Abstract
Batten disease is a group of mostly pediatric neurodegenerative lysosomal storage disorders caused by mutations in the CLN1–14 genes. We have recently shown that acidified drinking water attenuated neuropathological changes and improved motor function in the Cln1R151X and Cln3−/− mouse models of infantile CLN1 and juvenile CLN3 diseases. Here we tested if acidified drinking water has beneficial effects in Cln2R207X mice, a nonsense mutant model of late infantile CLN2 disease. Cln2R207X mice have motor deficits, muscle weakness, develop tremors, and die prematurely between 4 and 6 months of age. Acidified water administered to Cln2R207X male mice from postnatal day 21 significantly improved motor function, restored muscle strength and prevented tremors as measured at 3 months of age. Acidified drinking water also changed disease trajectory, slightly delaying the death of Cln2R207X males and females. The gut microbiota compositions of Cln2R207X and wild-type male mice were markedly different and acidified drinking water significantly altered the gut microbiota of Cln2R207X mice. This suggests that gut bacteria might contribute to the beneficial effects of acidified drinking water. Our study demonstrates that drinking water is a major environmental factor that can alter disease phenotypes and disease progression in rodent disease models.
Similar content being viewed by others
Introduction
Late infantile CLN2 disease is a recessively inherited, progressive neurodegenerative disorder that belongs to neuronal ceroid lipofuscinoses (also known as Batten disease), a group of lysosomal storage diseases with ceroid lipofuscin as storage material. CLN2 disease is caused by mutations in the CLN2 gene encoding the lysosomal protease, tripeptidyl peptidase 1 (TPP1)1. The disease begins at 2–4 years of age with seizures and ataxia followed by visual impairment and progressive cognitive and motor dysfunction, leading to death by 6–15 years of age2. It is still unknown how TPP1 deficiency results in brain atrophy. Since 2017, enzyme replacement therapy by intracerebroventricular infusion has been available for patients with CLN2 disease. This treatment with a recombinant TPP1 (cerliponase alfa by BioMarin Pharmaceutical Inc.) slows the decline of motor and language functions in symptomatic CLN2 disease patients 3 years of age and older3, 4.
We have recently generated a novel mouse model of the disease, Cln2R207X mice. The Cln2 mutation in these mice corresponds to the most frequent disease-causing human nonsense mutation. In Cln2R207X mice, due to the nonsense mutation that generates a premature stop codon, the mutant Cln2 mRNA level is markedly decreased by nonsense-mediated mRNA decay. Accordingly, TPP1 enzyme activity is dramatically reduced in the organs of Cln2R207X mice. Less than 1% of the wild-type TPP1 activity was measured in the liver, kidney, cerebral cortex and cerebellum of Cln2R207X mice5. Cln2R207X mice have a progressive disease course with significant neurological abnormalities, tremors, and neuropathological changes, such as accumulation of lysosomal storage material and astrocytic activation in the brain at 3 months of age. Cln2R207X mice prematurely die between 4 and 6 months of age5.
The type of drinking water (tap, distilled, autoclaved, acidified, neutral, alkaline) is a major environmental factor that can affect the physiology of experimental animals, but it is rarely reported in scientific publications. At numerous animal facilities, acidification of drinking water with HCl is used to eliminate pathogenic bacteria. We have recently shown in the Cln3−/− and Cln1R151X mouse models of juvenile CLN3 and infantile CLN1 diseases that acidified drinking water attenuated neuropathological changes in a disease-specific manner and provided disease-dependent beneficial effects on neurological function6, 7. In both Cln3−/− and Cln1R151X mice, the effects of acidified drinking water were accompanied by significant changes in the gut microbiota composition6, 7.
In the current study, we tested if acidified drinking water provides disease-modifying effects in the Cln2R207X nonsense mutant mouse model of late infantile CLN2 disease. The effect of acidified drinking water on the altered gut microbiota composition of Cln2R207X mice was also examined.
Materials and methods
Animals
We maintained Cln2R207X mice on a mixed 129S6/SvEv;C57BL/6J genetic background and 129S6/SvEv;C57BL/6J wild-type (WT) mice in our mouse colony. Cln2R207X mice and WT mice were not littermates; in our colony, Cln2R207X and WT mice were separately maintained. Mice were housed in ventilated microisolator cages (4–5 mice/cage), with ad libitum food (Teklad Global 2918 diet; Harlan Laboratories, Indianapolis, IN) and water (non-acidified tap water, pH 8.4), with a 14-h light, 10-h dark cycle. Cln2R207X and WT mice were randomly assigned to receive acidified drinking water from 21 days of age (weaning). To acidify tap water with HCl (pH 2.5–2.9; average pH: 2.8) a Technilab BMI BV water acidification system (Tecniplast USA, West Chester, PA) was used. In the behavioral and gut microbiota experiments, only male mice were used. In a comparative study, we previously demonstrated that male mouse models of juvenile CLN3 disease (Cln3−/− and Cln3Δex7/8 male mice) display more pronounced disease phenotypes than females and therefore, are more appropriate for testing new therapies8. Consequently, since we made the Cln2R207X mouse model to test novel therapies, when Cln2R207X mice were characterized, only the neurological phenotypes of male mice were determined5.
