailed deer and tested as CWD-positive in blood by PMCA57, and three samples identified as CWD-non-detect (Table 1). The CWD-positive deer carried two 96G PRNP alleles. Importantly, PrP 96G alleles are suggested to favor peripheral PrPSc replication14,15,25. Our data shows that both NaPTA and sarkosyl/ultracentrifugation methods were able to identify prions in two samples only (Fig. 1, Table 1). Interestingly, the method without pre-treatments showed positive detection of PK-resistant PrPSc in all the fecal specimens collected from the CWD-positive animals (Fig. 1, Table 1).
Pre-PMCA treatment on fecal samples results in variable detection outcomes. Fecal samples from CWD-detected (six, in red) and CWD-non-detect (three, in black) white-tailed deer by IHC (obex and retropharyngeal lymph node) were tested to detect seeding activity by PMCA. Analyses were performed comparing two pre-treatment methods (NaPTA and sarkosyl/ultracentrifugation) with no-treatment (direct spiking of feces homogenates in the PMCA reactions). PMCA products were treated with PK and evaluated as described in Materials and Methods. PrPC represents non-PK-treated brain extracts from Tg1536+/+ mice used as a molecular weight migration control. The numbers at the right represent molecular weight markers.
Screening of fecal samples from farmed white-tailed deer
Currently, official postmortem41 detection of CWD prions is performed by ELISA and/or IHC in obex and RPLN samples. However, several studies have shown that the accumulation of CWD prions in extra neural tissues and excreta occurs shortly after prion exposure, giving them potential diagnostic value14,39,40,61,66. In this study, we tested a set of 169 fecal samples from pre-clinical, CWD-infected white-tailed deer using the PMCA technique. From these samples, 104 specimens came from CWD-positive deer as tested by the conventional methods mentioned above. Importantly, the CWD status of the donor animals was blinded to the researchers performing the PMCA assay. CWD seeding activity was detected in 58 feces specimens from these CWD-positive animals, resulting in an overall diagnostic sensitivity of 54.81% (Table 2). The analysis of feces from CWD non-detect white-tailed deer provided a diagnostic specificity of 98.46% considering that a single sample from this group consistently presented with positive PMCA signals. This particular sample was evaluated by two different investigators, demonstrating seeding activity in 4/5 replicates. Considering this, results from this specific sample were considered as a false-positive.
Effect of genotyping and disease progression on CWD-prion detection using fecal samples
The progression of prion disease is correlated with PrPSc detection in different tissues14,66, secreta70 and excreta39,40. To evaluate the detection of the infectious agent in fecal samples across the course of the prion incubation period in naturally infected white-tailed deer, we evaluated samples from 169 animals regardless of their PrP polymorphic identity. The classification of the disease progression was defined according to the presence or absence of CWD-staining in obex and/or retropharyngeal lymph node (RPLN) tissues by IHC testing, as in previous reports56,57. Animals were considered (i) early pre-clinical (n = 49) if they were IHC-positive in the RPLN only, or (ii) late pre-clinical (n = 55) if they were IHC-positive in the RPLN and obex. Deer were classified as CWD-non-detect (n = 65) if they were IHC-negative in both tissues. It is relevant to note that a single animal showed PrPSc aggregates in the obex, but not in the RPLN. This deer was classified as late pre-clinical, given that neuroinvasion in white-tailed deer occurs in late stages of the CWD incubation period. Our PMCA analyses show CWD-seeding activity in fecal samples from 46 (83.64%) deer at the late pre-clinical stage. At the early pre-clinical stage, just eleven (22.45%) were accurately identified (Table 3). This drop of prion detection in feces at late vs. early stages of the CWD incubation period is consistent with what has been described in other deer cohorts using RT-QuIC16.
