Abstract
Several observational studies have investigated the association between cannabis use and intraocular pressure, but its association with primary open-angle glaucoma (POAG) remains unclear. In this study, we leveraged human genetic data to assess through Mendelian randomization (MR) whether cannabis use affects POAG. We used five single-nucleotide polymorphisms (SNPs) associated with lifetime cannabis use (P-value < 5 × 10–8) from a genome-wide association study (GWAS) (N = 184,765) by the International Cannabis Consortium, 23andMe, and UK Biobank and eleven SNPs associated with cannabis use disorder (P-value < 5 × 10–7) from a GWAS meta-analysis of (17,068 cases and 357,219 controls of European descent) from Psychiatric Genomics Consortium Substance Use Disorders working group, Lundbeck Foundation Initiative for Integrative Psychiatric Research, and deCode. We associated the selected five SNPs from the GWAS of lifetime cannabis use and the eleven SNPs from the GWAS of cannabis use disorder, with the largest to date GWAS meta-analysis of POAG (16,677 cases and 199,580 controls). MR analysis suggested no evidence for a causal association of lifetime cannabis use and cannabis use disorder with POAG (odds ratio (OR) of outcome per doubling of the odds of exposure (95% confidence interval): 1.04 (0.88; 1.23) for lifetime cannabis use and 0.97 (0.92; 1.03) for cannabis use disorder). Sensitivity analyses to address pleiotropy and weak instrument bias yielded similar estimates to the primary analysis. In conclusion, our results do not support a causal association between cannabis use and POAG.
Similar content being viewed by others
Glaucoma is the leading cause of irreversible blindness worldwide, and is estimated to affect more than 100 million in 20401. Primary open-angle glaucoma (POAG), the most common subtype of glaucoma, is a slowly progressing optic neuropathy that can remain undetected for years, for which intraocular pressure (IOP) has been identified as the most significant modifiable risk factor2. In most POAG cases the first line of therapy for lowering IOP, and thus slowing POAG progression, is topical treatment with eye drops3, which carry numerous risks for ocular side effects and usually require lifelong continuation4.
Given the high incidence of glaucoma and the limitations of the current anti-glaucoma agents, research has focused during the last decades on the identification of novel treatment modalities, including the use of cannabis5. Cannabinoids have been found to exert a lowering effect on IOP when administered intravenously, orally, or by smoking5. However long-term and adequately sized clinical trials testing cannabinoids treatment in POAG are lacking. Moreover, in the existing observational studies assessing the association between cannabis smoking and IOP, and subsequently risk of POAG, it is challenging to isolate the individual effects of cannabinoids and tobacco, since they are usually consumed together6.
One approach to strengthen the causal inference on the association of cannabis use and the risk of POAG is Mendelian randomization (MR), a form of instrumental variable analysis that uses genetic variants as instruments7. In the present study, we used MR to assess any potential causal association between cannabis use and the risk of POAG.
Materials and methods
Study design
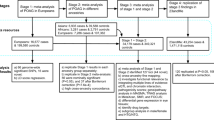
MR uses genetic variants as instrumental variables to assess causal associations between risk factors and diseases based on the random assignment of genetic variants in individuals at conception7. These genetic variants are usually single-nucleotide polymorphisms (SNPs) and since they are randomly allocated in individuals independently of other factors, MR studies can serve as naturally occurring randomized controlled trials7. Thus, MR association estimates are less prone to biases occurring from confounding and reverse causation than those derived from traditional observational studies. We conducted a two-sample, summary-based MR and utilized summary statistics from three genome-wide association studies (GWAS) of lifetime cannabis use8, cannabis use disorder9 and POAG10. Then by combining these estimates the causal association between cannabis use and cannabis use disorder with POAG was calculated. The recommendations by STROBE-MR11 and “Guidelines for performing Mendelian randomization investigations” were followed7. The study protocol was not pre-registered.