To determine the survival curves, both males and female mice were used.
Cln2R207X mice have a severe disease phenotype, they start trembling around 90 days of age and die within a few months. Therefore, from 90 days of age, Cln2R207X mice were monitored daily and were euthanized when morbidity criteria (i.e., immobility, difficulties in feeding) were observed. Mice were euthanized by carbon dioxide inhalation in accordance with the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition.
All animal procedures were approved by the Sanford Research Animal Care and Use Committee and were in compliance with NIH policies and the guidelines of the Animal Welfare Act. To report the animal experiments we followed the recommendations in the ARRIVE guidelines9.
Behavioral testing
Behavioral testing was carried out during the light phase. In the behavioral testing room, the lights were dimmed. Before starting the behavioral tests, the mice were weighed and let to adapt to the room for at least 20 min. The modified vertical pole test was the first test, followed by the wire hanging test and the force-plate actimeter.
The behavioral test results and the weight data were analyzed by 2-way ANOVA with Tukey’s post-test for multiple comparisons in GraphPad Prism 7.04 (GraphPad Software, San Diego, CA).
Modified vertical pole test
A modified version of the vertical pole test was used to assess motor function as we previously described6,7,8. The test started by putting the mouse at the top of the pole, head downward, and the time the mouse took to climb down the pole was measured in 5 consecutive trials. Following the climb down trials, the same mouse was put at the top of the pole, head upward, and the time the mouse took to turn completely downward was measured in 4 consecutive trials. Each test trial lasted a maximum of 60 s (to avoid fatigue). When a mouse fell, a 60 s score was recorded.
Wire hanging test
To test muscle strength, mice were placed in the center of a stainless-steel cooling rack for baking (20.32 cm × 29.85 cm; grid spacing: 1.27 cm square). The rack was inverted and held 46 cm above a cage containing bedding. The time mice could remain suspended was measured; 60 s was the cut-off time to prevent exhaustion. Each mouse was tested 5 consecutive times and the average time of staying suspended (latency to fall) was calculated.
Force-plate actimeter
The force-plate actimeter (BASi, West Lafayette, IN, USA) monitors several behavioral parameters in a freely moving mouse, such as locomotor activity (e.g., covered area, overall distance of movements, number and total degrees of turns, and distribution of the animal’s activity across the plate called spatial statistic), focused stereotypy score (number of occasions when the mouse is rearing, scratching, grooming or head bobbing), number of times during the test when a bout of low mobility occurred, and tremors over different frequency ranges can be measured5. Mice were tested in the force-plate actimeter as we previously described6 with some modification. Briefly, mice were put in the force-plate actimeter and the above-described behavioral parameters were recorded for 10.24 min. The recorded parameters were analyzed using FPAAnalysis software version 1.10.01 (BASi, West Lafayette, IN, USA; https://www.basinc.com/assets/library/manuals/FPA.pdf).
Analysis of the gut microbiota
When each mouse finished the modified vertical pole test, fecal pellets were aseptically collected from the pole base in a sterile 1.5-ml tube, and the tube was immediately put on dry ice. The collected fecal pellets (from 6 mice per experimental group) were stored at − 80 °C until they were shipped to MR DNA (www.mrdnalab.com, Shallowater, TX, USA) for DNA isolation and sequencing the V4 region of the bacterial 16S rRNA gene. DNA was isolated from the fecal samples using the Qiagen QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA). Sequencing was performed on the Illumina MiSeq platform with the adaptation of 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing. The sequence data were processed according to the method previously published6.
The sequence data provided by MR DNA underwent bioinformatic and statistical analyses using the Microbial Genomics Module in CLC Genomics Workbench vs 21.0.3 (Qiagen). Following quality trimming (Q = 20 and adapter trimming) and exclusion of chimeric reads, the obtained operational taxonomic units (OTUs) were aligned to the SILVA v132 database10 at 97% similarity. The Chao-1 bias-corrected index was used to measure alpha diversities11, 12. To determine the statistical significance for differences in alpha diversity, a Kruskal–Wallis test with Mann–Whitney U pairwise comparison was used. The Jaccard dissimilarity index was used for Principal Coordinate Analysis (PCoA) to compare beta diversities13, 14. To determine the statistical significance of differences in beta diversity between the experimental groups, a PERMANOVA analysis was applied. For pair-wise comparison of abundance at the different taxonomical levels, False Discovery Rate (FDR)-corrected p-values were calculated using a Wald test.