Subsequently, we explored the influence of polymorphic variation at codon 96 as this has been suggested as a modulator of peripheral prion replication and shedding13,14,18,24,39,40,70. A fraction (n = 123) of the total fecal samples were analyzed considering if the polymorphic identity at position 96 of PrP was available. In the PrP 96GG group, CWD-prion seeding activity was detected in 30 deer classified as late pre-clinical (93.75%). At the early pre-clinical stage, just 6 (26.09%) were accurately identified (Table 4). In the PrP 96GS late pre-clinical group, just 16 samples (65.57%) were detected as positive by PMCA. As expected, the diagnostic efficacy of PMCA decreased at the early pre-clinical stage as only 5 fecal samples (25.00%) were accurately diagnosed. In the PrP 96SS group, fecal samples from early pre-clinical animals only were collected. All samples from this group provided negative seeding activity in PMCA. In summary, these results clearly show that the presence of CWD-prions in feces is considerably more abundant in PrP 96GG white-tailed deer and at late pre-clinical stages.
Detection of CWD-prions in fecal samples and blood from matched animals
In order to compare the CWD detection and prion content of feces and blood, we compared the seeding activity of sample pairs which were collected from the same deer. For that purpose, we analyzed matched samples from 76 deer with known PrP polymorphic variation at position 96 and IHC CWD status available. The results of blood used to compare feces samples results were previously reported by our group56,57. Our results demonstrate that the highest CWD-prion detection was obtained at the late pre-clinical phase in PrP 96GG animals. For this group, the CWD-prion seeding activity in fecal and blood specimen was equal to 92.59%. For PrP 96GS animals at the late pre-clinical stage, the detection of the infectious agent was higher in blood (70.00%) than in feces (60.00%) (Table 5). Unfortunately, no samples from PrP 96SS were available at late-preclinical stages, so analyses were not possible for this group of animals. This data confirms previous reports suggesting that the PrP 96G allele increases prion shedding.
At the early pre-clinical stage, animals harboring the PrP 96GG variant displayed an expected decrease in CWD detection by PMCA. This ranged from 31.58 to 52.63% in fecal and blood specimens, respectively (Table 5). A similar trend was observed in PrP 96GS deer, considering the detection of CWD-prions was 30.77% in feces and 53.84% in blood. In contrast, in the less susceptible animals (PrP 96SS) the seeding activity was absent. These results further demonstrate the lower presence of CWD prions in excreta at early stages of the CWD incubation period.
Influence of sex on CWD-prion detection using blood and feces
Finally, we evaluated if seeding activity was affected by the sex of the donor animal in both feces and blood. A fraction of 104 fecal samples from 88 female and 16 males, and seventy-three samples of blood (61 females and 12 males) were compared (Table 6). Our results showed a detection of PK-resistant PrPSc in fecal samples comparable between does (54.55%) and bucks (56.25%). Similar results were found when testing whole blood (females: 68.85%; males: 58.33%). These results suggest that the detection of CWD-prion seeding activity in feces and blood is not influenced by the deer’s sex.
Discussion
CWD is continuously expanding in North America71,72, and it has been more recently reported in Northern Europe4,5,6,73,74. CWD is devastating socially and economically75, as there is no treatment or cure for this fatal disease and its zoonotic potential is still unknown. Significant practices have been implemented in surveilling this disease with the aim to reduce its spreading in both free-ranging and farmed populations. Additionally, considerable research efforts have been made towards the development of sensitive diagnostic tests75. A limitation of the currently accepted CWD diagnosis methods is their limited sensitivity and the fact that they need to be conducted postmortem14,15,41,48. Nonetheless, sensitive diagnostic methods such as RT-QuIC and PMCA have extensively demonstrated the detection of the infectious agent (i) in a wide range of biological and environmental samples14,16,17,18,19,20,22,23,27,31,39,40,50,52,59,76,77,78, (ii) long before the onset of clinical signs14,37,39,40, and (iii) in samples that harbor low amounts of CWD-prions. The main challenge for these tests is to optimize their use in biological samples suitable for antemortem collection. Candidate specimens for this include bodily fluids and excreta in which prions have been detected shortly post CWD prion exposure37,39. The purpose of this study was to compare the efficiency of PMCA to diagnose CWD in feces and blood. For that purpose, we considered multiple factors in white-tailed deer such as PrP polymorphic variability, prion incubation stages, and sex.