Data sources
We retrieved summary data from the largest GWAS to date for lifetime cannabis use comprising 184,765 individuals of European descent, by the International Cannabis Consortium, 23andMe, and UK Biobank8. The exposure was defined as any self-reported use of cannabis during a person’s lifetime. GWAS analysis were adjusted for sex, age, ancestry, and genotype batch. Genotyping and imputation methods have been described elsewhere8. We also retrieved summary statistics for cannabis use disorder from a GWAS meta-analysis of 17,068 cases and 357,219 controls of European descent, derived from the Psychiatric Genomics Consortium Substance Use Disorders working group, Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), and deCODE (Supplementary Table S1)9. Cases from the Psychiatric Genomics Consortium had the diagnosis of cannabis abuse or dependence according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV or DSM-III-R, from clinician ratings or semi-structured interviews. IPSYCH cases met the criteria for a diagnosis of cannabis abuse (F12.1) or cannabis dependence (F12.2) based on the ICD-10 criteria, while cases from the deCODE sample were diagnosed with lifetime cannabis abuse or dependence according to DSM-IV or DSM-III-R, or with cannabis use disorder according to DSM-V. Genotyping, quality control and imputation methods have been described elsewhere9. SNP-POAG associations were taken from a GWAS meta-analysis of 16,677 POAG cases and 199,580 controls of European ancestry10 from 16 participating studies (Supplementary Table S1). POAG was defined according to ICD9/ICD10 criteria. GWAS adjusted for age, sex, and study-specific principal components10. Genotyping, quality control and imputation have been described in detail elsewhere10. There was a 12.7% overlap between the GWAS of lifetime cannabis use and POAG, which does not significantly affect our association estimates.
Selection of genetic variants as instrumental variables
We adopted two approaches in the selection of genetic variants as instrumental variables. In the primary analysis we selected only SNPs reaching genome-wide significance (P-value < 5*10–8 for lifetime cannabis use and P-value < 5*10–7 for cannabis use disorder) following clumping for linkage disequilibrium (LD) at r2 < 0.001 across a 10mb window. In our secondary, more liberal approach12,13, we selected SNPs independently associated with lifetime cannabis use and cannabis use disorder at a GWAS P-value < 5*10–5 after accounting for LD at r2 < 0.1, in order to increase pooled instrument strength and power of the analysis. In both approaches, we calculated the percentage of phenotypic variance that is explained by our exposures of interest. By summing the coefficients of determination (R2) derived from the associations of the selected SNPs with our exposures of interest, we were able to calculate the proportion of variability in our exposure phenotypes that is explained by the selected SNPs. Finally, we performed the MR-Steiger directionality test to identify the direction of causality between lifetime cannabis use and POAG and removed SNPs that were more strongly correlated with the outcome than the exposure14. We excluded SNPs with highly influential data points in the funnel plots and scatter plots of SNP–exposure and outcome associations. Five SNPs associated with lifetime cannabis use and eleven SNPs associated with cannabis use disorder were selected in the primary analysis, while 267 and 157 SNPs associated with lifetime cannabis and cannabis use disorder, respectively, were selected in the secondary analysis.
Statistical analysis
After data harmonization, where SNPs were filtered according to HapMap315, excluded if they were strand-ambiguous and their effect sizes were aligned, we calculated Wald ratios by dividing the per-allele logarithm of odds ratio (logOR) for each selected SNP from the lifetime cannabis use and cannabis use disorder GWAS by the corresponding logOR from the same SNP in the POAG GWAS. Then, we estimated the effect of lifetime cannabis use and cannabis use disorder on the risk of POAG by pooling the Wald ratios with multiplicative random effects inverse-variance weighted (IVW) meta-analyses12.