Results
Acidified drinking water in Cln2 R207X mice improves motor function, prevents tremors and slightly delays death
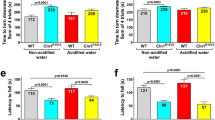
Cln2R207X mice have impaired motor function, muscle weakness, develop tremors, and die prematurely between 4 and 6 months of age5. To investigate the potential disease-modifying effects of acidified drinking water (average pH: 2.8), Cln2R207X and wild-type (WT) mice received acidified drinking water starting at weaning (postnatal day 21), and their motor function, muscle strength and tremors were evaluated at 3 months of age. In a modified vertical pole test, acidified drinking water improved the ability of Cln2R207X mice to climb down the pole; their performance became similar to WT mice receiving non-acidified drinking water (Fig. 1a). Another parameter measured in the vertical pole test, the time to turn downward at the top of the pole was not improved by acidified water (Fig. 1b). In the modified vertical pole test, the climbing down phase measures agility and motor coordination, whereas the turning downward task assesses spatial orientation and motor coordination. Accordingly, acidified drinking water improved the agility and motor coordination of Cln2R207X mice (Fig. 1a).
Acidified drinking water in Cln2R207X mice improves motor function. Cln2R207X and WT male mice either received non-acidified water or were given acidified drinking water from postnatal day 21 (weaning). At 3 months of age, the motor skills of mice were tested in a modified vertical pole test: time to climb down (a) and time to turn downward (b). Muscle strength was assessed in a wire hanging test (c). (d) Weight of the mice at 3 months. Acidified drinking water improved the ability of Cln2R207X mice to climb down the pole, their performance became similar to WT mice receiving non-acidified drinking water (a). Acidified drinking water restored the muscle strength of Cln2R207X mice to the WT level, as measured in a wire hanging test (c). In agreement with our previous results 7, 15, acidified drinking water compromised the performance of WT mice in the vertical pole test: they climbed down and turned downward significantly slower than WT mice receiving non-acidified water (a,b). Columns and bars represent mean + SEM and the symbols show the individual data (WT and Cln2R207X on non-acidified water: 28 and 20 mice in the modified vertical pole test, and 14 and 19 mice in the wire hanging test; WT and Cln2R207X on acidified water: 16 and 19 mice in both tests). Statistical significance was determined by 2-way ANOVA with Tukey’s post-test for multiple comparisons. N.S. not significant.
As measured in a wire hanging test, acidified drinking water restored the muscle strength of Cln2R207X mice to the WT level (Fig. 1c). Acidified drinking water also prevented the development of tremors in different frequency ranges (Fig. 2). Only Cln2R207X mice receiving non-acidified water displayed tremors.
Acidified drinking water in Cln2R207X mice prevents tremors. Cln2R207X and WT male mice either were kept on non-acidified water or received acidified drinking water from postnatal day 21 (weaning). At 3 months of age, tremors were measured in a force-plate actimeter. Tremors were quantified as force variations at different frequencies (tremor indices 1–4) (a–d). Cln2R207X mice on non-acidified water had intense tremors in every frequency range, and acidified drinking water prevented the development of these tremors. Columns and bars represent mean + SEM and the symbols show the individual data (WT and Cln2R207X on non-acidified water: 14 and 20 mice, respectively; WT and Cln2R207X on acidified water: 16 and 19 mice, respectively). Statistical significance was determined by 2-way ANOVA with Tukey’s post-test for multiple comparisons. N.S. not significant.
In line with our previous findings7, 15, acidified drinking water impaired the performance of WT mice in the vertical pole test: they climbed down and turned downward significantly slower than WT mice receiving non-acidified water (Fig. 1a,b), indicating an impairment in agility, motor coordination and spatial orientation. The differential effects of acidified drinking water on the motor function in Cln2R207X disease model and healthy WT mice is not surprising. While medications or treatments, especially those affecting the nervous system, have therapeutic results in disease states, they can cause adverse effects in healthy subjects.