The first step to efficiently detect CWD prions using PMCA in feces was to optimize the best protocol for detection. PMCA inhibitors or competitors are present in multiple matrices as shown in multiple publications35,52,55,56,67,68,69. The use of NaPTA to enhance the detection of PrPSc has been well documented14,15,37,39,50,66,67. In our experience, the use of the ionic detergent sarkosyl and ultracentrifugation enhance prion detection by PMCA detection in multiple biological samples, including blood56,57, semen58, retropharyngeal and submandibular lymph nodes, and brain (Morales et al. unpublished). In our hands, the direct spiking of the feces homogenates into the PMCA substrate supports a higher efficiency in contrast to NaPTA or sarkosyl/ultracentrifugation pre-treatments. In fact, the lower seeding activity exhibited by both enrichment methods in different replicates was consistent in multiple assays performed in our laboratory (data not shown). One explanation for these unexpected observations is that some fecal-associated PMCA inhibitors co-precipitate with PrPSc particles, decreasing the efficiency of the cyclic amplification of misfolded prions. Another plausible explanation may be found in Colby et al.79 who described that NaPTA strongly inhibits amyloid formation whereas sarkosyl either inhibits or accelerates amyloid formation depending on its concentration in amyloid seeding assays. Although the protocols used in this study include washing steps to remove traces of NaPTA and sarkosyl, it is possible that certain components particularly pelleting in fecal samples co-precipitate with these reagents in quantities that are incompatible with PMCA. It is important to highlight that the blood and fecal specimens used in the present study were processed using different methods: sarkosyl/ultracentrifugation and direct spiking, respectively. As discussed above, the presence of inhibitors/competitors in different specimens may have varying effects in PMCA. The data presented in this study highlights the differential pre-PMCA processing steps needed for optimal detection in seeding assays, depending on the sample type. Nonetheless, more studies are necessary to accurately explain the PrPSc interaction with the feces biological matrix. Directly spiking PMCA reactions may be advantageous for diagnostic purposes as this substantially reduces the time and costs associated with the screening of a large number of samples. Importantly, testing of fecal samples may not only be relevant for animal diagnosis, but also for screening of premises where this sample type is abundant.
In regards to animal diagnosis, PMCA screening of fecal samples for this white-tailed deer cohort provided an overall 55.77% of accurate diagnosis. Other groups, have attempted to diagnose CWD in bodily fluids and excreta using prion seeding assays. Results on those studies varied depending on the sample type: 25% to 28% in urine37,39, 34% to 56% in nasal secretions17,70, 56% in semen58, 50% to 82% in saliva35,37,55, 71% to 79.5% blood56,57, and 60% to 83% in feces16,39,40,61,68,69. Considering this, our results are comparable to those found in other sample types. However, if we restrict our cohort to the most CWD-susceptible PrP 96 polymorphic group, and to late stages of the pre-clinical phase, the detection approaches to 100% as compared to results from the official postmortem IHC testing. Our data also suggest a higher diagnostic power of PMCA over RT-QuIC using feces considering the data of Tewari et al.16 who describe seeding activity in 70.5% of the animals that harbored PrP 96G alleles and CWD status positive in RPLN and brain by IHC. Although the diagnostic potential of feces appears to be low, they may still provide some utility to screen animals at their late CWD stages. The important drop in detection at early stages of the pre-clinical phase is a relevant issue for diagnostic purposes, and future studies should be devoted to optimize the PMCA protocol to increase sensitivity in this subset of samples. Regardless of these limitations, the use of fecal specimens for environmental screening could be an effective option for surveillance efforts in areas where CWD has not been detected. In addition, the use of fecal samples is advantageous since they are easily accessible and non-invasive to collect. In contrast, postmortem specimens are rare or inconvenient to obtain.