Univariable two-sample MR was performed using summary-level statistics from the largest available GWAS on lifetime cannabis use and POAG. The two-sample MR approach rests on 3 core assumptions: (1) the genetic instruments should be robustly associated with the exposure of interest (“relevance” assumption), (2) the genetic instruments are not associated with confounders of the exposure-outcome association (“exchangeability” assumption), and (3) the genetic instruments are associated with the outcome exclusively through their effect on the exposure of interest (“exclusion restriction” assumption)16,17. The “relevance” assumption is satisfied by selecting SNPs, as instrumental variables, reaching the genome-wide significance (P-value < 5*10–8). Moreover, in order to quantify instrument strength, we calculated the proportion of variance of the exposure explained by the genetic instruments, as well as the F-statistic of our instruments18. Although, the “relevance” and “exclusion restriction” assumption cannot be proven, we performed sensitivity analyses to assess any possible violations of these assumptions. These can occur through horizontal pleiotropy, where the genetic variants affect the outcome via biological pathways other than the exposure under investigation. Thus, in the primary analysis, we utilized PhenoScanner19 to investigate associations between the selected genetic instruments with traits that could potentially confound our analysis and in case that pleotropic pathways were discovered, multivariable MR was used to adjust for these effects20. More specifically, one of our instrumental SNPs for lifetime cannabis use was associated with previously reported obesity-related phenotypes (Supplementary Table S3). Several studies have found an association between body mass index and POAG21,22, so this SNP might have been associated with POAG through pathways other than our exposure of interest and, thus, we performed multivariable IVW adjusting for BMI. Additionally, the associations of each selected SNP and its proxies (r2 > 0.8) with known risk factors for POAG were also checked. In the multivariable MR analyses the conditional F-statistic was used as a quantification of the strength of our genetic instruments23. Moreover, we assessed the heterogeneity among the selected genetic variants in the primary analysis through the Cochran Q heterogeneity test and IGX217 in order to detect pleiotropy. MR Egger regression was performed in order to assess the presence of directional pleiotropy17, as well as pleiotropy-robust methods24 (penalized weighted median, IVW radial regression and MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO)). Since only five SNPs for lifetime cannabis use were selected in our primary analysis, the IVW radial regression and the MR-PRESSO were not performed24. In order to assess whether the IVW estimate was driven by a single SNP, leave-one-out analysis was also conducted.
In our secondary analysis using a liberal threshold, we performed multiplicative random-effects IVW and pleiotropy-robust methods (penalized weighted median, IVW radial regression, MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO))24. The CAUSE MR analysis was additionally conducted as an additional method to improve statistical power and mitigate the risk of weak instrument bias7,25.
All MR estimates for the associations between our exposures and POAG were multiplied by loge2 (= 0.693), representing the change in log odds of POAG per doubling in the prevalence of our exposures26. All analyses were performed with R version 4.2.127 using the MendelianRandomization, TwoSampleMR, MVMR, MR-PRESSO and cause packages.
Results
Primary analysis results
In our primary analysis the selected 5 SNPs (Supplementary Fig. S1) explained 0.09% of the variance in the lifetime cannabis use and the F-statistics for all SNPs were ≥ 30.7 (Supplementary Table S2). The selected 11 SNPs (Supplementary Fig. S2) for cannabis use disorder explained 0.08% of the phenotypic variance and had an F-statistic of ≥ 25.5 (Supplementary Table S2). We found no evidence for an effect of the genetically predicted lifetime cannabis use on the POAG risk using the IVW method (OR = 1.04 per doubling odds of exposure; 95% CI = 0.88 to 1.23; P-value = 0.67) (Fig. 1 and Supplementary Fig. S3). The estimate from the penalized weighted median analysis was consistent with the estimate from the IVW analysis (Fig. 1). Similarly, estimates from the IVW analysis, as well as the pleiotropy-robust methods, did not support an association between genetically predicted cannabis use disorder and POAG (OR = 0.97 per doubling odds of exposure; 95% CI:0.92 to 1.03; P-value = 0.27) (Fig. 2 and Supplementary Fig. S4).
Mendelian randomization estimates for the effect of lifetime cannabis use on primary open-angle glaucoma. Estimates are reported as changes in odds of primary open-angle glaucoma per doubling in the prevalence of lifetime cannabis usea. aSNP single nucleotide polymorphism; CI confidence interval; MR-PRESSO Mendelian randomization pleiotropy residual sum and outlier; CAUSE causal analysis using summary effect estimates; BMI body mass index.
Mendelian randomization estimates for the effect of cannabis use disorder on primary open-angle glaucoma. Estimates are reported as changes in odds of primary open-angle glaucoma per doubling in the prevalence of cannabis use disordera. aSNP single nucleotide polymorphism; CI confidence interval; MR-PRESSO Mendelian randomization pleiotropy residual sum and outlier; CAUSE causal analysis using summary effect estimates; BMI body mass index.