Acidified water did not affect the muscle strength of WT mice (Fig. 1c). On non-acidified water, Cln2R207X mice were significantly heavier than WT mice, indicating an abnormal weight gain (Fig. 1d). Acidified drinking water in Cln2R207X mice prevented this abnormal weight gain, the weight of WT mice and Cln2R207X mice receiving acidified drinking water was very similar. The decreased weight by acidified drinking water in Cln2R207X mice, making them less fat and fitter, might contribute to the improved pole climbing and muscle strength. Correlation analysis, however, showed no correlation between the weight and pole climbing time in Cln2R207X mice receiving non-acidified or acidified drinking water, indicating that weight was not a contributing factor to the improvement in pole climbing. While the weight of Cln2R207X mice receiving acidified water did not correlate with muscle strength in the wire hanging test (p = 0.0671), the weight of Cln2R207X mice receiving non-acidified water showed a negative correlation with muscle strength (heavier mice fell sooner, p = 0.0008). This indicates that the decreased weight was a contributing factor to the increased muscle strength of Cln2R207X mice receiving acidified drinking water.
We also examined if acidified drinking water affected several locomotor and behavioral parameters using the force-plate actimeter (BASi, West Lafayette, IN). On non-acidified water, among all the parameters examined, only the distance traveled was significantly increased in Cln2R207X mice as compared to WT mice (Fig. S1). While acidified drinking water in Cln2R207X mice did not affect the distance traveled, focused stereotypies (head bobbing, grooming, rearing, scratching, etc.), or left and right turn counts, it changed the area covered, spatial statistic (space utilization), bout of low mobility and total degrees of left and right turns in comparison to WT mice receiving acidified water (Figs. S1, S2).
Cln2R207X mice die prematurely. Although, we did not examine neurological phenotypes in Cln2R207X female mice, the death of both male and female mice was of course recorded in our Cln2R207X colony, and we knew that Cln2R207X females also die prematurely around the same age as Cln2R207X males. Therefore, we tested the effect of acidified drinking water on the lifespan of both male and female Cln2R207X mice. The median survival ages of male and female Cln2R207X mice receiving non-acidified water were 15 and 16 weeks, respectively. Acidified drinking water changed disease trajectory, delaying the death of Cln2R207X females and males by 3 weeks [females: p = 0.0011 by Log-rank (Mantel-Cox) test and p = 0.0152 by Gehan–Breslow–Wilcoxon test; males: p = 0.0487 by Log-rank (Mantel-Cox) test and p = 0.0091 by Gehan–Breslow–Wilcoxon test] (Fig. 3). The ratios of median survivals and their 95% confidence intervals were 1.188 (0.695–2.030) for males on acidified/males on non-acidified, and 1.200 (0.629–2.287) for females on acidified/females on non-acidified.
Acidified drinking water changes disease trajectory, delaying the death of Cln2R207X mice. Survival curves of Cln2R207X male and female mice that either were kept on non-acidified water or received acidified drinking water from postnatal day 21 (weaning). The curves represent the survival of 45 males and 24 females that received non-acidified drinking water, and of 19 males and 15 females receiving acidified drinking water from weaning.
Acidified drinking water significantly changes the gut microbiota composition of Cln2 R207X mice
Accumulated evidence indicates that the gut microbiome plays a critical role in the development of neurodegenerative and neurological diseases16. We have previously shown that in comparison to WT mice, the gut microbiota composition of Cln2R207X mice is significantly altered17. Here we examined if acidified drinking water caused changes in the gut microbiota of Cln2R207X mice, and if there were specific changes that correlated with the beneficial neurological effects of acidified water. The microbiota composition of fecal samples was determined by 16S rRNA gene sequencing. Alpha diversity, the microbial diversity within a sample, was quantified using the Chao 1 bias-corrected diversity index. Alpha diversity was similar in Cln2R207X and WT mice receiving non-acidified drinking water, and acidified water did not cause statistically significant changes in either genotype (Fig. 4a). Alpha diversities in Cln2R207X and WT mice receiving acidified drinking water, however, were close to being different with a Kruskal–Wallis p = 0.05 (Fig. 4a). The global gut microbiota compositions in the different groups (beta diversity) were compared by principal coordinate analysis. Beta diversity in Cln2R207X mice was significantly different from that in WT mice on both types of drinking water (p = 0.00216 and 0.02814) (Fig. 4b). While acidified drinking water did not change the bacterial community structure in WT mice (p = 0.17749), it markedly altered the global microbiota composition in Cln2R207X mice (p = 0.01082) (Fig. 4b).