A relevant point in this screening involves the single false-positive result. Considering the consistent result in multiple replicates performed by different researchers, it is suggested that this sample was contaminated during the collection process. An alternative explanation is that this particular animal was at a very early stage of the disease and the accumulation of CWD prions within the lymph nodes was minimal and sparse (“patchy”). In addition, the fact that the animal was homozygous for serine at codon 96 of PRNP (a genotype that has been extensively described to be less susceptible to CWD) supports the assumption that low quantities of the infectious agent were present in this sample. Under this hypothetical scenario, this tissue may have displayed a patchy distribution of PrPSc and perhaps the portion used for IHC lacked PrPSc inclusions, making it histologically undetectable. However, it is important to note that blood from this animal lacked seeding activity or was under the PMCA threshold. Considering the PMCA data collected from the feces and blood from this animal, the most plausible explanation is that the fecal matter was contaminated during the collection process.
Different studies demonstrate that the genetic variability at the codon 96 of the white-tailed deer prion protein has a strong effect on both, the prevalence of CWD and the incubation period13,14,33,80,81. Likewise, the accumulation of PrPSc in different tissues across the course of the disease appears to be modulated by these polymorphic variations, affecting CWD detection13,14,19,24,42,82. In this study, we compared the PMCA seeding activity in feces and blood from white-tailed deer harboring different PrP 96 polymorphic variants and at different stages of the pre-clinical phase. Our results demonstrated that (i) CWD-prions are more available for detection in feces and blood at late stages of the pre-clinical phase, (ii) the PrP 96G allele favors detection in both sample types in a dose dependent manner, and (iii) at the early pre-clinical stage blood provides a better diagnostic power compared with feces. Considering that direct spiking of feces homogenates was the most efficient mean to detect prions using PMCA, it is suggested that the feces matrix contain less PMCA inhibitors compared with blood (sample that requires a sarkosyl/ultracentrifugation pre-treatment for PMCA detection). Along the same line, the data presented in this study suggests that feces contain lower quantities of PrPSc compared with blood. Alternatively, blood may harbor higher CWD-prion titers at late stages due to its role in the trafficking of the infectious agent from and to diverse peripheral tissues.
Our results also demonstrate that the overall presence of CWD in farmed white-tailed deer is not influenced by sex. This is different compared to free-ranging deer population where CWD incidence seems to be higher in males. These contradictory results may be due to the confinement experienced in farming settings. In addition, we acknowledge that our deer cohort includes a substantially higher number of females over males, fact that may have influenced these results. The analysis of larger and sex-equivalent deer cohorts from free-ranging and farmed deer may help to elucidate properly the effect of sex in CWD incidence, prevalence and diagnosis.
Conclusions
In summary, here we report the dynamics of CWD prion detection in fecal and blood specimens across the pre-clinical phase of infection. CWD-prions were best detected in deer carrying both copies of the PrP 96G codon, particularly during the late pre-clinical stage, in both fecal and blood samples. In addition, our results demonstrate that blood exhibited a higher diagnostic potential before neuroinvasion occur in infected deer. Overall, our findings contribute to the development of prion diagnosis methodologies and to the understanding of the prion distribution and burden across different biological samples during the course of the disease in naturally-infected, farmed deer.
Data availability
All data generated or analyzed during this study are included in this published article and Supplementary Information. Raw data are available upon reasonable request to the corresponding author.