In the multivariable IVW analysis adjusted for BMI, we found no evidence of horizontal pleiotropy introduced to the univariable estimates from lifetime cannabis use and cannabis use disorder (Figs. 1 and 2). The conditional F-statistics for lifetime cannabis use and cannabis use disorder were 12.5 and 11.6, respectively.
In our primary analysis there was no evidence of heterogeneity among Wald ratios for lifetime cannabis use and cannabis use disorder with POAG (Supplementary Table S4). The Cochran’s Q heterogeneity test yielded a value of 2.8 (P-value = 0.59) and 9.9 (P-value = 0.45), in our analyses of lifetime cannabis use and cannabis use disorder, respectively. The intercepts from the MR-Egger analyses did not deviate significantly from zero (0.029, P-value = 0.2 in the analysis including lifetime cannabis use as the exposure and − 0.036, P-value = 0.24 in the analysis including cannabis use disorder as the exposure), thus, no directional pleiotropy was present (Supplementary Table S4).
The intercepts from the MR-Egger analyses did not deviate from zero, thus, no directional pleiotropy was present (Supplementary Table S4). The leave-one-SNP-out analyses identified no SNPs with high influence on the IVW estimates for our exposures (Supplementary Table S5).
Secondary analysis results
In our secondary analysis using a liberal threshold, the selected 267 SNPs explained 2.99% of the variance in the lifetime cannabis use and the F-statistics for all SNPs were ≥ 16.4. The selected 157 SNPs for cannabis use disorder explained 0.82% of the phenotypic variance and had an F-statistic of ≥ 16.4. MR estimates from the IVW analyses, as well as from the pleiotropy-robust models showed no association between our exposures and POAG (Figs. 1 and 2). The MR-PRESSO global test provided evidence for one outlier SNP (P-value = 0.005) in the analysis with lifetime cannabis use as an exposure, which was removed (rs8140423) from the calculation of the final MR-PRESSO estimate. MR-PRESSO distortion test showed that the outlier corrected estimate did not differ significantly from the non-corrected estimate (P-value = 0.93). The CAUSE models included more instrumental SNPs in order to increase statistical power and did not reveal a causal effect of lifetime cannabis and cannabis use disorder on POAG (Figs. 1 and 2).
Discussion
In this two-sample MR we leveraged genetic data of lifetime cannabis use and cannabis use disorder from more than 180,000 and 370,000 individuals, respectively, and of 16,000 POAG cases, to assess the association of cannabis use with the risk of POAG. We found no evidence to support the hypothesis that cannabis use affects the development of POAG.
During the last decades, several lines of evidence have been put forward to elucidate the effect of cannabis on POAG. It has been postulated that cannabis consumption may have a protective effect on the risk of POAG, by a salutary effect on IOP5, without knowing the exact pathogenetic mechanism of this phenomenon. It has been hypothesized that IOP decrease results from the activation of the cannabinoid-related receptors in the ciliary body of the eye, by Δ9-tetrahydrocannabinol, the psychoactive constituent of cannabis28. As a result, the ciliary body reduces the production of aqueous humor and IOP decreases. An alternative hypothesis supports that the IOP lowering effect of cannabis is mediated through a decrease in blood pressure29. However, this hypothesized mechanism could potentially increase risk of POAG since it lowers ocular perfusion pressure and, thus may compromise perfusion of the optic nerve head30. Moreover, when cannabis is smoked, several toxic and carcinogenic compounds are inhaled31. The systemic absorption of these compounds may occur in higher concentrations than in tobacco smoking, mainly because of the way that cannabis is smoked. Usually, filters are lacking in cannabis cigarettes and a longer and deeper inhalation is required32. As a result, cannabis smoking may yield similar negative effects as tobacco smoking in POAG33. In our analysis none of our instrumental SNPs was associated with tobacco smoking, and thus, we did not adjust for it in the multivariable model.