Cln2R207X and WT mice have greatly different gut microbiota, and acidified drinking water causes significant alteration in the global gut microbiota composition of Cln2R207X mice only. A group of Cln2R207X and WT male mice was given acidified drinking water from 21 days of age (weaning). Another group of Cln2R207X and WT male mice were kept on non-acidified drinking water. At 3 months of age, fecal pellets were collected for the analysis of the gut microbiota via 16S rRNA gene sequencing. (a) Alpha diversity, the microbial diversity within a sample, was quantified using the Chao 1 bias-corrected diversity index. Alpha diversity was similar in Cln2R207X and WT mice receiving non-acidified drinking water, and acidified water did not cause statistically significant changes in either genotype. Alpha diversities in Cln2R207X and WT mice receiving acidified drinking water, however, were close to be different with a p-value of 0.05. Box and whisker plot: each black dot represents a mouse (n = 6 mice). To determine the statistical significance for differences in alpha diversity, a Kruskal–Wallis test with Mann–Whitney U pairwise comparison was used. (b) The global gut microbiota compositions in the different groups (beta diversity) were compared by principal coordinate (PCo) analysis. Beta diversity in Cln2R207X mice was significantly different from that in WT mice on both types of drinking water (p = 0.00216 and 0.02814). While acidified drinking water did not change the bacterial community structure in WT mice (p = 0.17749), it markedly altered the global microbiota composition in Cln2R207X mice (p = 0.01082). Each symbol represents an individual mouse (n = 6 mice in each group). A PERMANOVA analysis was used to determine the statistical significance in beta diversity (Bray–Curtis).
Gut microbiota analysis at the individual taxonomic categories showed numerous differences (Supplementary Table 1, Figs. S3, S4). Acidified drinking water significantly increased the abundance of the Bacteroidetes and Patescibacteria phyla (2.2- and 3.5-fold) in Cln2R207X mice, whereas in WT mice, it reduced the abundance of the Tenericutes phylum (− 11.2-fold). Acidified water in Cln2R207X mice markedly altered the abundance of 3 orders (Flavobacteriales, Betaproteobacteriales, Pasteurellales), 13 families (Atopobiaceae, Muribaculaceae , Rikenellaceae, Flavobacteriaceae, Uncultured bacterium-1, Enterococcaceae, Uncultured-1, Clostridiaceae 1, Burkholderiaceae, Nitrosomonadaceae, Pasteurellaceae, Anaeroplasmataceae, Ambiguous_taxa-4), and 17 genera (Coriobacteriaceae UCG-002, Parvibacter, Uncultured-3, Uncultured bacterium-3, Tetragenococcus, Clostridium sensu stricto 1, Tyzzerella, Eubacterium coprostanoligenes group, Ruminiclostridium 6, Ruminococcaceae UCG-005, {Unknown Genus} Erysipelotrichaceae, Dubosiella, Faecalibaculum, Uncultured-9, Parasutterella, Rodentibacter, Ambiguous_taxa-8) (Supplementary Table 1). Acidified drinking water in WT mice significantly changed the abundance of 3 classes (Saccharimonadia, Alphaproteobacteria, Mollicutes), 2 orders (Rickettsiales, Anaeroplasmatales), 2 families (Saccharimonadaceae Anaeroplasmataceae), and 1 genus (Anaeroplasma) (Supplementary Table 1).
Discussion
Our study demonstrates that acidified drinking water administered to Cln2R207X mice from postnatal day 21 significantly improves motor function, restores muscle strength to the WT level and prevents tremors as measured at 3 months of age. Acidified drinking water also changed disease trajectory, delaying the death of Cln2R207X mice by 3 weeks. The gut microbiota of Cln2R207X and WT mice significantly differed and acidified drinking water caused pronounced changes in the gut microbiota composition of Cln2R207X mice.
The complete prevention of tremors by acidified drinking water in Cln2R207X mice was surprising. However, we have previously found that acidified drinking water can cause striking changes in serum metabolite levels in WT mice that included amino acids and their metabolites, fatty acids and their metabolites and many glycerophospholipids15. Similar acidified water-induced changes in neuroactive metabolite levels in Cln2R207X mice may be responsible for the prevention of tremor development. For example, the serum level of palmitic amide, a primary fatty acid amide derived from palmitic acid, was increased 43.4-fold by acidified drinking water in WT mice15. Fatty acid amides compete with endocannabinoids for binding to the active site of fatty acid amide hydrolase and thus, increase the concentration of endocannabinoids by preventing their degradation18. Cannabinoids are known to reduce tremors associated with neurodegenerative diseases19.
Acidified drinking water in Cln2R207X mice, in comparison to WT mice, changed several locomotor and behavioral parameters as measured in the force-plate actimeter: the area covered and total degrees of left and right turns were increased, whereas space utilization (spatial statistic) and bout of low mobility were decreased (see Figs. S1, S2). Although, the significance of these parameters is not clear, the changes in them may represent potentially negative effects of acidified water in Cln2R207X mice.