Abbreviations
- (w/v):
-
(Weight/volume)
- 96G:
-
Glycine at position 96 of the prion protein
- 96GG:
-
Homozygous for glycine at position 96 of the prion protein
- 96GS:
-
Heterozygous for glycine and serine at position 96 of the prion protein
- 96SS:
-
Homozygous for serine at position 96 of the prion protein
- CWD:
-
Chronic wasting disease
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISAs:
-
Enzyme-linked immunosorbent assays
- IHC:
-
Immunohistochemistry
- MRLPN:
-
Medial retropharyngeal lymph node
- NaPTA:
-
Sodium phosphotungstic acid
- PBS:
-
Phosphate-buffered saline
- PK:
-
Proteinase K
- PMCA:
-
Protein misfolded cyclic amplification
- PRNP :
-
Prion protein gene
- PrP:
-
Prion protein
- PrPC :
-
Host cellular prion protein
- PrPSc :
-
Infectious prion protein
- RAMALT:
-
Recto anal mucosa-associated lymphoid tissue
- RPLN:
-
Retropharyngeal lymph node
- RT-QuIC:
-
Real-time quaking-induced conversion
- SDS:
-
Sodium dodecyl sulfate
- USDA:
-
U.S. Department of Agriculture
- UTHealth:
-
The University of Texas Health Science Center at Houston
References
Spraker, T. R. et al. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J. Wildl. Dis. 33, 1–6 (1997).
Joly, D. O. et al. Chronic wasting disease in free-ranging Wisconsin White-tailed Deer. Emerg. Infect. Dis. 9, 599–601 (2003).
Spraker, T. R. et al. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive M. Vet. Pathol. 39, 110–119 (2002).
Pirisinu, L. et al. Novel type of chronic wasting disease detected in moose (Alces alces), Norway. Emerg. Infect. Dis. 24, 2210–2218 (2018).
Benestad, S. L., Mitchell, G., Simmons, M., Ytrehus, B. & Vikøren, T. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet. Res. 47, 88 (2016).
Vikøren, T. et al. First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J. Wildl. Dis. 55, 970–972 (2019).
Peters, J., Miller, J. M., Jenny, A. L., Peterson, T. L. & Carmichael, K. P. Immunohistochemical diagnosis of chronic wasting disease in preclinically affected elk from a captive herd. J. Vet. Diagn. Investig. 12, 579–582 (2000).
Williams, E. S. & Young, S. Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and Elk (Cervus elaphus nelsoni). Vet. Pathol. 30, 36–45 (1993).
Williams, E. S. & Young, S. Spongiform encephalopathies in Cervidae. Rev. Sci. Tech. 11, 551–567. https://doi.org/10.20506/rst.11.2.611 (1992).
Guiroy, D. C., Williams, E. S., Song, K. J., Yanagihara, R. & Gajdusek, D. C. Fibrils in brains of Rocky Mountain elk with chronic wasting disease contain scrapie amyloid. Acta Neuropathol. 86, 77–80 (1993).
Prusiner, S. B. Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982).
Griffith, J. S. Nature of the scrapie agent: Self-replication and scrapie. Nature 215, 1043–1044 (1967).
Otero, A. et al. Prion protein polymorphisms associated with reduced CWD susceptibility limit peripheral PrPCWD deposition in orally infected white-tailed deer. BMC Vet. Res. 15, 50 (2019).
Hoover, C. E. et al. Pathways of prion spread during early chronic wasting disease in deer. J. Virol. 91, 17 (2017).
Henderson, D. M. et al. Progression of chronic wasting disease in white-tailed deer analyzed by serial biopsy RT-QuIC and immunohistochemistry. PLoS ONE 15, e0228327 (2020).
Tewari, D. et al. Detection of chronic wasting disease in feces and recto-anal mucosal associated lymphoid tissues with RT-QuIC in a naturally infected farmed white-tailed deer herd. Front. Vet. Sci. 9, 555 (2022).
Haley, N. J. et al. Seeded amplification of chronic wasting disease prions in nasal brushings and recto-anal mucosa-associated lymphoid tissues from elk by real-time quaking-induced conversion. J. Clin. Microbiol. 54, 1117–1126 (2016).
Haley, N. J. et al. Antemortem detection of chronic wasting disease prions in nasal brush collections and rectal biopsy specimens from white-tailed deer by real-time quaking-induced conversion. J. Clin. Microbiol. 54, 1108–1116 (2016).