Although, several interventional studies have investigated the effects of cannabis in IOP, evidence on the effect of cannabis in POAG are scarce and usually limited by small sample sizes. In a recent prospective study of the UK Biobank cohort34, lifetime cannabis use was not associated with POAG in multivariable analysis adjusted for tobacco smoking and other confounders. Similar to our MR estimates, the OR of POAG for cannabis use versus never-cannabis use was 1.03 (95% CI: 0.91–1.17). On the contrary, in the same study, it was found that participants who used cannabis 11 to 100 times in their lifetime had lower mean IOP compared to those who had never consumed cannabis, without, however, adjusting for potential confounders. The existing interventional studies on the association between cannabis use and IOP35 have several limitations including, small sample sizes and duration of studies, inclusion of patients with various types of glaucoma, which limits the generalization of the result, and lack of specification of the time that IOP was measured.
The key strength of this study was the utilization of the largest to date GWAS meta-analysis of POAG, which increased the statistical precision of our estimates. Moreover, our MR estimates have been shown to be robust to model violations in sensitivity analyses. The study does though have several limitations. First, because of the binary nature of our exposure we were not able to assess any dose-dependent changes in POAG. A more detailed description of our exposure was not available, so we were also not able to assess the chemical composition of cannabis consumed nor its route of administration. Second, we did not investigate the association of cannabis use on other types of glaucoma (e.g., low tension glaucoma).
In conclusion, our data provided evidence for a lack of association of genetic liability to lifetime cannabis use and cannabis use disorder with POAG. Triangulation of evidence from different types of research studies, with different key sources of bias, is warranted to confirm these results.
Data availability
The summary statistics for the lifetime cannabis use GWAS are available at https://www.ru.nl/bsi/research/group-pages/substance-use-addiction-food-saf/vm-saf/genetics/international-cannabis-consortium-icc/ (access date: 2022/10/17). The cannabis use disorder data are available at https://ipsych.dk/en/research/downloads/data-download-agreement-ipsych-secondary-phenotypes-cannabis-2019/ (access date: 2022/10/17). The primary open-angle glaucoma summary data are available at https://www.ebi.ac.uk/gwas/publications/33627673 (access date: 2023/07/20).
References
Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121, 2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013 (2014).
Le, A., Mukesh, B. N., McCarty, C. A. & Taylor, H. R. Risk factors associated with the incidence of open-angle glaucoma: The visual impairment project. Invest. Ophthalmol. Vis. Sci. 44, 3783–3789. https://doi.org/10.1167/iovs.03-0077 (2003).
Prum, B. E. Jr. et al. Primary open-angle glaucoma preferred practice pattern(®) guidelines. Ophthalmology 123, P41-p111. https://doi.org/10.1016/j.ophtha.2015.10.053 (2016).
Beckers, H. J. M., Schouten, J. S. A. G., Webers, C. A. B., van der Valk, R. & Hendrikse, F. Side effects of commonly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefe’s Archiv. Clin. Exp. Ophthalmol. 246, 1485–1490. https://doi.org/10.1007/s00417-008-0875-7 (2008).
Novack, G. D. Cannabinoids for treatment of glaucoma. Curr. Opin. Ophthalmol. 27, 146–150. https://doi.org/10.1097/icu.0000000000000242 (2016).
Agrawal, A., Budney, A. J. & Lynskey, M. T. The co-occurring use and misuse of cannabis and tobacco: A review. Addiction 107, 1221–1233. https://doi.org/10.1111/j.1360-0443.2012.03837.x (2012).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Primers 2, 6. https://doi.org/10.1038/s43586-021-00092-5 (2022).
Pasman, J. A. et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability. Nat. Neurosci. 21, 1161–1170. https://doi.org/10.1038/s41593-018-0206-1 (2018).
Johnson, E. C. et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry 7, 1032–1045. https://doi.org/10.1016/s2215-0366(20)30339-4 (2020).
Gharahkhani, P. et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nature Communications 12, 1258. https://doi.org/10.1038/s41467-020-20851-4 (2021).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ 375, n2233. https://doi.org/10.1136/bmj.n2233 (2021).
Burgess, S., Dudbridge, F. & Thompson, S. G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 35, 1880–1906 (2015).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res 4, 186. https://doi.org/10.12688/wellcomeopenres.15555.2 (2019).
Hemani, G., Tilling, K. & Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13, e1007081. https://doi.org/10.1371/journal.pgen.1007081 (2017).
Frazer, K. A. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861. https://doi.org/10.1038/nature06258 (2007).