Since Cln2R207X mice die very early, starting around 4 months of age, our study focused on the effects of acidified drinking water on neurological functions and life span, which are more important outcome measures than the pathological changes in the brain (accumulation of lysosomal storage material, astrocytosis and microglial activation) common in mouse models of the different Batten disease forms. Most Cln2R207X mice in our colony died suddenly: 1 day they were fine or just had tremors and the next day they were found dead. Examination of the organs of Cln2R207X mice by a veterinary pathologist could not identify a definitive causes of death5. A recent study, however, showed a temporal relationship between seizure activity and death in Cln2R207X mice20. The most likely cause of sudden death associated with neurodegeneration and brain dysfunction is respiratory arrest as observed in epileptic patients21 and in a mouse model of Leigh syndrome, a progressive encephalomyelopathy22. The underlying cause is the suppression/dysfunction of respiratory centers in the brainstem. While acidified drinking water in Cln2R207X males improved motor function, restored muscle strength to the WT level and prevented tremors, it only delayed the death by 3 weeks: the median survival age was increased from 16 to 19 weeks. In Cln2R207X females, acidified water increased the median survival age from 15 to 18 weeks (see Fig. 3). Despite the similar effects on median survival in Cln2R207X males and females, acidified drinking water changed the survival curves of males and females differently. While the survival curves of Cln2R207X males crossed each other at 21 weeks of age, the female survival curves did not cross each other. At 18 weeks of age, all Cln2R207X females receiving non-acidified water were dead but 47% of Cln2R207X females receiving acidified water were still alive, indicating a more pronounced effect of acidified water on the survival of Cln2R207X females. Future studies will determine if the delayed death in Cln2R207X females is associated with neurological improvements like in Cln2R207X males.
We have previously tested the effects of acidified drinking water on neurological functions in Cln3−/− and Cln1R151X mice, models of juvenile CLN3 and infantile CLN1 diseases6, 7. While Cln3−/− mice were on the 129S6/SvEv genetic background, Cln1R151X mice were on the same mixed 129S6/SvEv;C57BL/6J background as Cln2R207X mice. Acidified drinking water in Cln3−/− mice had a temporary effect, improving the pole climbing ability at 3 months but not at 6 months of age6. In Cln1R151X mice, however, acidified water prevented the motor impairment at both 3 and 6 months as measured in the pole climbing test7. Acidified drinking water had a similar effect on the pole climbing ability of Cln2R207X mice; they could climb down the vertical pole as quickly as WT mice at 3 months of age (see Fig. 1). These results indicate that acidified drinking water is more beneficial for Cln1 and Cln2 mutations than for a Cln3 deletion, and/or the genetic background has a strong influence on the disease-modifying effects of acidified water.
Accumulated evidence shows that an altered gut microbiota, by its secreted metabolites and via the vagus nerve, contributes to disease development and progression in neurological and neurodegenerative disorders23. We have previously demonstrated markedly changed gut microflora in Cln3−/− and Cln1R151X mice6, 7, and now confirmed that the gut microbiota is also altered in Cln2R207X mice (see Fig. 4). While acidified drinking water did not change alpha diversity of the gut microbiota in Cln3−/− and Cln2R207X mice, it temporarily decreased alpha diversity in Cln1R151X mice at 3 months of age7. Beta diversity, the global gut microbiota composition, was prominently changed by acidified water in both Cln1R151X and Cln2R207X mice, but still remained different from that in WT mice. In contrast, acidified drinking water did not alter beta diversity in Cln3−/− mice6. At the individual taxonomic levels, acidified drinking water caused significant changes in Cln3−/−, Cln1R151X and Cln2R207X mice, but the changes were specific to each mouse model. For instance, while the abundance of the short-chain fatty acid-producing bacterial family, Erysipelotrichaceae was markedly increased by acidified water in the gut of Cln1R151X mice, it was unchanged in Cln2R207X mice. Similarly, while acidified water highly elevated the abundance of the Eubacterium coprostanoligenes group genus (105.6-fold) in Cln2R207X mice, it did not change the abundance of this genus in Cln1R151X mice.