Cooper, S. K. et al. Detection of CWD in cervids by RT-QuIC assay of third eyelids. PLoS ONE 14, 1–14 (2019).
Ness, A. et al. Chronic wasting disease prions in mule deer interdigital glands. PLoS ONE 17, e0275375 (2022).
Sigurdson, C. J. et al. Oral transmission and early lymphoid tropism of chronic wasting disease PrP(res) in mule deer fawns (Odocoileus hemionus). J. Gen. Virol. 80, 2757–2764 (1999).
Daus, M. L. et al. Presence and seeding activity of pathological prion protein (PrPTSE) in skeletal muscles of white-tailed deer infected with chronic wasting disease. PLoS ONE 6, e18345 (2011).
Bravo-Risi, F. et al. Detection of CWD prions in naturally infected white-tailed deer fetuses and gestational tissues by PMCA. Sci. Rep. 11, 18385 (2021).
Thomsen, B. V. et al. Diagnostic accuracy of rectal mucosa biopsy testing for chronic wasting disease within white-tailed deer (Odocoileus virginianus) herds in North America: Effects of age, sex, polymorphism at PRNP codon 96, and disease progression. J. Vet. Diagn. Investig. 24, 878–887 (2012).
Wolfe, L. L. et al. PrPCWD in rectal lymphoid tissue of deer (Odocoileus spp.). J. Gen. Virol. 88, 2078–2082 (2007).
Jewell, J. E., Brown, J., Kreeger, T. & Williams, E. S. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J. Gen. Virol. 87, 3443–3450 (2006).
Li, M. et al. RT-QuIC detection of CWD prion seeding activity in white-tailed deer muscle tissues. Sci. Rep. 11, 16759 (2021).
Ferreira, N. C. et al. Detection of chronic wasting disease in mule and white-tailed deer by RT-QuIC analysis of outer ear. Sci. Rep. 11, 7702 (2021).
Tamgüney, G. et al. Asymptomatic deer excrete infectious prions in faeces. Nature 461, 529–532 (2009).
Mathiason, C. K. et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314, 133–136 (2006).
Haley, N. J., Mathiason, C. K., Zabel, M. D., Telling, G. C. & Hoover, E. A. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS ONE 4, e7990 (2009).
Haley, N. J., Seelig, D. M., Zabel, M. D., Telling, G. C. & Hoover, E. A. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE 4, e4848 (2009).
Mammadova, N., Cassmann, E. & Greenlee, J. J. Successful transmission of the chronic wasting disease (CWD) agent to white-tailed deer by intravenous blood transfusion. Res. Vet. Sci. 133, 304–306 (2020).
Nichols, T. A., Fischer, J. W., Spraker, T. R., Kong, Q. & VerCauteren, K. C. CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans). Prion 9, 367–375 (2015).
Henderson, D. M. et al. Rapid antemortem detection of CWD prions in deer saliva. PLoS ONE 8, e74377 (2013).
Mathiason, C. K. et al. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS ONE 4, e5916 (2009).
Henderson, D. M. et al. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J. Virol. 89, 9338–9347 (2015).
Hwang, S., Greenlee, J. J. & Nicholson, E. M. Real-time quaking-induced conversion detection of PrPSc in fecal samples from chronic wasting disease infected white-tailed deer using bank vole substrate. Front. Vet. Sci. 8, 643754 (2021).
Plummer, I. H., Wright, S. D., Johnson, C. J., Pedersen, J. A. & Samuel, M. D. Temporal patterns of chronic wasting disease prion excretion in three cervid species. J. Gen. Virol. 98, 1932–1942 (2017).
Tennant, J. M. et al. Shedding and stability of CWD prion seeding activity in cervid feces. PLoS ONE 15, e0227094 (2020).