Burgess, S., Foley, C. N. & Zuber, V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu. Rev. Genomics Hum. Genet. 19, 303–327. https://doi.org/10.1146/annurev-genom-083117-021731 (2018).
Hemani, G., Bowden, J. & Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 27, R195–R208. https://doi.org/10.1093/hmg/ddy163 (2018).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163. https://doi.org/10.1002/sim.3034 (2008).
Kamat, M. A. et al. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 35, 4851–4853. https://doi.org/10.1093/bioinformatics/btz469 (2019).
Sanderson, E. Multivariable Mendelian randomization and mediation. Cold Spring Harb. Perspect. Med. 11, 038984. https://doi.org/10.1101/cshperspect.a038984 (2021).
Marshall, H. et al. Association between body mass index and primary open angle glaucoma in three cohorts. Am. J. Ophthalmol. 245, 126–133. https://doi.org/10.1016/j.ajo.2022.08.006 (2022).
Lin, S. C., Pasquale, L. R., Singh, K. & Lin, S. C. The association between body mass index and open-angle glaucoma in a South Korean population-based sample. J. Glaucoma 27, 239–245. https://doi.org/10.1097/ijg.0000000000000867 (2018).
Sanderson, E., Spiller, W. & Bowden, J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat. Med. 40, 5434–5452. https://doi.org/10.1002/sim.9133 (2021).
Slob, E. A. W. & Burgess, S. A comparison of robust Mendelian randomization methods using summary data. Genet. Epidemiol. 44, 313–329. https://doi.org/10.1002/gepi.22295 (2020).
Morrison, J., Knoblauch, N., Marcus, J. H., Stephens, M. & He, X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 52, 740–747. https://doi.org/10.1038/s41588-020-0631-4 (2020).
Burgess, S. & Labrecque, J. A. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur. J. Epidemiol. 33, 947–952. https://doi.org/10.1007/s10654-018-0424-6 (2018).
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Miller, S., Daily, L., Leishman, E., Bradshaw, H. & Straiker, A. Δ9-Tetrahydrocannabinol and cannabidiol differentially regulate intraocular pressure. Invest. Ophthalmol. Vis. Sci. 59, 5904–5911. https://doi.org/10.1167/iovs.18-24838 (2018).
Sun, X., Xu, C. S., Chadha, N., Chen, A. & Liu, J. Marijuana for glaucoma: A recipe for disaster or treatment?. Yale J. Biol. Med. 88, 265–269 (2015).
Leske, M. C. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr. Opin. Ophthalmol. 20, 73–78. https://doi.org/10.1097/ICU.0b013e32831eef82 (2009).
Moir, D. et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 21, 494–502. https://doi.org/10.1021/tx700275p (2008).
Wu, T. C., Tashkin, D. P., Djahed, B. & Rose, J. E. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 318, 347–351. https://doi.org/10.1056/nejm198802113180603 (1988).
Jain, V., Jain, M., Abdull, M. M. & Bastawrous, A. The association between cigarette smoking and primary open-angle glaucoma: A systematic review. Int. Ophthalmol. 37, 291–301. https://doi.org/10.1007/s10792-016-0245-0 (2017).
Lehrer, S. & Rheinstein, P. H. Cannabis smoking and glaucoma in the UK Biobank cohort. J. Fr. Ophtalmol. 45, 423–429. https://doi.org/10.1016/j.jfo.2021.12.012 (2022).
MacMillan, K., Keddy, A. & Furlong, J. Cannabis and glaucoma: A literature review. Dalhousie Med J 46, 5. https://doi.org/10.15273/dmj.Vol46No1.9830 (2019).
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support from the Open Access Publication Fund of the University of Münster.
Author information
Authors and Affiliations
Contributions
K.A., B.S.E. and N.M. contributed to the study conception and design, drafted the manuscript and analyzed the data. All authors critically revised the manuscript for important intellectual content, provided administrative, technical, or material support and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Katsimpris, A., Baumeister, SE., Baurecht, H. et al. Cannabis use and the risk of primary open-angle glaucoma: a Mendelian randomization study. Sci Rep 13, 19605 (2023). https://doi.org/10.1038/s41598-023-45872-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45872-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.