The acidified drinking water-caused changes in the gut microbiota of Cln2R207X mice might contribute to the improved neurological function and delayed death, although it cannot be assumed that these events are causally related. Acidified water caused an 8.6-fold increase in the abundance of the Ruminococcaceae UCG-005 genus in Cln2R207X mice (see Supplementary Table 1). Bacteria in the Ruminococcaceae UCG-005 genus produce butyrate and other short-chain fatty acids24 that prevent neuroinflammation by inhibiting microglial activation and secretion of pro-inflammatory cytokines25. Short-chain fatty acids from gut bacteria also have beneficial effects on neuronal function by modulating the levels of neurotransmitters and neurotrophic factors25, and provide neuroprotection in mouse models of Parkinson’s disease, stroke and encephalopathy26,27,28,29. Increased plasma cholesterol levels have been associated with neuroinflammation and impaired brain function30. Gut bacteria in the Eubacterium coprostanoligenes group genus reduce the host’s cholesterol level by converting cholesterol to coprostanol31, and acidified drinking water in Cln2R207X mice increased the abundance of the Eubacterium coprostanoligenes group genus by 105.6-fold (Supplementary Table 1). The Tetragenococcus genus contains five lactic acid-producing species with probiotic potential32, and the Tetragenococcus halophilus species has been shown to have beneficial immunomodulatory effects in vitro and in vivo33. Acidified water in Cln2R207X mice increased the abundance of the Tetragenococcus genus by 10.9-fold (Supplementary Table 1). Another change that might contribute to the beneficial effects of acidified water was a pronounced 34.9-fold decrease in the abundance of the pathogenic Clostridium sensu stricto 1 genus (Supplementary Table 1). Bacteria in the Clostridium sensu stricto 1 genus cause intestinal inflammation34, 35.
In summary, our results show that acidified drinking water had clear beneficial effects in Cln2R207X mice. It improved the pole climbing ability to the WT level, restored muscle strength to the WT level, prevented the development of tremors, and slightly delayed death. Our study emphasizes that drinking water is a major environmental factor that can alter neurological functions and pathology in rodent disease models.
Data availability
The 16S rRNA gene sequencing data generated and analyzed during the current study are available in the Sequence Read Archive (SRA) of NCBI (https://www.ncbi.nlm.nih.gov/sra), with accession number PRJNA984750.
Abbreviations
- OTUs:
-
Operational taxonomic units
- WT:
-
Wild-type
References
Sleat, D. E. et al. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science 277, 1802–1805 (1997).
Carcel-Trullols, J., Kovacs, A. D. & Pearce, D. A. Cell biology of the NCL proteins: What they do and don’t do. Biochim. Biophys. Acta 1852, 2242. https://doi.org/10.1016/j.bbadis.2015.04.027 (2015).
Lewis, G., Morrill, A. M., Conway-Allen, S. L. & Kim, B. Review of cerliponase alfa: Recombinant human enzyme replacement therapy for late-infantile neuronal ceroid lipofuscinosis type 2. J. Child Neurol. 35, 348–353. https://doi.org/10.1177/0883073819895694 (2020).
Schulz, A. et al. Study of intraventricular cerliponase alfa for CLN2 disease. N. Engl. J. Med. 378, 1898–1907. https://doi.org/10.1056/NEJMoa1712649 (2018).
Geraets, R. D. et al. A tailored mouse model of CLN2 disease: A nonsense mutant for testing personalized therapies. PLoS ONE 12, e0176526. https://doi.org/10.1371/journal.pone.0176526 (2017).
Johnson, T. B. et al. Changes in motor behavior, neuropathology, and gut microbiota of a Batten disease mouse model following administration of acidified drinking water. Sci. Rep. 9, 14962. https://doi.org/10.1038/s41598-019-51488-z (2019).
Kovacs, A. D., Langin, L. M., Hernandez, J. L. G. & Pearce, D. A. Acidified drinking water attenuates motor deficits and brain pathology in a mouse model of a childhood neurodegenerative disorder. Sci. Rep. 12, 9025. https://doi.org/10.1038/s41598-022-12981-0 (2022).
Kovacs, A. D. & Pearce, D. A. Finding the most appropriate mouse model of juvenile CLN3 (Batten) disease for therapeutic studies: the importance of genetic background and gender. Dis. Models Mech. 8, 351–361. https://doi.org/10.1242/dmm.018804 (2015).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412. https://doi.org/10.1371/journal.pbio.1000412 (2010).
Yilmaz, P. et al. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 42, D643–D648. https://doi.org/10.1093/nar/gkt1209 (2014).
Chao, A. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 11, 265–270 (1984).
Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43, 783–791 (1987).
Jaccard, P. The distribution of the flora in the Alpine zone. New Phytol. 11, 37–50 (1912).
Koleff, P., Gaston, K. J. & Lennon, J. J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 72, 367–382 (2003).
Whipple, B., Agar, J., Zhao, J., Pearce, D. A. & Kovacs, A. D. The acidified drinking water-induced changes in the behavior and gut microbiota of wild-type mice depend on the acidification mode. Sci. Rep. 11, 2877. https://doi.org/10.1038/s41598-021-82570-0 (2021).