Agriculture, United States Department of Service, Animal and Plant Health Inspection Services, V. Chronic Wasting Disease (CWD) Program Standards 1–91. https://www.aphis.usda.gov/animal_health/animal_diseases/cwd/downloads/cwd-program-standards.pdf (2019).
Keane, D. et al. Validation of use of rectoanal mucosa-associated lymphoid tissue for immunohistochemical diagnosis of chronic wasting disease in white-tailed deer (Odocoileus virginianus). J. Clin. Microbiol. 47, 1412–1417 (2009).
Saborio, G. P., Permanne, B. & Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).
Kurt, T. D. et al. Efficient in vitro amplification of chronic wasting disease PrP RES. J. Virol. 81, 9605–9608 (2007).
Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 17, 175 (2011).
Wilham, J. M. et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 6, e1001217 (2010).
Castilla, J., Saá, P. & Soto, C. Detection of prions in blood. Nat. Med. 11, 982–985 (2005).
McNulty, E. et al. Comparison of conventional, amplification and bio-assay detection methods for a chronic wasting disease inoculum pool. PLoS ONE 14, e0216621 (2019).
Haley, N. J. et al. Sensitivity of protein misfolding cyclic amplification versus immunohistochemistry in ante-mortem detection of chronic wasting disease. J. Gen. Virol. 93, 1141–1150 (2012).
Plummer, I. H., Johnson, C. J., Chesney, A. R., Pedersen, J. A. & Samuel, M. D. Mineral licks as environmental reservoirs of chronic wasting disease prions. PLoS ONE 13, e0196745 (2018).
Pritzkow, S. et al. Efficient prion disease transmission through common environmental materials. J. Biol. Chem. 293, 3363–3373 (2018).
Pritzkow, S. et al. Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep. 11, 1168–1175 (2015).
Pulford, B. et al. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J. Wildl. Dis. 48, 425–434 (2012).
Haley, N. J. et al. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: Potential mechanisms of prion shedding and transmission. J. Virol. 85, 6309–6318 (2011).
Davenport, K. A., Hoover, C. E., Denkers, N. D., Mathiason, C. K. & Hoover, E. A. Modified protein misfolding cyclic amplification overcomes real-time quaking-induced conversion assay inhibitors in deer saliva to detect chronic wasting disease prions. J. Clin. Microbiol. 56, 18 (2018).
Kramm, C., Soto, P., Nichols, T. A. & Morales, R. Chronic wasting disease (CWD) prion detection in blood from pre-symptomatic white-tailed deer harboring PRNP polymorphic variants. Sci. Rep. 10, 19763 (2020).
Kramm, C. et al. Detection of prions in blood of cervids at the asymptomatic stage of chronic wasting disease. Sci. Rep. 7, 17241 (2017).
Kramm, C. et al. In vitro detection of chronic wasting disease (CWD) prions in semen and reproductive tissues of white tailed deer bucks (Odocoileus virginianus). PLoS ONE 14, 1–12 (2019).
Inzalaco, H. N. et al. Ticks harbor and excrete chronic wasting disease prions. Sci. Rep. 13, 7838 (2023).
Miller, M. W., Williams, E. S., Hobbs, N. T. & Wolfe, L. L. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 10, 1003–1006 (2004).
Cheng, Y. C. et al. Early and non-invasive detection of chronic wasting disease prions in elk feces by real-time quaking induced conversion. PLoS ONE 11, e0166187 (2016).
McNulty, E. E. et al. In vitro detection of haematogenous prions in white-tailed deer orally dosed with low concentrations of chronic wasting disease. J. Gen. Virol. 101, 347–361 (2020).
Elder, A. M. et al. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS ONE 8, e80203 (2013).
Browning, S. R. et al. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J. Virol. 78, 13345–13350 (2004).
Parikh, R., Mathai, A., Parikh, S., Sekhar, G. C. & Thomas, R. Understanding and using sensitivity, specificity and predictive values. Indian J. Ophthalmol. 56, 45–50 (2008).
Davenport, K. A. et al. PrPC expression and prion seeding activity in the alimentary tract and lymphoid tissue of deer. PLoS ONE 12, e0183927 (2017).
Henderson, D. M. et al. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J. Gen. Virol. 96, 210–219 (2015).
Cheng, Y. C. et al. Real-time quaking-induced conversion assay for detection of cwd prions in fecal material. J. Vis. Exp. 2017, 1–9 (2017).
Denkers, N. D., Henderson, D. M., Mathiason, C. K. & Hoover, E. A. Enhanced prion detection in biological samples by magnetic particle extraction and real-time quaking-induced conversion. J. Gen. Virol. 97, 2023–2029 (2016).
Kraft, C. N., Denkers, N. D., Mathiason, C. K. & Hoover, E. A. Longitudinal detection of prion shedding in nasal secretions of CWD-infected white-tailed deer. J. Gen. Virol. 104, 1–6 (2023).
National Wildlife Health Center. Expanding Distribution of Chronic Wasting Disease. USGS. https://www.usgs.gov/centers/nwhc/science/expanding-distribution-chronic-wasting-disease (2022).
Rivera, N. A., Brandt, A. L., Novakofski, J. E. & Mateus-Pinilla, N. E. Chronic wasting disease in cervids: Prevalence, impact and management strategies. Vet. Med. Res. Rep. 10, 123–139 (2019).
Tranulis, M. A. et al. Chronic wasting disease in Europe: New strains on the horizon. Acta Vet. Scand. 63, 48 (2021).
Sun, J. L. et al. Novel prion strain as cause of chronic wasting disease in a Moose, Finland. Emerg. Infect. Dis. 29, 323–332 (2023).
Chiavacci, S. J. The economic costs of chronic wasting disease in the United States. PLoS ONE 17, e0278366 (2022).
Nichols, T. A. et al. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 3, 171–183 (2009).
Henderson, D. M. et al. Detection of chronic wasting disease prion seeding activity in deer and elk feces by real-time quaking-induced conversion. J. Gen. Virol. 98, 1953–1962 (2017).
Kuznetsova, A., McKenzie, D., Ytrehus, B., Utaaker, K. S. & Aiken, J. M. Movement of chronic wasting disease prions in Prairie, Boreal and Alpine soils. Pathogens 12, 269 (2023).
Colby, D. W. et al. Prion detection by an amyloid seeding assay. Proc. Natl. Acad. Sci. 104, 20914–20919 (2007).
Johnson, C., Johnson, J., Clayton, M., McKenzie, D. & Aiken, J. Prion protein gene heterogeneity in free-ranging white-tailed deer within the chronic wasting disease affected region of Wisconsin. J. Wildl. Dis. 39, 576–581 (2003).
Johnson, C. et al. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J. Gen. Virol. 87, 2109–2114 (2006).
Schneider, D. A. et al. Tonsil biopsy to detect chronic wasting disease in white-tailed deer (Odocoileus virginianus) by immunohistochemistry. PLoS ONE 18, 1–11 (2023).
Acknowledgements
The authors would like to thank Mr. Reece McGinn for editing and proof-reading the original manuscript.
Funding
This work was funded by a Grant from NIH (1R01AI132695) to RM.
Author information
Authors and Affiliations
Contributions
F.B.-R., R.B., and P.S. performed the PMCA experiments. F.B.-R. and R.M. analyzed the data and wrote the manuscript. F.B.-R. prepared the figures. T.N. collected the samples. T.N. and R.M. designed the experiments. R.M. supervised the research work and was responsible for the funding. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.M. is listed as an inventor in one patent related to the PMCA technology. F. B-R, P.S., R.B., and T.N. declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bravo‐Risi, F., Soto, P., Benavente, R. et al. Dynamics of CWD prion detection in feces and blood from naturally infected white-tailed deer. Sci Rep 13, 20170 (2023). https://doi.org/10.1038/s41598-023-46929-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46929-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.