Bicknell, B. et al. Neurodegenerative and neurodevelopmental diseases and the gut-brain axis: The potential of therapeutic targeting of the microbiome. Int. J. Mol. Sci. 24, 9577. https://doi.org/10.3390/ijms24119577 (2023).
Parker, C., Zhao, J., Pearce, D. A. & Kovacs, A. D. Comparative analysis of the gut microbiota composition in the Cln 1(R151X) and Cln2(R207X) mouse models of Batten disease and in three wild-type mouse strains. Arch. Microbiol. 203, 85–96. https://doi.org/10.1007/s00203-020-02007-6 (2021).
McKinney, M. K. & Cravatt, B. F. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 74, 411–432. https://doi.org/10.1146/annurev.biochem.74.082803.133450 (2005).
Carlsen, E. M. M. et al. Spinal astroglial cannabinoid receptors control pathological tremor. Nat. Neurosci. 24, 658–666. https://doi.org/10.1038/s41593-021-00818-4 (2021).
Takahashi, K. et al. Gene therapy ameliorates spontaneous seizures associated with cortical neuron loss in a Cln2R207X mouse model. J. Clin. Investig. 133, 5908. https://doi.org/10.1172/JCI165908 (2023).
Pathak, S. J., Yousaf, M. I. K. & Shah, V. B. StatPearls (2022).
Quintana, A. et al. Fatal breathing dysfunction in a mouse model of Leigh syndrome. J. Clin. Investig. 122, 2359–2368. https://doi.org/10.1172/JCI62923 (2012).
Liu, L., Huh, J. R. & Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 77, 103908. https://doi.org/10.1016/j.ebiom.2022.103908 (2022).
Deleu, S., Machiels, K., Raes, J., Verbeke, K. & Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 66, 103293. https://doi.org/10.1016/j.ebiom.2021.103293 (2021).
Silva, Y. P., Bernardi, A. & Frozza, R. L. The role of short-chain fatty acids from gut microbiota in gut–brain communication. Front. Endocrinol. 11, 25. https://doi.org/10.3389/fendo.2020.00025 (2020).
Hou, Y. et al. Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson’s disease. Exp. Gerontol. 150, 111376. https://doi.org/10.1016/j.exger.2021.111376 (2021).
Lee, J. et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ. Res. 127, 453–465. https://doi.org/10.1161/CIRCRESAHA.119.316448 (2020).
Liu, J. et al. The neuroprotective effect of short chain fatty acids against sepsis-associated encephalopathy in mice. Front. Immunol. 12, 626894. https://doi.org/10.3389/fimmu.2021.626894 (2021).
Sadler, R. et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. 40, 1162–1173. https://doi.org/10.1523/JNEUROSCI.1359-19.2019 (2020).
Thirumangalakudi, L. et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 106, 475–485. https://doi.org/10.1111/j.1471-4159.2008.05415.x (2008).
Mukherjee, A., Lordan, C., Ross, R. P. & Cotter, P. D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 12, 1802866. https://doi.org/10.1080/19490976.2020.1802866 (2020).
Link, T., Vogel, R. F. & Ehrmann, M. A. The diversity among the species Tetragenococcus halophilus including new isolates from a lupine seed fermentation. BMC Microbiol. 21, 320. https://doi.org/10.1186/s12866-021-02381-1 (2021).
Kumazawa, T., Nishimura, A., Asai, N. & Adachi, T. Isolation of immune-regulatory Tetragenococcus halophilus from miso. PLoS ONE 13, e0208821. https://doi.org/10.1371/journal.pone.0208821 (2018).
Hu, C. et al. A comprehensive analysis of the colonic flora diversity, short chain fatty acid metabolism, transcripts, and biochemical indexes in heat-stressed pigs. Front. Immunol. 12, 717723. https://doi.org/10.3389/fimmu.2021.717723 (2021).
Yang, W. Y., Lee, Y., Lu, H., Chou, C. H. & Wang, C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS ONE 14, e0205784. https://doi.org/10.1371/journal.pone.0205784 (2019).
Acknowledgements
The authors thank Logan Langin for maintaining our mouse colony.
Funding
This work was funded by Sanford Health.
Author information
Authors and Affiliations
Contributions
A.D.K. designed the study, A.D.K. performed the experiments, A.D.K., J.L.G.H. and D.A.P. analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kovács, A.D., Gonzalez Hernandez, J.L. & Pearce, D.A. Acidified drinking water improves motor function, prevents tremors and changes disease trajectory in Cln2R207X mice, a model of late infantile Batten disease. Sci Rep 13, 19229 (2023). https://doi.org/10.1038/s41598-023-46283-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46283-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.